[1]
WU J, CAI J T. Dilemma and challenge of immunotherapy for pancreatic cancer[J]. Dig Dis Sci, 2021, 66(2): 359-368.
[本文引用: 1]
[2]
TSUCHIKAWA T, TAKEUCHI S, NAKAMURA T, et al. Clinical impact of chemotherapy to improve tumor microenvironment of pancreatic cancer[J]. World J Gastrointest Oncol, 2016, 8(11): 786-792.
[本文引用: 1]
[3]
GILLEN S, SCHUSTER T, MEYER ZUM BÜSCHENFELDE C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages[J]. PLoS Med, 2010, 7(4): e1000267.
[本文引用: 1]
[4]
SHARMA V, AGGARWAL A, JACOB J, et al. Myeloid-derived suppressor cells: bridging the gap between inflammation and pancreatic adenocarcinoma[J]. Scand J Immunol, 2021, 93(5): e13021.
[本文引用: 2]
[5]
SARVEPALLI D, RASHID M U, RAHMAN A U, et al. Gemcitabine: a review of chemoresistance in pancreatic cancer[J]. Crit Rev Oncog, 2019, 24(2): 199-212.
[本文引用: 1]
[6]
THYAGARAJAN A, ALSHEHRI M S A, MILLER K L R, et al. Myeloid-derived suppressor cells and pancreatic cancer: implications in novel therapeutic approaches[J]. Cancers, 2019, 11(11): 1627.
[本文引用: 4]
[7]
DYSTHE M, PARIHAR R. Myeloid-derived suppressor cells in the tumor microenvironment[J]. Adv Exp Med Biol, 2020, 1224: 117-140.
[本文引用: 1]
[8]
KHALED Y S, AMMORI B J, ELKORD E. Increased levels of granulocytic myeloid-derived suppressor cells in peripheral blood and tumour tissue of pancreatic cancer patients[J]. J Immunol Res, 2014, 2014: 879897.
[本文引用: 3]
[9]
GARGETT T, CHRISTO S N, HERCUS T R, et al. GM-CSF signalling blockade and chemotherapeutic agents act in concert to inhibit the function of myeloid-derived suppressor cells in vitro [J]. Clin Transl Immunology, 2016, 5(12): e119.
[本文引用: 1]
[10]
NAGARAJ S, GABRILOVICH D I. Tumor escape mechanism governed by myeloid-derived suppressor cells[J]. Cancer Res, 2008, 68(8): 2561-2563.
[本文引用: 1]
[11]
BRONTE V, BRANDAU S, CHEN S H, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards[J]. Nat Commun, 2016, 7: 12150.
[本文引用: 1]
[12]
YOUN J I, NAGARAJ S, COLLAZO M, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice[J]. J Immunol, 2008, 181(8): 5791-5802.
[本文引用: 1]
[13]
YOUN J I, GABRILOVICH D I. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity[J]. Eur J Immunol, 2010, 40(11): 2969-2975.
[14]
GABRILOVICH D I, NAGARAJ S. Myeloid-derived suppressor cells as regulators of the immune system[J]. Nat Rev Immunol, 2009, 9(3): 162-174.
[本文引用: 1]
[15]
PENG M Y, ZHANG Q, LIU Y Q, et al. Apolipoprotein A-I mimetic peptide L-4F suppresses granulocytic-myeloid-derived suppressor cells in mouse pancreatic cancer[J]. Front Pharmacol, 2020, 11: 576.
[本文引用: 2]
[16]
MARVEL D, GABRILOVICH D I. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected[J]. J Clin Invest, 2015, 125(9): 3356-3364.
[本文引用: 2]
[17]
OSIPOV A, SAUNG M T, ZHENG L, et al. Small molecule immunomodulation: the tumor microenvironment and overcoming immune escape[J]. J Immunother Cancer, 2019, 7(1): 224.
[本文引用: 1]
[18]
PADOAN A, PLEBANI M, BASSO D. Inflammation and pancreatic cancer: focus on metabolism, cytokines, and immunity[J]. Int J Mol Sci, 2019, 20(3): 676.
[本文引用: 1]
[19]
APTE M V, XU Z, POTHULA S, et al. Pancreatic cancer: the microenvironment needs attention too![J]. Pancreatology, 2015, 15(4): S32-S38.
[本文引用: 1]
[20]
THAKUR A, SCHALK D, TOMASZEWSKI E, et al. Microenvironment generated during EGFR targeted killing of pancreatic tumor cells by ATC inhibits myeloid-derived suppressor cells through COX2 and PGE2 dependent pathway[J]. J Transl Med, 2013, 11: 35.
[本文引用: 1]
[21]
UZUNPARMAK B, SAHIN I H. Pancreatic cancer microenvironment: a current dilemma[J]. Clin Transl Med, 2019, 8(1): 2.
[本文引用: 1]
[22]
POREMBKA M R, MITCHEM J B, BELT B A, et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth[J]. Cancer Immunol Immunother, 2012, 61(9): 1373-1385.
[本文引用: 1]
[23]
GABRILOVICH D I, OSTRAND-ROSENBERG S, BRONTE V. Coordinated regulation of myeloid cells by tumours[J]. Nat Rev Immunol, 2012, 12(4): 253-268.
[本文引用: 1]
[24]
LI H Q, HAN Y M, GUO Q L, et al. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1[J]. J Immunol, 2009, 182(1): 240-249.
[本文引用: 1]
[25]
SERAFINI P, MGEBROFF S, NOONAN K, et al. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells[J]. Cancer Res, 2008, 68(13): 5439-5449.
[本文引用: 1]
[26]
OSTRAND-ROSENBERG S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity[J]. Cancer Immunol Immunother, 2010, 59(10): 1593-1600.
[本文引用: 1]
[27]
SZEFEL J, DANIELAK A, KRUSZEWSKI W J. Metabolic pathways of L-arginine and therapeutic consequences in tumors[J]. Adv Med Sci, 2019, 64(1): 104-110.
[本文引用: 1]
[28]
RODRÍGUEZ P C, OCHOA A C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives[J]. Immunol Rev, 2008, 222: 180-191.
[本文引用: 1]
[29]
GALLEGO-ORTEGA D, LEDGER A, RODEN D L, et al. ELF5 drives lung metastasis in luminal breast cancer through recruitment of Gr1+ CD11b+ myeloid-derived suppressor cells[J]. PLoS Biol, 2015, 13(12): e1002330.
[本文引用: 1]
[30]
TALMADGE J E, GABRILOVICH D I. History of myeloid-derived suppressor cells[J]. Nat Rev Cancer, 2013, 13(10): 739-752.
[本文引用: 1]
[31]
BIAN Z, SHI L, VENKATARAMANI M, et al. Tumor conditions induce bone marrow expansion of granulocytic, but not monocytic, immunosuppressive leukocytes with increased CXCR2 expression in mice[J]. Eur J Immunol, 2018, 48(3): 532-542.
[本文引用: 2]
[32]
UMANSKY V, BLATTNER C, GEBHARDT C, et al. The role of myeloid-derived suppressor cells (MDSC) in cancer progression[J]. Vaccines, 2016, 4(4): 36.
[本文引用: 1]
[33]
TCYGANOV E, MASTIO J, CHEN E, et al. Plasticity of myeloid-derived suppressor cells in cancer[J]. Curr Opin Immunol, 2018, 51: 76-82.
[本文引用: 1]
[34]
HOLOKAI L, CHAKRABARTI J, LUNDY J, et al. Murine- and human-derived autologous organoid/immune cell co-cultures as pre-clinical models of pancreatic ductal adenocarcinoma[J]. Cancers, 2020, 12(12): 3816.
