
Journal of Shanghai Jiao Tong University (Medical Science) ›› 2024, Vol. 44 ›› Issue (11): 1391-1405.doi: 10.3969/j.issn.1674-8115.2024.11.006
• Basic research • Previous Articles
SHI Zelun1( ), WANG Qing1(
), WANG Qing1( ), HE Wen1, FU Weijia1, WANG Yingwen2, HAN Xiao3(
), HE Wen1, FU Weijia1, WANG Yingwen2, HAN Xiao3( ), ZHANG Xiaobo1(
), ZHANG Xiaobo1( )
)
Received:2024-03-11
Accepted:2024-05-21
Online:2024-11-28
Published:2024-11-28
Contact:
HAN Xiao,ZHANG Xiaobo
E-mail:21211240020@m.fudan.edu.cn;wq141269@163.com;sqhx12@126.com;zhangxiaobo0307@163.com
Supported by:CLC Number:
SHI Zelun, WANG Qing, HE Wen, FU Weijia, WANG Yingwen, HAN Xiao, ZHANG Xiaobo. Nanoplastics aggravate severe asthma by inducing DNA damage of alveolar type Ⅱ epithelial cells[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2024, 44(11): 1391-1405.
Tab 1 Primer sequences for qPCR

Fig 2 Effect of exposure to PS-NPs on BALF inflammatory cell counts, lung tissue pathological sections and histopathological scores in mice with severe asthma
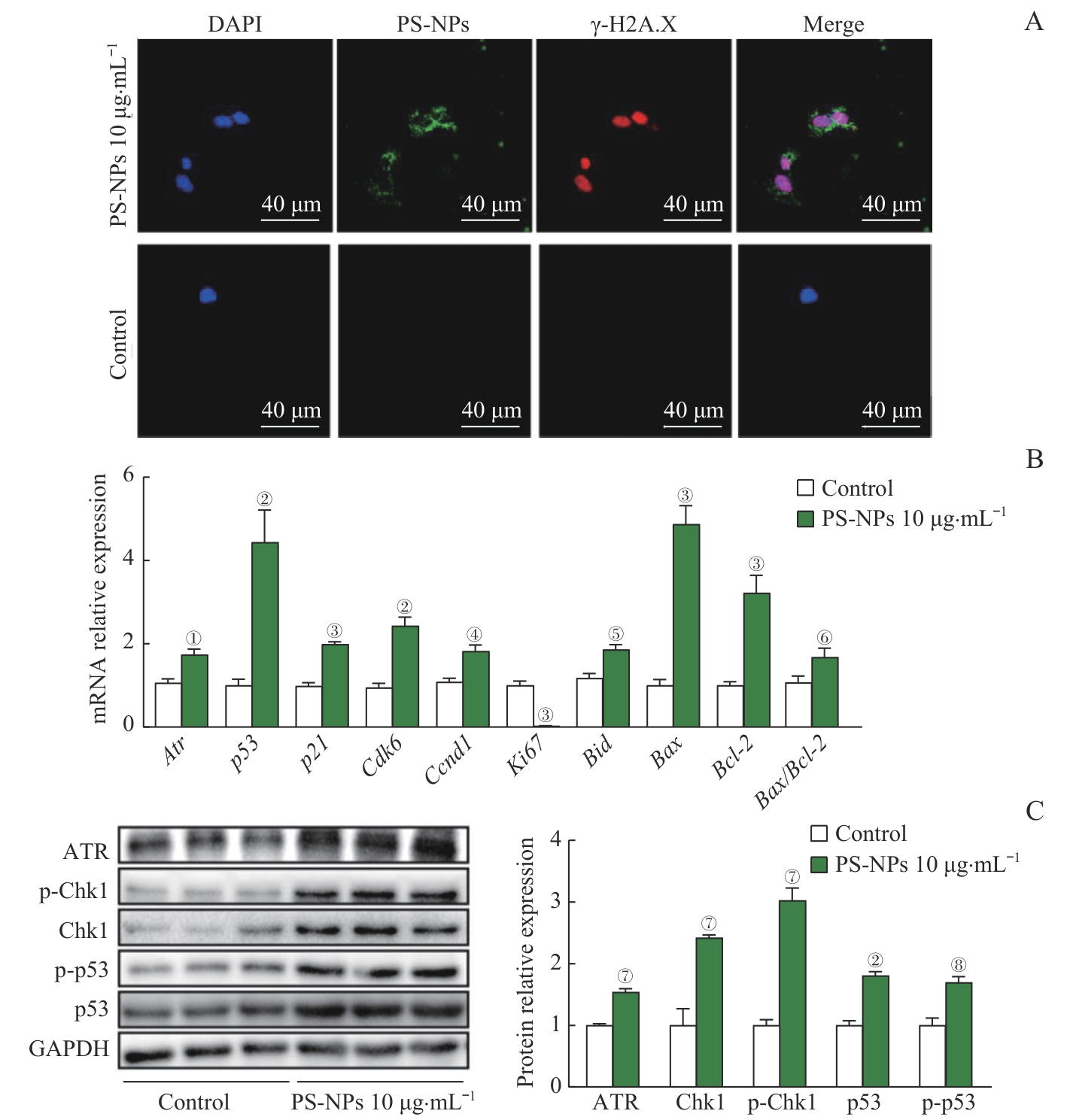
Fig 6 In vitro experiments confirmed the DNA damage induction and the upregulation of the ATR/Chk1/p53 signaling pathway in AT2 cells after PS-NPs exposure
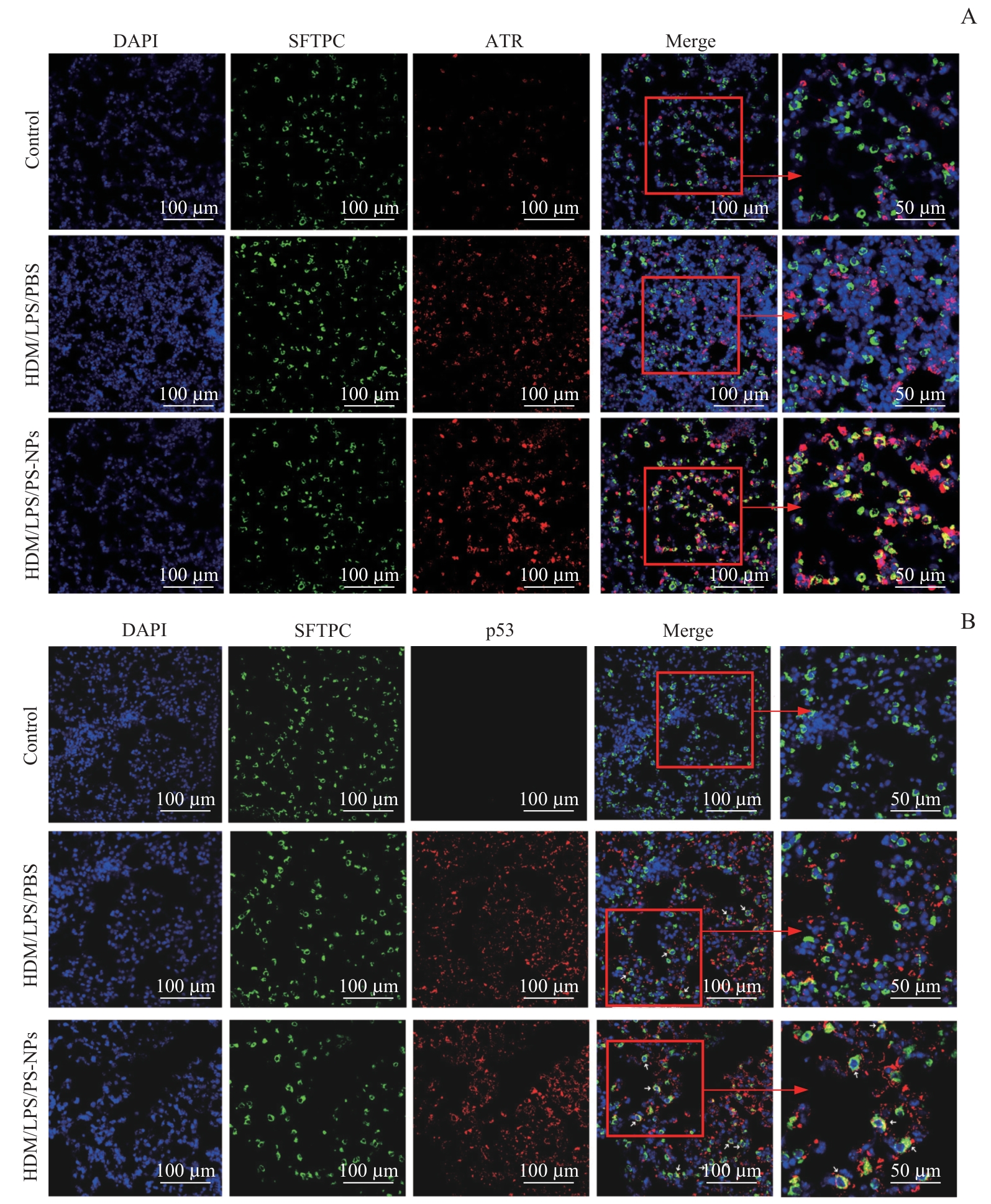
Fig 7 In vivo experiments confirmed that the upregulated expression levels of ATR and p53 in Sftpc+ AT2 cells of severe asthma mice after PS-NPs exposure
| 1 | 中华医学会儿科学分会呼吸学组, 《中华儿科杂志》编辑委员会. 儿童支气管哮喘诊断与防治指南(2016年版)[J]. 中华儿科杂志, 2016, 54(3): 167-181. |
| Respiratory Group, Pediatrics Society of Chinese Medical Association, Editorial Board of Chinese Journal of Pediatrics. Guidelines for diagnosis and prevention of bronchial asthma in children (2016 edition)[J]. Chinese Journal of Pediatrics, 2016, 54(3): 167-181. | |
| 2 | 全国儿科哮喘协作组, 中国疾病预防控制中心环境与健康相关产品安全所. 第三次中国城市儿童哮喘流行病学调查[J]. 中华儿科杂志, 2013, 51(10): 729-735. |
| The National Cooperative Group on Childhood Asthma, Institute of Environmental Health and Related Product Safety, Chinese Center for Disease Control and Prevention. Third nationwide survey of childhood asthma in urban areas of China[J]. Chinese Journal of Pediatrics, 2013, 51(10): 729-735. | |
