
JOURNAL OF SHANGHAI JIAOTONG UNIVERSITY (MEDICAL SCIENCE) ›› 2022, Vol. 42 ›› Issue (1): 82-89.doi: 10.3969/j.issn.1674-8115.2022.01.012
• Clinical research • Previous Articles Next Articles
Yu ZHANG1( ), Xiaoyuan WU2(
), Xiaoyuan WU2( ), Lihua GUAN3, Yiyuan LIU4, Xingyue PENG4, Haiyan XIE1, Wei HU1, Keke HAO1, Ning XIA1, Guojun LU1, Zhibo HOU1
), Lihua GUAN3, Yiyuan LIU4, Xingyue PENG4, Haiyan XIE1, Wei HU1, Keke HAO1, Ning XIA1, Guojun LU1, Zhibo HOU1
Received:2021-08-04
Online:2022-01-28
Published:2022-02-18
Contact:
Yu ZHANG
E-mail:zhangyu2113_nj@163.com
Supported by:CLC Number:
Yu ZHANG, Xiaoyuan WU, Lihua GUAN, Yiyuan LIU, Xingyue PENG, Haiyan XIE, Wei HU, Keke HAO, Ning XIA, Guojun LU, Zhibo HOU. Application of high-throughput drug sensitivity screening system in the treatment of non-small cell lung cancer with malignant pleural effusion[J]. JOURNAL OF SHANGHAI JIAOTONG UNIVERSITY (MEDICAL SCIENCE), 2022, 42(1): 82-89.
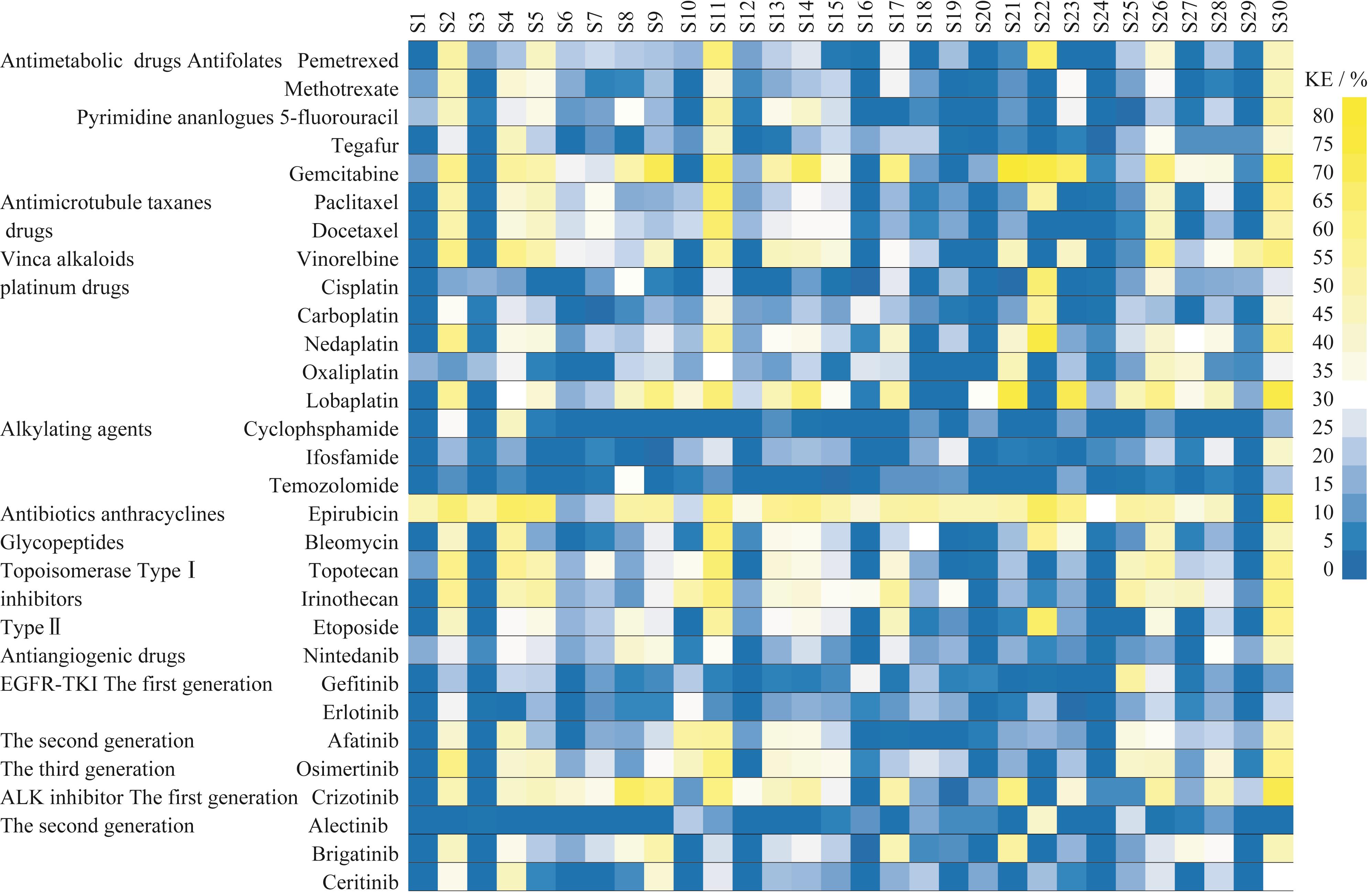
Fig 3 Heat map and hierarchical clustering analysis of the killing efficiency of 30 drugs in primary tumor cells cultured from 30 MPE patients with NSCLC
| Sample | Gender | Age /year | Therapy line | Histology | EGFR/ALK/ ROS1 testing | Treatment | Clinical efficacy | High-throughput drug sensitivity test in vitro | Consistency analysis |
|---|---|---|---|---|---|---|---|---|---|
| S1 | Male | 72 | 1 | Adenocarcinoma | EGFR19DEL | Gefitinib | PR | Gefitinib resistance | Inconsistent |
| S3 | Female | 48 | 1 | Adenocarcinoma | EGFR19DEL | Gefitinib | PR | Gefitinib resistance | Inconsistent |
| S4 | Female | 78 | 1 | Adenocarcinoma | Negative | Pemetrexed + Carboplatin | SD | Low sensitive to Pemetrexed and Carboplatin | Consistent |
| S5 | Female | 48 | 1 | Adenocarcinoma | EGFR19DEL | Gefitinib | PR | Low sensitive to Gefitinib | Consistent |
| S7 | Female | 48 | 1 | Adenocarcinoma | Negative | Pemetrexed + Cisplatin + Bevacizumab | PR | Low sensitive to Pemetrexed and Cisplatin | Consistent |
| S10 | Female | 60 | 1 | Adenocarcinoma | Negative | Pemetrexed + Carboplatin | SD | Low sensitive to Pemetrexed and Carboplatin | Consistent |
| S15 | Female | 67 | 1 | Adenocarcinoma | Negative | Pemetrexed + Cisplatin | PR | Resistant to Pemetrexed and Cisplatin | Inconsistent |
| S16 | Male | 63 | 1 | Adenocarcinoma | EGFR21L858R | Gefitinib | SD | Low sensitive to Gefitinib | Consistent |
| S19 | Male | 74 | 1 | Adenosquamous | Negative | Docetaxel + Nedaplatin | SD | Low sensitive to Docetaxel and Nedaplatin | Consistent |