[本文引用: 1]
[35]
FARAJZADEH VALILOU S, KESHAVARZ-FATHI M, SILVESTRIS N, et al. The role of inflammatory cytokines and tumor associated macrophages (TAMs) in microenvironment of pancreatic cancer[J]. Cytokine Growth Factor Rev, 2018, 39: 46-61.
[本文引用: 1]
[36]
TADMOR T, ATTIAS D, POLLIACK A. Myeloid-derived suppressor cells: their role in haemato-oncological malignancies and other cancers and possible implications for therapy[J]. Br J Haematol, 2011, 153(5): 557-567.
[本文引用: 1]
[37]
DONG P, YAN Y, FAN Y J, et al. The role of myeloid-derived suppressor cells in the treatment of pancreatic cancer[J]. Technol Cancer Res Treat, 2022, 21: 15330338221142472.
[本文引用: 1]
[38]
HALABY M J, HEZAVEH K, LAMORTE S, et al. GCN2 drives macrophage and MDSC function and immunosuppression in the tumor microenvironment[J]. Sci Immunol, 2019, 4(42): eaax8189.
[本文引用: 1]
[39]
LAW A M K, VALDES-MORA F, GALLEGO-ORTEGA D. Myeloid-derived suppressor cells as a therapeutic target for cancer[J]. Cells, 2020, 9(3): 561.
[本文引用: 2]
[40]
SUZUKI E, SUN J, KAPOOR V, et al. Gemcitabine has significant immunomodulatory activity in murine tumor models independent of its cytotoxic effects[J]. Cancer Biol Ther, 2007, 6(6): 880-885.
[本文引用: 1]
[41]
SEVKO A, MICHELS T, VROHLINGS M, et al. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model[J]. J Immunol, 2013, 190(5): 2464-2471.
[本文引用: 1]
[42]
GHANSAH T, VOHRA N, KINNEY K, et al. Dendritic cell immunotherapy combined with gemcitabine chemotherapy enhances survival in a murine model of pancreatic carcinoma[J]. Cancer Immunol Immunother, 2013, 62(6): 1083-1091.
[本文引用: 2]
[43]
MELANI C, SANGALETTI S, BARAZZETTA F M, et al. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma[J]. Cancer Res, 2007, 67(23): 11438-11446.
[本文引用: 1]
[44]
GOEDEGEBUURE P, MITCHEM J B, POREMBKA M R, et al. Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer[J]. Curr Cancer Drug Targets, 2011, 11(6): 734-751.
[本文引用: 1]
[45]
ISHERWOOD J, ARSHAD A, CHUNG W Y, et al. Myeloid derived suppressor cells are reduced and T regulatory cells stabilised in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega 3[J]. Ann Transl Med, 2020, 8(5): 172.
[本文引用: 1]
[46]
GHIRINGHELLI F, APETOH L. Enhancing the anticancer effects of 5-fluorouracil: current challenges and future perspectives[J]. Biomed J, 2015, 38(2): 111-116.
[本文引用: 1]
[47]
VINCENT J, MIGNOT G, CHALMIN F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity[J]. Cancer Res, 2010, 70(8): 3052-3061.
[本文引用: 1]
[48]
ANNELS N E, SHAW V E, GABITASS R F, et al. The effects of gemcitabine and capecitabine combination chemotherapy and of low-dose adjuvant GM-CSF on the levels of myeloid-derived suppressor cells in patients with advanced pancreatic cancer[J]. Cancer Immunol Immunother, 2014, 63(2): 175-183.
[本文引用: 1]
[49]
CONDAMINE T, KUMAR V, RAMACHANDRAN I R, et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis[J]. J Clin Invest, 2014, 124(6): 2626-2639.
[本文引用: 1]
[50]
DOMINGUEZ G A, CONDAMINE T, MONY S, et al. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody[J]. Clin Cancer Res, 2017, 23(12): 2942-2950.
[本文引用: 1]
[51]
PENG M Y, HUANG B Q, ZHANG Q, et al. Embelin inhibits pancreatic cancer progression by directly inducing cancer cell apoptosis and indirectly restricting IL-6 associated inflammatory and immune suppressive cells[J]. Cancer Lett, 2014, 354(2): 407-416.
[本文引用: 1]
[52]
GHANSAH T. A novel strategy for modulation of MDSC to enhance cancer immunotherapy[J]. Oncoimmunology, 2012, 1(6): 984-985.
[本文引用: 1]
[53]
VEGLIA F, PEREGO M, GABRILOVICH D. Myeloid-derived suppressor cells coming of age[J]. Nat Immunol, 2018, 19(2): 108-119.
[本文引用: 1]
[54]
SONG J, LEE J, KIM J, et al. Pancreatic adenocarcinoma up-regulated factor (PAUF) enhances the accumulation and functional activity of myeloid-derived suppressor cells (MDSCs) in pancreatic cancer[J]. Oncotarget, 2016, 7(32): 51840-51853.
[本文引用: 2]
[55]
NAGARAJ S, YOUN J I, WEBER H, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer[J]. Clin Cancer Res, 2010, 16(6): 1812-1823.
[本文引用: 2]
[56]
SANCTIS F D, SOLITO S, UGEL S, et al. MDSCs in cancer: conceiving new prognostic and therapeutic targets[J]. Biochim Biophys Acta, 2016, 1865(1): 35-48.
[本文引用: 1]
[57]
FLEMING V, HU X Y, WEBER R, et al. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression[J]. Front Immunol, 2018, 9: 398.
[本文引用: 1]
[58]
SANFORD D E, BELT B A, PANNI R Z, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis[J]. Clin Cancer Res, 2013, 19(13): 3404-3415.
[本文引用: 1]
1
... 胰腺癌(pancreatic cancer,PC)是一种侵袭性极强的恶性肿瘤,患者生存率极低.预计到2030年,它将成为全球第二位死亡病因[1 -2 ] .大多数胰腺癌患者在疾病早期均无明显临床表现;等发现时,已是疾病的中晚期[3 -4 ] .目前,胰腺癌的治疗手段包括手术切除、各种药物治疗[如化学治疗(化疗)药物、靶向药物]、放射治疗等[5 ] .虽然这些治疗方案是胰腺癌常用的,但正在进行的一些临床研究[6 ] 表明,药物治疗有可能会由于系统和肿瘤微环境(tumor microenvironment,TME)的免疫抑制而受到阻碍.特别是发生癌症后,一些免疫抑制细胞尤其是骨髓源性抑制细胞(myeloid-derived suppressor cell,MDSC)会异常产生并被招募到TME,去促进建立免疫抑制TME,帮助肿瘤逃逸[7 ] .一致认为肿瘤逃避免疫系统的关键机制就是肿瘤诱导的免疫抑制[8 ] .现如今,肿瘤通过免疫抑制逃避免疫系统已被确定为癌症的一个特点.研究[9 ] 发现在胰腺癌的免疫抑制中,MDSC发挥了关键作用,与胰腺癌的发展和预后有着非常密切的关系.本文就以MDSC为切入点,对其在胰腺癌TME中发挥的作用以及作为潜在靶点免疫治疗胰腺癌的相关进展进行综述. ...