| 3 | CARR T F, BLEECKER E. Asthma heterogeneity and severity[J]. World Allergy Organ J, 2016, 9(1): 41. |
| 4 | 中华医学会呼吸病学分会哮喘学组. 支气管哮喘防治指南(2020年版)[J]. 中华结核和呼吸杂志, 2020, 43(12): 1023-1048. |
| Chinese Medical Association Respiratory Diseases Branch Asthma Group. Guidelines for prevention and treatment of bronchial asthma (2020 edition)[J]. Chinese Journal of Tuberculosis and Respiratory Diseases, 2020, 43(12): 1023-1048. | |
| 5 | LACHOWICZ-SCROGGINS M E, DUNICAN E M, CHARBIT A R, et al. Extracellular DNA, neutrophil extracellular traps, and inflammasome activation in severe asthma[J]. Am J Respir Crit Care Med, 2019, 199(9): 1076-1085. |
| 6 | LIU T, WANG F P, WANG G, et al. Role of neutrophil extracellular traps in asthma and chronic obstructive pulmonary disease[J]. Chin Med J (Engl), 2017, 130(6): 730-736. |
| 7 | COSTA-GÓMEZ I, SUAREZ-SUAREZ M, MORENO J M, et al. A novel application of thermogravimetry-mass spectrometry for polystyrene quantification in the PM10 and PM2.5 fractions of airborne microplastics[J]. Sci Total Environ, 2023, 856(Pt 2): 159041. |
| 8 | SUN J, HO S S H, NIU X Y, et al. Explorations of tire and road wear microplastics in road dust PM2.5 at eight megacities in China[J]. Sci Total Environ, 2022, 823: 153717. |
| 9 | AMATO-LOURENÇO L F, CARVALHO-OLIVEIRA R, JÚNIOR G R, et al. Presence of airborne microplastics in human lung tissue[J]. J Hazard Mater, 2021, 416: 126124. |
| 10 | WANG L L, NETTO K G, ZHOU L J, et al. Single-cell transcriptomic analysis reveals the immune landscape of lung in steroid-resistant asthma exacerbation[J]. Proc Natl Acad Sci USA, 2021, 118(2): e2005590118. |
| 11 | HADJIGOL S, NETTO K G, MALTBY S, et al. Lipopolysaccharide induces steroid-resistant exacerbations in a mouse model of allergic airway disease collectively through IL-13 and pulmonary macrophage activation[J]. Clin Exp Allergy, 2020, 50(1): 82-94. |
| 12 | KRISHNAMOORTHY N, DOUDA D N, BRÜGGEMANN T R, et al. Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma[J]. Sci Immunol, 2018, 3(26): eaao4747. |