| S20 | Female | 57 | 1 | Adenocarcinoma | EGFR19DEL | Afatinib | PR | Afatinib resistance | Inconsistent |
| S23 | Male | 65 | 1 | Adenocarcinoma | EGFR19DEL | Gefitinib | PR | Gefitinib resistance | Inconsistent |
| S24 | Male | 77 | 1 | Adenocarcinoma | Negative | Pemetrexed + Carboplatin | SD | Resistant to Pemetrexed and Carboplatin | Inconsistent |
| S25 | Male | 74 | 1 | Adenocarcinoma | EGFR21L858R | Gefitinib | PR | Moderate sensitive to Gefitinib | Consistent |
| S26 | Female | 66 | 1 | Adenocarcinoma | EGFR21L861Q | Afatinib | PR | Low sensitive to Afatinib | Consistent |
| S27 | Male | 92 | 1 | Adenocarcinoma | EGFR19DEL | Gefitinib | SD | Gefitinib resistance | Inconsistent |
| S8 | Male | 45 | 3 | Adenocarcinoma | Negative | Vinorelbine + Lobaplatin | SD | Low sensitive to Vinorelbine, moderate sensitive to Lobaplatin | Consistent |
| S11 | Female | 48 | 3 | Adenocarcinoma | EGFR19DEL | Pemetrexed + Carboplatin + Bevacizumab | PR | Moderate sensitive to Pemetrexed and Carboplatin | Consistent |
| S13 | Male | 55 | 2 | Squamous | Negative | Gemcitabine + Nedaplatin | SD | Moderate sensitive to Gemcitabine, low sensitive to Nedaplatin | Consistent |
| S14 | Female | 82 | 3 | Adenocarcinoma | EGFR21L858R/T790M/C797S(trans) | S1+Anlotinib | SD | Low sensitive to Tegafur and Nintedanib | Consistent |
| S18 | Male | 72 | 3 | Squamous | Negative | S1 | SD | Low sensitive to Tegafur | Consistent |
| S21 | Male | 45 | 5 | Adenocarcinoma | EGFR19DEL/ T790M/C797S(trans) | Gemcitabine + Lobaplatin | PR | High sensitive to Gemcitabine, moderate sensitive to Lobaplatin | Consistent |
| S22 | Female | 71 | 3 | Adenocarcinoma | EGFR21L858R/T790M/C797S(trans) | Pemetrexed + Carboplatin +Bevacizumab | PR | Moderate sensitive to Pemetrexed and Carboplatin | Consistent |
| S29 | Female | 60 | 3 | Adenocarcinoma | EGFR21L858R+BRAF V600E | Pemetrexed + Carboplatin | PD | Resistant to Pemetrexed and Carboplatin | Consistent |
Tab 1 Clinical characteristics of 23 NSCLC patients with evaluable clinical efficacy and the consistency between the results of high-throughput drug sensitivity test in vitro and the clinical efficacy
| Sample | Gender | Age /year | Therapy line | Histology | EGFR/ALK/ ROS1 testing | Treatment | Clinical efficacy | High-throughput drug sensitivity test in vitro | Consistency analysis |
|---|---|---|---|---|---|---|---|---|---|
| S1 | Male | 72 | 1 | Adenocarcinoma | EGFR19DEL | Gefitinib | PR | Gefitinib resistance | Inconsistent |
| S3 | Female | 48 | 1 | Adenocarcinoma | EGFR19DEL | Gefitinib | PR | Gefitinib resistance | Inconsistent |