1
... 胰腺癌(pancreatic cancer,PC)是一种侵袭性极强的恶性肿瘤,患者生存率极低.预计到2030年,它将成为全球第二位死亡病因[1 -2 ] .大多数胰腺癌患者在疾病早期均无明显临床表现;等发现时,已是疾病的中晚期[3 -4 ] .目前,胰腺癌的治疗手段包括手术切除、各种药物治疗[如化学治疗(化疗)药物、靶向药物]、放射治疗等[5 ] .虽然这些治疗方案是胰腺癌常用的,但正在进行的一些临床研究[6 ] 表明,药物治疗有可能会由于系统和肿瘤微环境(tumor microenvironment,TME)的免疫抑制而受到阻碍.特别是发生癌症后,一些免疫抑制细胞尤其是骨髓源性抑制细胞(myeloid-derived suppressor cell,MDSC)会异常产生并被招募到TME,去促进建立免疫抑制TME,帮助肿瘤逃逸[7 ] .一致认为肿瘤逃避免疫系统的关键机制就是肿瘤诱导的免疫抑制[8 ] .现如今,肿瘤通过免疫抑制逃避免疫系统已被确定为癌症的一个特点.研究[9 ] 发现在胰腺癌的免疫抑制中,MDSC发挥了关键作用,与胰腺癌的发展和预后有着非常密切的关系.本文就以MDSC为切入点,对其在胰腺癌TME中发挥的作用以及作为潜在靶点免疫治疗胰腺癌的相关进展进行综述. ...
1
... 胰腺癌(pancreatic cancer,PC)是一种侵袭性极强的恶性肿瘤,患者生存率极低.预计到2030年,它将成为全球第二位死亡病因[1 -2 ] .大多数胰腺癌患者在疾病早期均无明显临床表现;等发现时,已是疾病的中晚期[3 -4 ] .目前,胰腺癌的治疗手段包括手术切除、各种药物治疗[如化学治疗(化疗)药物、靶向药物]、放射治疗等[5 ] .虽然这些治疗方案是胰腺癌常用的,但正在进行的一些临床研究[6 ] 表明,药物治疗有可能会由于系统和肿瘤微环境(tumor microenvironment,TME)的免疫抑制而受到阻碍.特别是发生癌症后,一些免疫抑制细胞尤其是骨髓源性抑制细胞(myeloid-derived suppressor cell,MDSC)会异常产生并被招募到TME,去促进建立免疫抑制TME,帮助肿瘤逃逸[7 ] .一致认为肿瘤逃避免疫系统的关键机制就是肿瘤诱导的免疫抑制[8 ] .现如今,肿瘤通过免疫抑制逃避免疫系统已被确定为癌症的一个特点.研究[9 ] 发现在胰腺癌的免疫抑制中,MDSC发挥了关键作用,与胰腺癌的发展和预后有着非常密切的关系.本文就以MDSC为切入点,对其在胰腺癌TME中发挥的作用以及作为潜在靶点免疫治疗胰腺癌的相关进展进行综述. ...
2
... 胰腺癌(pancreatic cancer,PC)是一种侵袭性极强的恶性肿瘤,患者生存率极低.预计到2030年,它将成为全球第二位死亡病因[1 -2 ] .大多数胰腺癌患者在疾病早期均无明显临床表现;等发现时,已是疾病的中晚期[3 -4 ] .目前,胰腺癌的治疗手段包括手术切除、各种药物治疗[如化学治疗(化疗)药物、靶向药物]、放射治疗等[5 ] .虽然这些治疗方案是胰腺癌常用的,但正在进行的一些临床研究[6 ] 表明,药物治疗有可能会由于系统和肿瘤微环境(tumor microenvironment,TME)的免疫抑制而受到阻碍.特别是发生癌症后,一些免疫抑制细胞尤其是骨髓源性抑制细胞(myeloid-derived suppressor cell,MDSC)会异常产生并被招募到TME,去促进建立免疫抑制TME,帮助肿瘤逃逸[7 ] .一致认为肿瘤逃避免疫系统的关键机制就是肿瘤诱导的免疫抑制[8 ] .现如今,肿瘤通过免疫抑制逃避免疫系统已被确定为癌症的一个特点.研究[9 ] 发现在胰腺癌的免疫抑制中,MDSC发挥了关键作用,与胰腺癌的发展和预后有着非常密切的关系.本文就以MDSC为切入点,对其在胰腺癌TME中发挥的作用以及作为潜在靶点免疫治疗胰腺癌的相关进展进行综述. ...
... 除免疫抑制作用外,MDSC也可通过促进肿瘤血管生成以及肿瘤侵袭转移等机制参与肿瘤的发生发展[36 ] .MDSC可通过下调γ干扰素(interferon-γ,IFN-γ)、过表达炎症因子、表达基质金属蛋白酶9(matrix metalloproteinase-9,MMP9)和其他重塑因子降低细胞外基质(extracellular matrix,ECM)和基底膜的完整性,诱导血管渗漏而促进胰腺癌在微环境中的发展和转移[31 ] .上皮-间充质转化(epithelial-mesenchymal transition,EMT)是一种可逆的细胞过程,上皮细胞瞬间进入准间充质细胞状态,可使肿瘤细胞具有更大的侵袭和转移潜力,并产生更大的治疗耐药性.EMT的增加被认为是肿瘤转移的主要标志之一.在肿瘤形成的早期M-MDSC可通过IL-6介导的STAT3磷酸化诱导EMT,EMT和转移也会随着G-MDSC的增多而增多.在胰腺癌患者中可根据EMT标志物与MDSC亚群频率的比较确定这些MDSC在胰腺癌组织中的作用[4 ,37 ] . ...
1
... 胰腺癌(pancreatic cancer,PC)是一种侵袭性极强的恶性肿瘤,患者生存率极低.预计到2030年,它将成为全球第二位死亡病因[1 -2 ] .大多数胰腺癌患者在疾病早期均无明显临床表现;等发现时,已是疾病的中晚期[3 -4 ] .目前,胰腺癌的治疗手段包括手术切除、各种药物治疗[如化学治疗(化疗)药物、靶向药物]、放射治疗等[5 ] .虽然这些治疗方案是胰腺癌常用的,但正在进行的一些临床研究[6 ] 表明,药物治疗有可能会由于系统和肿瘤微环境(tumor microenvironment,TME)的免疫抑制而受到阻碍.特别是发生癌症后,一些免疫抑制细胞尤其是骨髓源性抑制细胞(myeloid-derived suppressor cell,MDSC)会异常产生并被招募到TME,去促进建立免疫抑制TME,帮助肿瘤逃逸[7 ] .一致认为肿瘤逃避免疫系统的关键机制就是肿瘤诱导的免疫抑制[8 ] .现如今,肿瘤通过免疫抑制逃避免疫系统已被确定为癌症的一个特点.研究[9 ] 发现在胰腺癌的免疫抑制中,MDSC发挥了关键作用,与胰腺癌的发展和预后有着非常密切的关系.本文就以MDSC为切入点,对其在胰腺癌TME中发挥的作用以及作为潜在靶点免疫治疗胰腺癌的相关进展进行综述. ...
4
... 胰腺癌(pancreatic cancer,PC)是一种侵袭性极强的恶性肿瘤,患者生存率极低.预计到2030年,它将成为全球第二位死亡病因[1 -2 ] .大多数胰腺癌患者在疾病早期均无明显临床表现;等发现时,已是疾病的中晚期[3 -4 ] .目前,胰腺癌的治疗手段包括手术切除、各种药物治疗[如化学治疗(化疗)药物、靶向药物]、放射治疗等[5 ] .虽然这些治疗方案是胰腺癌常用的,但正在进行的一些临床研究[6 ] 表明,药物治疗有可能会由于系统和肿瘤微环境(tumor microenvironment,TME)的免疫抑制而受到阻碍.特别是发生癌症后,一些免疫抑制细胞尤其是骨髓源性抑制细胞(myeloid-derived suppressor cell,MDSC)会异常产生并被招募到TME,去促进建立免疫抑制TME,帮助肿瘤逃逸[7 ] .一致认为肿瘤逃避免疫系统的关键机制就是肿瘤诱导的免疫抑制[8 ] .现如今,肿瘤通过免疫抑制逃避免疫系统已被确定为癌症的一个特点.研究[9 ] 发现在胰腺癌的免疫抑制中,MDSC发挥了关键作用,与胰腺癌的发展和预后有着非常密切的关系.本文就以MDSC为切入点,对其在胰腺癌TME中发挥的作用以及作为潜在靶点免疫治疗胰腺癌的相关进展进行综述. ...