| 13 | HUANG S M, HUANG X X, BI R, et al. Detection and analysis of microplastics in human sputum[J]. Environ Sci Technol, 2022, 56(4): 2476-2486. |
| 14 | LIM D, JEONG J, SONG K S, et al. Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412[J]. Chemosphere, 2021, 262: 128330. |
| 15 | LUO H J, XIAO T, SUN X X, et al. The regulation of circRNA_kif26b on alveolar epithelial cell senescence via miR-346-3p is involved in microplastics-induced lung injuries[J]. Sci Total Environ, 2023, 882: 163512. |
| 16 | SCHOLZEN T, GERDES J. The Ki-67 protein: from the known and the unknown[J]. J Cell Physiol, 2000, 182(3): 311-322. |
| 17 | ROOS W P, THOMAS A D, KAINA B. DNA damage and the balance between survival and death in cancer biology[J]. Nat Rev Cancer, 2016, 16: 20-33. |
| 18 | LIU J H, ZHANG J, REN L H, et al. Fine particulate matters induce apoptosis via the ATM/P53/CDK2 and mitochondria apoptosis pathway triggered by oxidative stress in rat and GC-2spd cell[J]. Ecotoxicol Environ Saf, 2019, 180: 280-287. |
| 19 | JIN Y, LI Y T, HE S Y, et al. ATM participates in fine particulate matter-induced airway inflammation through regulating DNA damage and DNA damage response[J]. Environ Toxicol, 2023, 38(11): 2668-2678. |
| 20 | QUEZADA-MALDONADO E M, SÁNCHEZ-PÉREZ Y, CHIRINO Y I, et al. Airborne particulate matter induces oxidative damage, DNA adduct formation and alterations in DNA repair pathways[J]. Environ Pollut, 2021, 287: 117313. |
| 21 | MESSIER E M, BAHMED K, TUDER R M, et al. Trolox contributes to Nrf2-mediated protection of human and murine primary alveolar type Ⅱ cells from injury by cigarette smoke[J]. Cell Death Dis, 2013, 4(4): e573. |
| 22 | KIM Y H, KANG M K, LEE E J, et al. Dried yeast extracts curtails pulmonary oxidative stress, inflammation and tissue destruction in a model of experimental emphysema[J]. Antioxidants, 2019, 8(9): 349. |
| 23 | BOROK Z, HORIE M, FLODBY P, et al. Grp78 loss in epithelial progenitors reveals an age-linked role for endoplasmic reticulum stress in pulmonary fibrosis[J]. Am J Respir Crit Care Med, 2020, 201(2): 198-211. |