| S4 | Female | 78 | 1 | Adenocarcinoma | Negative | Pemetrexed + Carboplatin | SD | Low sensitive to Pemetrexed and Carboplatin | Consistent |
| S5 | Female | 48 | 1 | Adenocarcinoma | EGFR19DEL | Gefitinib | PR | Low sensitive to Gefitinib | Consistent |
| S7 | Female | 48 | 1 | Adenocarcinoma | Negative | Pemetrexed + Cisplatin + Bevacizumab | PR | Low sensitive to Pemetrexed and Cisplatin | Consistent |
| S10 | Female | 60 | 1 | Adenocarcinoma | Negative | Pemetrexed + Carboplatin | SD | Low sensitive to Pemetrexed and Carboplatin | Consistent |
| S15 | Female | 67 | 1 | Adenocarcinoma | Negative | Pemetrexed + Cisplatin | PR | Resistant to Pemetrexed and Cisplatin | Inconsistent |
| S16 | Male | 63 | 1 | Adenocarcinoma | EGFR21L858R | Gefitinib | SD | Low sensitive to Gefitinib | Consistent |
| S19 | Male | 74 | 1 | Adenosquamous | Negative | Docetaxel + Nedaplatin | SD | Low sensitive to Docetaxel and Nedaplatin | Consistent |
| S20 | Female | 57 | 1 | Adenocarcinoma | EGFR19DEL | Afatinib | PR | Afatinib resistance | Inconsistent |
| S23 | Male | 65 | 1 | Adenocarcinoma | EGFR19DEL | Gefitinib | PR | Gefitinib resistance | Inconsistent |
| S24 | Male | 77 | 1 | Adenocarcinoma | Negative | Pemetrexed + Carboplatin | SD | Resistant to Pemetrexed and Carboplatin | Inconsistent |
| S25 | Male | 74 | 1 | Adenocarcinoma | EGFR21L858R | Gefitinib | PR | Moderate sensitive to Gefitinib | Consistent |
| S26 | Female | 66 | 1 | Adenocarcinoma | EGFR21L861Q | Afatinib | PR | Low sensitive to Afatinib | Consistent |
| S27 | Male | 92 | 1 | Adenocarcinoma | EGFR19DEL | Gefitinib | SD | Gefitinib resistance | Inconsistent |
| S8 | Male | 45 | 3 | Adenocarcinoma | Negative | Vinorelbine + Lobaplatin | SD | Low sensitive to Vinorelbine, moderate sensitive to Lobaplatin | Consistent |
| S11 | Female | 48 | 3 | Adenocarcinoma | EGFR19DEL | Pemetrexed + Carboplatin + Bevacizumab | PR | Moderate sensitive to Pemetrexed and Carboplatin | Consistent |
| S13 | Male | 55 | 2 | Squamous | Negative | Gemcitabine + Nedaplatin | SD | Moderate sensitive to Gemcitabine, low sensitive to Nedaplatin | Consistent |
| S14 | Female | 82 | 3 | Adenocarcinoma | EGFR21L858R/T790M/C797S(trans) | S1+Anlotinib | SD | Low sensitive to Tegafur and Nintedanib | Consistent |
| S18 | Male | 72 | 3 | Squamous | Negative | S1 | SD | Low sensitive to Tegafur | Consistent |
| S21 | Male | 45 | 5 | Adenocarcinoma | EGFR19DEL/ T790M/C797S(trans) | Gemcitabine + Lobaplatin | PR | High sensitive to Gemcitabine, moderate sensitive to Lobaplatin | Consistent |