... 但值得关注的是,联合GEM靶向MDSC治疗胰腺癌这种策略正在被探索[6 ] .GHANSAH等[42 ] 在同源小鼠胰腺癌细胞Panc02种植模型中发现,GEM化疗可以明显缩小肿瘤,但并没有提高其生存期;树突细胞治疗对肿瘤大小及生存期更是无明显影响,但将GEM与树突细胞结合治疗不但可以明显缩小肿瘤体积,明显降低脾脏中的MDSC水平,还可显著提高总体生存期,所以考虑树突状细胞免疫治疗联合GEM化疗可提高胰腺癌小鼠的生存.在一项GEM化疗联合静脉注射ω-3脂肪酸(ω-3 fatty acids,ω-3FAs)与单独GEM化疗对胰腺癌患者免疫细胞的影响研究中发现,ω-3FAs脂肪酸可以抑制胰腺癌的生长,增强GEM的治疗作用.GEM联合应用ω-3FAs治疗胰腺癌明显减少了患者MDSC的数量,从而验证了ω-3FAs的积极作用[45 ] .5-FU是一种嘧啶类似物,研究发现它可强效抑制体内MDSC的细胞毒性[46 ] ,可选择性地杀死MDSC,从而促进抗肿瘤免疫反应[47 ] .值得注意的是,在GEM联合卡培他滨(一种5-氟尿嘧啶前药)对胰腺癌患者MDSC的影响研究中发现,GEM和卡培他滨联合治疗并没有一致的降低胰腺癌患者的MDSC[48 ] .也就是说,虽然这两种药物都可以治疗胰腺癌,但随着患者数量以及患病程度的增加联合效果并不理想.因此联合治疗也并不是最佳的治疗手段,也存在一定局限性.正是由于癌症治疗的复杂化,所以未来更应该注重联合治疗的科学合理性. ...
... 目前,MDSC主要是通过分泌或表达一些抑制性因子发挥免疫抑制作用,比如,Arg-1、iNOS和ROS等[53 ] .因此,通过阻断这些抑制性因子的表达从而消除MDSC的免疫抑制功能不失为一种有效的手段.在胰腺癌小鼠模型中一种胰腺癌上调因子(pancreatic adenocarcinoma up-regulated factor,PAUF)可通过toll样受体4(Toll-like receptor 4,TLR4)信号转导参与MAPK/ERK信号通路升高Arg-1、NO和ROS的水平,从而增强MDSC的免疫抑制活性[54 ] .当使用人抗PAUF单克隆抗体PMAb83治疗后,明显降低了MDSC的数量以及免疫抑制活性[6 ,54 ] ,继而表明通过靶向PAUF治疗胰腺癌有巨大的潜力.三萜类人工合成物CDDO-Me也可抑制荷瘤小鼠以及癌症患者的MDSC活性,并改善抗肿瘤免疫[55 ] .但联合GEM治疗并没有明显影响MDSC,并未达到预期效果[6 ,55 ] .我们前期的研究[15 ] 发现用载脂蛋白AI(apolipoprotein A-I,ApoA-I)的模拟肽L-4F可以通过降低G-MDSC的数量和功能来抑制小鼠胰腺癌的发展. ...
... [6 ,55 ].我们前期的研究[15 ] 发现用载脂蛋白AI(apolipoprotein A-I,ApoA-I)的模拟肽L-4F可以通过降低G-MDSC的数量和功能来抑制小鼠胰腺癌的发展. ...
1
... 胰腺癌(pancreatic cancer,PC)是一种侵袭性极强的恶性肿瘤,患者生存率极低.预计到2030年,它将成为全球第二位死亡病因[1 -2 ] .大多数胰腺癌患者在疾病早期均无明显临床表现;等发现时,已是疾病的中晚期[3 -4 ] .目前,胰腺癌的治疗手段包括手术切除、各种药物治疗[如化学治疗(化疗)药物、靶向药物]、放射治疗等[5 ] .虽然这些治疗方案是胰腺癌常用的,但正在进行的一些临床研究[6 ] 表明,药物治疗有可能会由于系统和肿瘤微环境(tumor microenvironment,TME)的免疫抑制而受到阻碍.特别是发生癌症后,一些免疫抑制细胞尤其是骨髓源性抑制细胞(myeloid-derived suppressor cell,MDSC)会异常产生并被招募到TME,去促进建立免疫抑制TME,帮助肿瘤逃逸[7 ] .一致认为肿瘤逃避免疫系统的关键机制就是肿瘤诱导的免疫抑制[8 ] .现如今,肿瘤通过免疫抑制逃避免疫系统已被确定为癌症的一个特点.研究[9 ] 发现在胰腺癌的免疫抑制中,MDSC发挥了关键作用,与胰腺癌的发展和预后有着非常密切的关系.本文就以MDSC为切入点,对其在胰腺癌TME中发挥的作用以及作为潜在靶点免疫治疗胰腺癌的相关进展进行综述. ...
3
... 胰腺癌(pancreatic cancer,PC)是一种侵袭性极强的恶性肿瘤,患者生存率极低.预计到2030年,它将成为全球第二位死亡病因[1 -2 ] .大多数胰腺癌患者在疾病早期均无明显临床表现;等发现时,已是疾病的中晚期[3 -4 ] .目前,胰腺癌的治疗手段包括手术切除、各种药物治疗[如化学治疗(化疗)药物、靶向药物]、放射治疗等[5 ] .虽然这些治疗方案是胰腺癌常用的,但正在进行的一些临床研究[6 ] 表明,药物治疗有可能会由于系统和肿瘤微环境(tumor microenvironment,TME)的免疫抑制而受到阻碍.特别是发生癌症后,一些免疫抑制细胞尤其是骨髓源性抑制细胞(myeloid-derived suppressor cell,MDSC)会异常产生并被招募到TME,去促进建立免疫抑制TME,帮助肿瘤逃逸[7 ] .一致认为肿瘤逃避免疫系统的关键机制就是肿瘤诱导的免疫抑制[8 ] .现如今,肿瘤通过免疫抑制逃避免疫系统已被确定为癌症的一个特点.研究[9 ] 发现在胰腺癌的免疫抑制中,MDSC发挥了关键作用,与胰腺癌的发展和预后有着非常密切的关系.本文就以MDSC为切入点,对其在胰腺癌TME中发挥的作用以及作为潜在靶点免疫治疗胰腺癌的相关进展进行综述. ...