| 24 | PRATA J C. Airborne microplastics: consequences to human health?[J]. Environ Pollut, 2018, 234: 115-126. |
| 25 | CRISFORD H, SAPEY E, ROGERS G B, et al. Neutrophils in asthma: the good, the bad and the bacteria[J]. Thorax, 2021, 76(8): 835-844. |
| 26 | RAY A, KOLLS J K. Neutrophilic inflammation in asthma and association with disease severity[J]. Trends Immunol, 2017, 38(12): 942-954. |
| 27 | WANG J Y. The innate immune response in house dust mite-induced allergic inflammation[J]. Allergy Asthma Immunol Res, 2013, 5(2): 68-74. |
| 28 | GOLDBERG M S, THÉRIAULT G. Retrospective cohort study of workers of a synthetic textiles plant in Quebec: Ⅰ. General mortality[J]. Am J Ind Med, 1994, 25(6): 889-907. |
| 29 | MASTRANGELO G, BOMBANA S, PRIANTE E, et al. Repeated case-control studies as a method of surveillance for asthma in occupations[J]. J Occup Environ Med, 1997, 39(1): 51-57. |
| 30 | FAN Z, XIAO T, LUO H J, et al. A study on the roles of long non-coding RNA and circular RNA in the pulmonary injuries induced by polystyrene microplastics[J]. Environ Int, 2022, 163: 107223. |
| 31 | ZHA H, XIA J F, LI S J, et al. Airborne polystyrene microplastics and nanoplastics induce nasal and lung microbial dysbiosis in mice[J]. Chemosphere, 2023, 310: 136764. |
| 32 | DO D C, ZHANG Y, TU W, et al. Type Ⅱ alveolar epithelial cell-specific loss of RhoA exacerbates allergic airway inflammation through SLC26A4[J]. JCI Insight, 2021, 6(14): e148147. |
| 33 | WANG J, ZHAO Y L, ZHANG X, et al. Type Ⅱ alveolar epithelial cell aryl hydrocarbon receptor protects against allergic airway inflammation through controlling cell autophagy[J]. Front Immunol, 2022, 13: 964575. |
| 34 | KWON M, JUNG J, PARK H S, et al. Diesel exhaust particle exposure accelerates oxidative DNA damage and cytotoxicity in normal human bronchial epithelial cells through PD-L1[J]. Environ Pollut, 2023, 317: 120705. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 1121
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 239
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||