| S22 | Female | 71 | 3 | Adenocarcinoma | EGFR21L858R/T790M/C797S(trans) | Pemetrexed + Carboplatin +Bevacizumab | PR | Moderate sensitive to Pemetrexed and Carboplatin | Consistent |
| S29 | Female | 60 | 3 | Adenocarcinoma | EGFR21L858R+BRAF V600E | Pemetrexed + Carboplatin | PD | Resistant to Pemetrexed and Carboplatin | Consistent |
| 1 | BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. |
| 2 | SIEGEL R L, MILLER K D, JEMAL A. Cancer statistics, 2018[J]. CA Cancer J Clin, 2018, 68(1): 7-30. |
| 3 | BURROWS C M, MATHEWS W C, COLT H G. Predicting survival in patients with recurrent symptomatic malignant pleural effusions: an assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease[J]. Chest, 2000, 117(1): 73-78. |
| 4 | CUFER T, KNEZ L. Update on systemic therapy of advanced non-small-cell lung cancer[J]. Expert Rev Anticancer Ther, 2014, 14(10): 1189-1203. |
| 5 | SZULKIN A, OTVÖS R, HILLERDAL C O, et al. Characterization and drug sensitivity profiling of primary malignant mesothelioma cells from pleural effusions[J]. BMC Cancer, 2014, 14: 709. |
| 6 | ÖTVÖS R, SZULKIN A, HILLERDAL C O, et al. Drug sensitivity profiling and molecular characteristics of cells from pleural effusions of patients with lung adenocarcinoma[J]. Genes Cancer, 2015, 6(3/4): 119-128. |
| 7 | 中国临床肿瘤学会指南工作委员会组织. 中国临床肿瘤学会(CSCO)原发性肺癌诊疗指南-2019[M]. 北京: 人民卫生出版社, 2019. |
| 8 | LEE S H. Chemotherapy for lung cancer in the era of personalized medicine[J]. Tuberc Respir Dis (Seoul), 2019, 82(3): 179-189. |
| 9 | FRIBOULET L, OLAUSSEN K A, PIGNON J P, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer[J]. N Engl J Med, 2013, 368(12): 1101-1110. |
| 10 | OLAUSSEN K A, POSTEL-VINAY S. Predictors of chemotherapy efficacy in non-small-cell lung cancer: a challenging landscape[J]. Ann Oncol, 2016, 27(11): 2004-2016. |
| 11 | SEUFFERLEIN T, AHN J, KRNDIJA D, et al. Tumor biology and cancer therapy: an evolving relationship[J]. Cell Commun Signal, 2009, 7: 19. |
| 12 | BEN-DAVID U, SIRANOSIAN B, HA G, et al. Genetic and transcriptional evolution alters cancer cell line drug response[J]. Nature, 2018, 560(7718): 325-330. |
| 13 | JIN K T, TENG L S, SHEN Y P, et al. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review[J]. Clin Transl Oncol, 2010, 12(7): 473-480. |
| 14 | FULCHER M L, RANDELL S H. Human nasal and tracheo-bronchial respiratory epithelial cell culture[J]. Methods Mol Biol, 2013, 945: 109-121. |
| 15 | WILDING J L, BODMER W F. Cancer cell lines for drug discovery and development[J]. Cancer Res, 2014, 74(9): 2377-2384. |