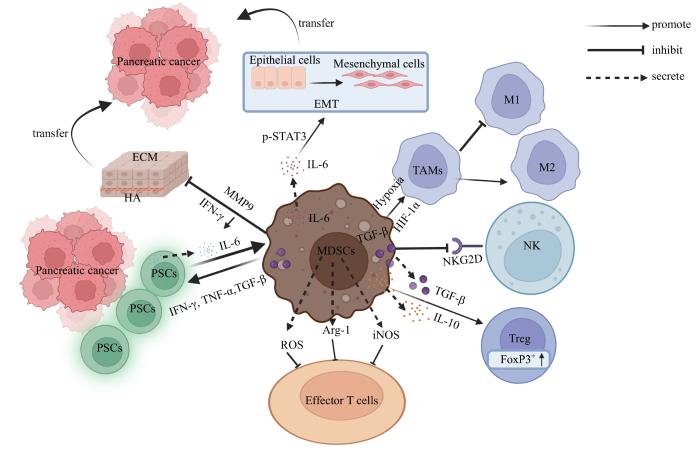
... 研究[8 ] 发现胰腺癌患者体内循环的MDSC(主要为G-MDSC)可高度表达Arg-1并抑制T细胞反应,这证实了Arg-1是胰腺癌中G-MDSC的特征性表达.当然,MDSC也可通过分泌活性氧(reactive oxygen species,ROS)来抑制T细胞免疫应答反应.与MDSC共培养之后,T细胞的功能和增殖明显受到抑制[28 ] .不同亚型的MDSC,通过不同的机制抑制T细胞:如M-MDSC主要通过STAT1途径,上调Arg-1、iNOS和TGF-β的表达等,实现非特异性T细胞失活;相对而言,G-MDSC则会通过STAT3信号通路产生较高水平的ROS,并通过与T淋巴细胞的接触发挥免疫抑制功能,使T细胞表现出对抗原特异性刺激的不敏感性,但对非特异性刺激有反应的现象[16 ,29 ,30 ] . ...
... 总的来说,M-MDSC和G-MDSC均具有免疫抑制作用.但在具体分析MDSC用于消除免疫抑制的主要机制时,要着重注意M-MDSC和G-MDSC的所占比例.在胰腺癌中,肿瘤组织中浸润的MDSC主要是G-MDSC,以其升高为主[8 ] .在胰腺癌TME中,MDSC可参与嗜中性粒细胞形成和进化[31 ] .MDSC也可促进M1型巨噬细胞(M1 type macrophage,M1)向M2型转化,从而加快肿瘤生长,促进肿瘤转移[32 ] .研究表明,在肿瘤环境下缺氧,特别是缺氧诱导因子(hypoxia inducible factor-1α,HIF-1α)可驱动MDSC分化为肿瘤相关巨噬细胞(tumor-associated macrophage,TAM)[33 ] 参与胰腺导管腺癌(pancreatic ductaladenocarcinoma,PDAC)中嗜神经侵袭(perineural invasion,PNI)的形成,其增多会导致PDAC预后不良[34 ] .在胰腺癌组织中,可观察到丰富的M2型TAM,与肿瘤的TNM分期密切相关,可用来评估患者的预后以及肿瘤的进展情况[35 ] . ...
1
... 胰腺癌(pancreatic cancer,PC)是一种侵袭性极强的恶性肿瘤,患者生存率极低.预计到2030年,它将成为全球第二位死亡病因[1 -2 ] .大多数胰腺癌患者在疾病早期均无明显临床表现;等发现时,已是疾病的中晚期[3 -4 ] .目前,胰腺癌的治疗手段包括手术切除、各种药物治疗[如化学治疗(化疗)药物、靶向药物]、放射治疗等[5 ] .虽然这些治疗方案是胰腺癌常用的,但正在进行的一些临床研究[6 ] 表明,药物治疗有可能会由于系统和肿瘤微环境(tumor microenvironment,TME)的免疫抑制而受到阻碍.特别是发生癌症后,一些免疫抑制细胞尤其是骨髓源性抑制细胞(myeloid-derived suppressor cell,MDSC)会异常产生并被招募到TME,去促进建立免疫抑制TME,帮助肿瘤逃逸[7 ] .一致认为肿瘤逃避免疫系统的关键机制就是肿瘤诱导的免疫抑制[8 ] .现如今,肿瘤通过免疫抑制逃避免疫系统已被确定为癌症的一个特点.研究[9 ] 发现在胰腺癌的免疫抑制中,MDSC发挥了关键作用,与胰腺癌的发展和预后有着非常密切的关系.本文就以MDSC为切入点,对其在胰腺癌TME中发挥的作用以及作为潜在靶点免疫治疗胰腺癌的相关进展进行综述. ...
1
... MDSC是一群未成熟的且可调节免疫应答的异质性细胞,包括幼稚的单核细胞、粒细胞和树突状细胞(dendritic cell,DC)等[10 ] .研究[11 ] 表明,MDSC的表型特征在人类和小鼠中并不完全相同.在小鼠中,通过标识细胞表面分子CD11b和GR-1可鉴定和区分MDSC;按照GR-1的不同(Ly6C和Ly6G),MDSC分为 CD11b+ Ly6G- Ly6Chi 的单核细胞型(monocytic MDSC,M-MDSC)和CD11b+ Ly6G+ Ly6Clow 的粒细胞型(granulocytic MDSC,G-MDSC)[12 ] .而通过标识细胞表面分子CD11b+ CD33+ HLA-DR- ,人类M-MDSC的表型为CD14+ CD11b+ CD33+ HLA-DR- ,人类G-MDSC表型为CD15+ CD11b+ CD33+ HLA-DR-[13] .正常情况下,健康人外周血中存在较低水平的MDSC,但在机体出现感染、炎症和癌症等时,MDSC会大量增加并参与免疫反应[14 -15 ] .这些异常产生的MDSC会被招募到肿瘤部位,从而构造出可以抑制宿主免疫应答的微环境[16 ] . ...
1
... MDSC是一群未成熟的且可调节免疫应答的异质性细胞,包括幼稚的单核细胞、粒细胞和树突状细胞(dendritic cell,DC)等[10 ] .研究[11 ] 表明,MDSC的表型特征在人类和小鼠中并不完全相同.在小鼠中,通过标识细胞表面分子CD11b和GR-1可鉴定和区分MDSC;按照GR-1的不同(Ly6C和Ly6G),MDSC分为 CD11b+ Ly6G- Ly6Chi 的单核细胞型(monocytic MDSC,M-MDSC)和CD11b+ Ly6G+ Ly6Clow 的粒细胞型(granulocytic MDSC,G-MDSC)[12 ] .而通过标识细胞表面分子CD11b+ CD33+ HLA-DR- ,人类M-MDSC的表型为CD14+ CD11b+ CD33+ HLA-DR- ,人类G-MDSC表型为CD15+ CD11b+ CD33+ HLA-DR-[13] .正常情况下,健康人外周血中存在较低水平的MDSC,但在机体出现感染、炎症和癌症等时,MDSC会大量增加并参与免疫反应[14 -15 ] .这些异常产生的MDSC会被招募到肿瘤部位,从而构造出可以抑制宿主免疫应答的微环境[16 ] . ...
1
... MDSC是一群未成熟的且可调节免疫应答的异质性细胞,包括幼稚的单核细胞、粒细胞和树突状细胞(dendritic cell,DC)等[10 ] .研究[11 ] 表明,MDSC的表型特征在人类和小鼠中并不完全相同.在小鼠中,通过标识细胞表面分子CD11b和GR-1可鉴定和区分MDSC;按照GR-1的不同(Ly6C和Ly6G),MDSC分为 CD11b+ Ly6G- Ly6Chi 的单核细胞型(monocytic MDSC,M-MDSC)和CD11b+ Ly6G+ Ly6Clow 的粒细胞型(granulocytic MDSC,G-MDSC)[12 ] .而通过标识细胞表面分子CD11b+ CD33+ HLA-DR- ,人类M-MDSC的表型为CD14+ CD11b+ CD33+ HLA-DR- ,人类G-MDSC表型为CD15+ CD11b+ CD33+ HLA-DR-[13] .正常情况下,健康人外周血中存在较低水平的MDSC,但在机体出现感染、炎症和癌症等时,MDSC会大量增加并参与免疫反应[14 -15 ] .这些异常产生的MDSC会被招募到肿瘤部位,从而构造出可以抑制宿主免疫应答的微环境[16 ] . ...
1
... MDSC是一群未成熟的且可调节免疫应答的异质性细胞,包括幼稚的单核细胞、粒细胞和树突状细胞(dendritic cell,DC)等[10 ] .研究[11 ] 表明,MDSC的表型特征在人类和小鼠中并不完全相同.在小鼠中,通过标识细胞表面分子CD11b和GR-1可鉴定和区分MDSC;按照GR-1的不同(Ly6C和Ly6G),MDSC分为 CD11b+ Ly6G- Ly6Chi 的单核细胞型(monocytic MDSC,M-MDSC)和CD11b+ Ly6G+ Ly6Clow 的粒细胞型(granulocytic MDSC,G-MDSC)[12 ] .而通过标识细胞表面分子CD11b+ CD33+ HLA-DR- ,人类M-MDSC的表型为CD14+ CD11b+ CD33+ HLA-DR- ,人类G-MDSC表型为CD15+ CD11b+ CD33+ HLA-DR-[13] .正常情况下,健康人外周血中存在较低水平的MDSC,但在机体出现感染、炎症和癌症等时,MDSC会大量增加并参与免疫反应[14 -15 ] .这些异常产生的MDSC会被招募到肿瘤部位,从而构造出可以抑制宿主免疫应答的微环境[16 ] . ...
2
... MDSC是一群未成熟的且可调节免疫应答的异质性细胞,包括幼稚的单核细胞、粒细胞和树突状细胞(dendritic cell,DC)等[10 ] .研究[11 ] 表明,MDSC的表型特征在人类和小鼠中并不完全相同.在小鼠中,通过标识细胞表面分子CD11b和GR-1可鉴定和区分MDSC;按照GR-1的不同(Ly6C和Ly6G),MDSC分为 CD11b+ Ly6G- Ly6Chi 的单核细胞型(monocytic MDSC,M-MDSC)和CD11b+ Ly6G+ Ly6Clow 的粒细胞型(granulocytic MDSC,G-MDSC)[12 ] .而通过标识细胞表面分子CD11b+ CD33+ HLA-DR- ,人类M-MDSC的表型为CD14+ CD11b+ CD33+ HLA-DR- ,人类G-MDSC表型为CD15+ CD11b+ CD33+ HLA-DR-[13] .正常情况下,健康人外周血中存在较低水平的MDSC,但在机体出现感染、炎症和癌症等时,MDSC会大量增加并参与免疫反应[14 -15 ] .这些异常产生的MDSC会被招募到肿瘤部位,从而构造出可以抑制宿主免疫应答的微环境[16 ] . ...
... 目前,MDSC主要是通过分泌或表达一些抑制性因子发挥免疫抑制作用,比如,Arg-1、iNOS和ROS等[53 ] .因此,通过阻断这些抑制性因子的表达从而消除MDSC的免疫抑制功能不失为一种有效的手段.在胰腺癌小鼠模型中一种胰腺癌上调因子(pancreatic adenocarcinoma up-regulated factor,PAUF)可通过toll样受体4(Toll-like receptor 4,TLR4)信号转导参与MAPK/ERK信号通路升高Arg-1、NO和ROS的水平,从而增强MDSC的免疫抑制活性[54 ] .当使用人抗PAUF单克隆抗体PMAb83治疗后,明显降低了MDSC的数量以及免疫抑制活性[6 ,54 ] ,继而表明通过靶向PAUF治疗胰腺癌有巨大的潜力.三萜类人工合成物CDDO-Me也可抑制荷瘤小鼠以及癌症患者的MDSC活性,并改善抗肿瘤免疫[55 ] .但联合GEM治疗并没有明显影响MDSC,并未达到预期效果[6 ,55 ] .我们前期的研究[15 ] 发现用载脂蛋白AI(apolipoprotein A-I,ApoA-I)的模拟肽L-4F可以通过降低G-MDSC的数量和功能来抑制小鼠胰腺癌的发展. ...
2
... MDSC是一群未成熟的且可调节免疫应答的异质性细胞,包括幼稚的单核细胞、粒细胞和树突状细胞(dendritic cell,DC)等[10 ] .研究[11 ] 表明,MDSC的表型特征在人类和小鼠中并不完全相同.在小鼠中,通过标识细胞表面分子CD11b和GR-1可鉴定和区分MDSC;按照GR-1的不同(Ly6C和Ly6G),MDSC分为 CD11b+ Ly6G- Ly6Chi 的单核细胞型(monocytic MDSC,M-MDSC)和CD11b+ Ly6G+ Ly6Clow 的粒细胞型(granulocytic MDSC,G-MDSC)[12 ] .而通过标识细胞表面分子CD11b+ CD33+ HLA-DR- ,人类M-MDSC的表型为CD14+ CD11b+ CD33+ HLA-DR- ,人类G-MDSC表型为CD15+ CD11b+ CD33+ HLA-DR-[13] .正常情况下,健康人外周血中存在较低水平的MDSC,但在机体出现感染、炎症和癌症等时,MDSC会大量增加并参与免疫反应[14 -15 ] .这些异常产生的MDSC会被招募到肿瘤部位,从而构造出可以抑制宿主免疫应答的微环境[16 ] . ...
... 研究[8 ] 发现胰腺癌患者体内循环的MDSC(主要为G-MDSC)可高度表达Arg-1并抑制T细胞反应,这证实了Arg-1是胰腺癌中G-MDSC的特征性表达.当然,MDSC也可通过分泌活性氧(reactive oxygen species,ROS)来抑制T细胞免疫应答反应.与MDSC共培养之后,T细胞的功能和增殖明显受到抑制[28 ] .不同亚型的MDSC,通过不同的机制抑制T细胞:如M-MDSC主要通过STAT1途径,上调Arg-1、iNOS和TGF-β的表达等,实现非特异性T细胞失活;相对而言,G-MDSC则会通过STAT3信号通路产生较高水平的ROS,并通过与T淋巴细胞的接触发挥免疫抑制功能,使T细胞表现出对抗原特异性刺激的不敏感性,但对非特异性刺激有反应的现象[16 ,29 ,30 ] . ...
1
... 胰腺癌TME不仅包括大量的基质成分,如透明质酸、肿瘤相关纤维母细胞(cancer associated fibroblast,CAF)、可溶性抑制因子,还包括各种免疫细胞[17 ] ,且周围生长着十分密集的胰腺星状细胞(pancreatic stellate cell,PSC).研究[18 ] 发现,不管是胰腺癌患者还是小鼠胰腺癌模型中,其肿瘤组织中数量最多的炎性细胞都是MDSC,其对胰腺癌TME调节起重要作用(图1 ).PSC会产生大量基质元素,可直接接触免疫细胞并与之相互作用,如PSC可通过分泌白细胞介素-6(interleukin-6,IL-6)而激活人外周血单核细胞(peripheral blood mononuclear cell,PBMC)中信号转导及转录激活蛋白3(signal transducer and activator of transcription 3,STAT3)信号通路,从而诱导MDSC的产生[19 ] .体外研究[20 ] 发现,人胰腺癌细胞MiaPaCa-2与PBMC共培养,也会诱导产生MDSC.也有研究[21 ] 表明,MDSC通常存在于胰腺癌基质中,PSC可由MDSC分泌的促炎细胞因子而激活.随着胰腺癌的发展,MDSC的数量在骨髓、外周血以及脾脏组织中逐渐增多[22 ] . ...