| 16 | BYRNE A T, ALFÉREZ D G, AMANT F, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts[J]. Nat Rev Cancer, 2017, 17(4): 254-268. |
| 17 | GAO H, KORN J M, FERRETTI S, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response[J]. Nat Med, 2015, 21(11): 1318-1325. |
| 18 | ROSSI G, MANFRIN A, LUTOLF M P. Progress and potential in organoid research[J]. Nat Rev Genet, 2018, 19(11): 671-687. |
| 19 | DE WETERING MVAN, FRANCIES H E, FRANCIS J M, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients[J]. Cell, 2015, 161(4): 933-945. |
| 20 | CUNDERLÍKOVÁ B. Issues to be considered when studying cancer in vitro[J]. Crit Rev Oncol Hematol, 2013, 85(2): 95-111. |
| 21 | YIN S Y, XI R B, WU A W, et al. Patient-derived tumor-like cell clusters for drug testing in cancer therapy[J]. Sci Transl Med, 2020, 12(549): eaaz1723. |
| 22 | MARKASZ L, KIS L L, STUBER G, et al. Hodgkin-lymphoma-derived cells show high sensitivity to dactinomycin and paclitaxel[J]. Leuk Lymphoma, 2007, 48(9): 1835-1845. |
| 23 | SKRIBEK H, OTVOS R, FLABERG E, et al. Chronic lymphoid leukemia cells are highly sensitive to the combination of prednisolone and daunorubicin, but much less to doxorubicin or epirubicin[J]. Exp Hematol, 2010, 38(12): 1219-1230. |
| 24 | DEDINSZKI D, KISS A, MÁRKÁSZ L, et al. Inhibition of protein phosphatase-1 and-2A decreases the chemosensitivity of leukemic cells to chemotherapeutic drugs[J]. Cell Signal, 2015, 27(2): 363-372. |
| 25 | MANCINI R, GIARNIERI E, DE VITIS C, et al. Spheres derived from lung adenocarcinoma pleural effusions: molecular characterization and tumor engraftment[J]. PLoS One, 2011, 6(7): e21320. |
| 26 | SZULKIN A, NILSONNE G, MUNDT F, et al. Variation in drug sensitivity of malignant mesothelioma cell lines with substantial effects of selenite and bortezomib, highlights need for individualized therapy[J]. PLoS One, 2013, 8(6): e65903. |
| 27 | ROSCILLI G, DE VITIS C, FERRARA F F, et al. Human lung adenocarcinoma cell cultures derived from malignant pleural effusions as model system to predict patients chemosensitivity[J]. J Transl Med, 2016, 14: 61. |
| 28 | ALIZADEH A A, ARANDA V, BARDELLI A, et al. Toward understanding and exploiting tumor heterogeneity[J]. Nat Med, 2015, 21(8): 846-853. |
| 29 | 王丹. 高通量体外药敏检测技术在晚期肺癌伴恶性胸水中的临床研究[D]. 合肥: 安徽医科大学, 2018: 1-41. |
| 30 | 中华医学会, 中华医学会肿瘤学分会, 中华医学会杂志社. 中华医学会肺癌临床诊疗指南(2018版)[J]. 中华肿瘤杂志, 2018, 40(12): 935-964. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 667
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 698
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||