1
... 胰腺癌TME不仅包括大量的基质成分,如透明质酸、肿瘤相关纤维母细胞(cancer associated fibroblast,CAF)、可溶性抑制因子,还包括各种免疫细胞[17 ] ,且周围生长着十分密集的胰腺星状细胞(pancreatic stellate cell,PSC).研究[18 ] 发现,不管是胰腺癌患者还是小鼠胰腺癌模型中,其肿瘤组织中数量最多的炎性细胞都是MDSC,其对胰腺癌TME调节起重要作用(图1 ).PSC会产生大量基质元素,可直接接触免疫细胞并与之相互作用,如PSC可通过分泌白细胞介素-6(interleukin-6,IL-6)而激活人外周血单核细胞(peripheral blood mononuclear cell,PBMC)中信号转导及转录激活蛋白3(signal transducer and activator of transcription 3,STAT3)信号通路,从而诱导MDSC的产生[19 ] .体外研究[20 ] 发现,人胰腺癌细胞MiaPaCa-2与PBMC共培养,也会诱导产生MDSC.也有研究[21 ] 表明,MDSC通常存在于胰腺癌基质中,PSC可由MDSC分泌的促炎细胞因子而激活.随着胰腺癌的发展,MDSC的数量在骨髓、外周血以及脾脏组织中逐渐增多[22 ] . ...
1
... 胰腺癌TME不仅包括大量的基质成分,如透明质酸、肿瘤相关纤维母细胞(cancer associated fibroblast,CAF)、可溶性抑制因子,还包括各种免疫细胞[17 ] ,且周围生长着十分密集的胰腺星状细胞(pancreatic stellate cell,PSC).研究[18 ] 发现,不管是胰腺癌患者还是小鼠胰腺癌模型中,其肿瘤组织中数量最多的炎性细胞都是MDSC,其对胰腺癌TME调节起重要作用(图1 ).PSC会产生大量基质元素,可直接接触免疫细胞并与之相互作用,如PSC可通过分泌白细胞介素-6(interleukin-6,IL-6)而激活人外周血单核细胞(peripheral blood mononuclear cell,PBMC)中信号转导及转录激活蛋白3(signal transducer and activator of transcription 3,STAT3)信号通路,从而诱导MDSC的产生[19 ] .体外研究[20 ] 发现,人胰腺癌细胞MiaPaCa-2与PBMC共培养,也会诱导产生MDSC.也有研究[21 ] 表明,MDSC通常存在于胰腺癌基质中,PSC可由MDSC分泌的促炎细胞因子而激活.随着胰腺癌的发展,MDSC的数量在骨髓、外周血以及脾脏组织中逐渐增多[22 ] . ...
1
... 胰腺癌TME不仅包括大量的基质成分,如透明质酸、肿瘤相关纤维母细胞(cancer associated fibroblast,CAF)、可溶性抑制因子,还包括各种免疫细胞[17 ] ,且周围生长着十分密集的胰腺星状细胞(pancreatic stellate cell,PSC).研究[18 ] 发现,不管是胰腺癌患者还是小鼠胰腺癌模型中,其肿瘤组织中数量最多的炎性细胞都是MDSC,其对胰腺癌TME调节起重要作用(图1 ).PSC会产生大量基质元素,可直接接触免疫细胞并与之相互作用,如PSC可通过分泌白细胞介素-6(interleukin-6,IL-6)而激活人外周血单核细胞(peripheral blood mononuclear cell,PBMC)中信号转导及转录激活蛋白3(signal transducer and activator of transcription 3,STAT3)信号通路,从而诱导MDSC的产生[19 ] .体外研究[20 ] 发现,人胰腺癌细胞MiaPaCa-2与PBMC共培养,也会诱导产生MDSC.也有研究[21 ] 表明,MDSC通常存在于胰腺癌基质中,PSC可由MDSC分泌的促炎细胞因子而激活.随着胰腺癌的发展,MDSC的数量在骨髓、外周血以及脾脏组织中逐渐增多[22 ] . ...
1
... 胰腺癌TME不仅包括大量的基质成分,如透明质酸、肿瘤相关纤维母细胞(cancer associated fibroblast,CAF)、可溶性抑制因子,还包括各种免疫细胞[17 ] ,且周围生长着十分密集的胰腺星状细胞(pancreatic stellate cell,PSC).研究[18 ] 发现,不管是胰腺癌患者还是小鼠胰腺癌模型中,其肿瘤组织中数量最多的炎性细胞都是MDSC,其对胰腺癌TME调节起重要作用(图1 ).PSC会产生大量基质元素,可直接接触免疫细胞并与之相互作用,如PSC可通过分泌白细胞介素-6(interleukin-6,IL-6)而激活人外周血单核细胞(peripheral blood mononuclear cell,PBMC)中信号转导及转录激活蛋白3(signal transducer and activator of transcription 3,STAT3)信号通路,从而诱导MDSC的产生[19 ] .体外研究[20 ] 发现,人胰腺癌细胞MiaPaCa-2与PBMC共培养,也会诱导产生MDSC.也有研究[21 ] 表明,MDSC通常存在于胰腺癌基质中,PSC可由MDSC分泌的促炎细胞因子而激活.随着胰腺癌的发展,MDSC的数量在骨髓、外周血以及脾脏组织中逐渐增多[22 ] . ...
1
... 胰腺癌TME不仅包括大量的基质成分,如透明质酸、肿瘤相关纤维母细胞(cancer associated fibroblast,CAF)、可溶性抑制因子,还包括各种免疫细胞[17 ] ,且周围生长着十分密集的胰腺星状细胞(pancreatic stellate cell,PSC).研究[18 ] 发现,不管是胰腺癌患者还是小鼠胰腺癌模型中,其肿瘤组织中数量最多的炎性细胞都是MDSC,其对胰腺癌TME调节起重要作用(图1 ).PSC会产生大量基质元素,可直接接触免疫细胞并与之相互作用,如PSC可通过分泌白细胞介素-6(interleukin-6,IL-6)而激活人外周血单核细胞(peripheral blood mononuclear cell,PBMC)中信号转导及转录激活蛋白3(signal transducer and activator of transcription 3,STAT3)信号通路,从而诱导MDSC的产生[19 ] .体外研究[20 ] 发现,人胰腺癌细胞MiaPaCa-2与PBMC共培养,也会诱导产生MDSC.也有研究[21 ] 表明,MDSC通常存在于胰腺癌基质中,PSC可由MDSC分泌的促炎细胞因子而激活.随着胰腺癌的发展,MDSC的数量在骨髓、外周血以及脾脏组织中逐渐增多[22 ] . ...
1
... 目前胰腺癌TME中的免疫逃逸机制是胰腺癌靶向治疗的主要难点.这是由MDSC的免疫抑制功能形成的,其可通过多种途径发挥免疫抑制作用,如消耗T细胞增殖所需氨基酸的代谢、分泌免疫抑制性细胞因子、减少抗肿瘤效应细胞的运输,以及诱导调节性T细胞(regulatory T cell,Treg细胞)等[23 ] .特别是对免疫细胞,MDSC更显示出其强大的免疫抑制作用.固有性免疫方面,MDSC可通过膜型转化生长因子β1(transforming growth factor β1,TGF-β1)下调自然杀伤细胞(natural killer cell,NK细胞)表面家族2成员D(NK cells group 2,member D,NKG2D)的表达,从而抑制NK细胞对肿瘤的杀伤功能[24 ] .也可通过释放细胞因子TGF-β1和IL-10等诱导TME中CD25+ FoxP3+ Treg细胞的扩增,从而促进Treg细胞对免疫的负向调控[25 ] .适应性免疫方面,MDSC可通过分泌精氨酸-1(arginine-1,Arg-1)和一氧化氮合酶(inducible nitric oxide synthase,iNOS)将T细胞受体(T cell receptor,TCR)ξ链的重要组成部分——L-精氨酸(L-arginine,L-Arg)分解为尿素和鸟氨酸或一氧化氮(nitric oxide,NO)和瓜氨酸,竞争性消耗微环境中L-Arg.这样不仅影响了TCR上cD3ξ链的合成,而且还抑制了细胞周期蛋白D3(cyclin D3,CCND3)和细胞周期依赖性激酶4(cyclin-dependent kinase 4,CDK4)的表达,导致T细胞停留在G0—G1期,最终抑制T细胞的增殖[26 -27 ] . ...
1
... 目前胰腺癌TME中的免疫逃逸机制是胰腺癌靶向治疗的主要难点.这是由MDSC的免疫抑制功能形成的,其可通过多种途径发挥免疫抑制作用,如消耗T细胞增殖所需氨基酸的代谢、分泌免疫抑制性细胞因子、减少抗肿瘤效应细胞的运输,以及诱导调节性T细胞(regulatory T cell,Treg细胞)等[23 ] .特别是对免疫细胞,MDSC更显示出其强大的免疫抑制作用.固有性免疫方面,MDSC可通过膜型转化生长因子β1(transforming growth factor β1,TGF-β1)下调自然杀伤细胞(natural killer cell,NK细胞)表面家族2成员D(NK cells group 2,member D,NKG2D)的表达,从而抑制NK细胞对肿瘤的杀伤功能[24 ] .也可通过释放细胞因子TGF-β1和IL-10等诱导TME中CD25+ FoxP3+ Treg细胞的扩增,从而促进Treg细胞对免疫的负向调控[25 ] .适应性免疫方面,MDSC可通过分泌精氨酸-1(arginine-1,Arg-1)和一氧化氮合酶(inducible nitric oxide synthase,iNOS)将T细胞受体(T cell receptor,TCR)ξ链的重要组成部分——L-精氨酸(L-arginine,L-Arg)分解为尿素和鸟氨酸或一氧化氮(nitric oxide,NO)和瓜氨酸,竞争性消耗微环境中L-Arg.这样不仅影响了TCR上cD3ξ链的合成,而且还抑制了细胞周期蛋白D3(cyclin D3,CCND3)和细胞周期依赖性激酶4(cyclin-dependent kinase 4,CDK4)的表达,导致T细胞停留在G0—G1期,最终抑制T细胞的增殖[26 -27 ] . ...
1
... 目前胰腺癌TME中的免疫逃逸机制是胰腺癌靶向治疗的主要难点.这是由MDSC的免疫抑制功能形成的,其可通过多种途径发挥免疫抑制作用,如消耗T细胞增殖所需氨基酸的代谢、分泌免疫抑制性细胞因子、减少抗肿瘤效应细胞的运输,以及诱导调节性T细胞(regulatory T cell,Treg细胞)等[23 ] .特别是对免疫细胞,MDSC更显示出其强大的免疫抑制作用.固有性免疫方面,MDSC可通过膜型转化生长因子β1(transforming growth factor β1,TGF-β1)下调自然杀伤细胞(natural killer cell,NK细胞)表面家族2成员D(NK cells group 2,member D,NKG2D)的表达,从而抑制NK细胞对肿瘤的杀伤功能[24 ] .也可通过释放细胞因子TGF-β1和IL-10等诱导TME中CD25+ FoxP3+ Treg细胞的扩增,从而促进Treg细胞对免疫的负向调控[25 ] .适应性免疫方面,MDSC可通过分泌精氨酸-1(arginine-1,Arg-1)和一氧化氮合酶(inducible nitric oxide synthase,iNOS)将T细胞受体(T cell receptor,TCR)ξ链的重要组成部分——L-精氨酸(L-arginine,L-Arg)分解为尿素和鸟氨酸或一氧化氮(nitric oxide,NO)和瓜氨酸,竞争性消耗微环境中L-Arg.这样不仅影响了TCR上cD3ξ链的合成,而且还抑制了细胞周期蛋白D3(cyclin D3,CCND3)和细胞周期依赖性激酶4(cyclin-dependent kinase 4,CDK4)的表达,导致T细胞停留在G0—G1期,最终抑制T细胞的增殖[26 -27 ] . ...
1
... 目前胰腺癌TME中的免疫逃逸机制是胰腺癌靶向治疗的主要难点.这是由MDSC的免疫抑制功能形成的,其可通过多种途径发挥免疫抑制作用,如消耗T细胞增殖所需氨基酸的代谢、分泌免疫抑制性细胞因子、减少抗肿瘤效应细胞的运输,以及诱导调节性T细胞(regulatory T cell,Treg细胞)等[23 ] .特别是对免疫细胞,MDSC更显示出其强大的免疫抑制作用.固有性免疫方面,MDSC可通过膜型转化生长因子β1(transforming growth factor β1,TGF-β1)下调自然杀伤细胞(natural killer cell,NK细胞)表面家族2成员D(NK cells group 2,member D,NKG2D)的表达,从而抑制NK细胞对肿瘤的杀伤功能[24 ] .也可通过释放细胞因子TGF-β1和IL-10等诱导TME中CD25+ FoxP3+ Treg细胞的扩增,从而促进Treg细胞对免疫的负向调控[25 ] .适应性免疫方面,MDSC可通过分泌精氨酸-1(arginine-1,Arg-1)和一氧化氮合酶(inducible nitric oxide synthase,iNOS)将T细胞受体(T cell receptor,TCR)ξ链的重要组成部分——L-精氨酸(L-arginine,L-Arg)分解为尿素和鸟氨酸或一氧化氮(nitric oxide,NO)和瓜氨酸,竞争性消耗微环境中L-Arg.这样不仅影响了TCR上cD3ξ链的合成,而且还抑制了细胞周期蛋白D3(cyclin D3,CCND3)和细胞周期依赖性激酶4(cyclin-dependent kinase 4,CDK4)的表达,导致T细胞停留在G0—G1期,最终抑制T细胞的增殖[26 -27 ] . ...
1
... 目前胰腺癌TME中的免疫逃逸机制是胰腺癌靶向治疗的主要难点.这是由MDSC的免疫抑制功能形成的,其可通过多种途径发挥免疫抑制作用,如消耗T细胞增殖所需氨基酸的代谢、分泌免疫抑制性细胞因子、减少抗肿瘤效应细胞的运输,以及诱导调节性T细胞(regulatory T cell,Treg细胞)等[23 ] .特别是对免疫细胞,MDSC更显示出其强大的免疫抑制作用.固有性免疫方面,MDSC可通过膜型转化生长因子β1(transforming growth factor β1,TGF-β1)下调自然杀伤细胞(natural killer cell,NK细胞)表面家族2成员D(NK cells group 2,member D,NKG2D)的表达,从而抑制NK细胞对肿瘤的杀伤功能[24 ] .也可通过释放细胞因子TGF-β1和IL-10等诱导TME中CD25+ FoxP3+ Treg细胞的扩增,从而促进Treg细胞对免疫的负向调控[25 ] .适应性免疫方面,MDSC可通过分泌精氨酸-1(arginine-1,Arg-1)和一氧化氮合酶(inducible nitric oxide synthase,iNOS)将T细胞受体(T cell receptor,TCR)ξ链的重要组成部分——L-精氨酸(L-arginine,L-Arg)分解为尿素和鸟氨酸或一氧化氮(nitric oxide,NO)和瓜氨酸,竞争性消耗微环境中L-Arg.这样不仅影响了TCR上cD3ξ链的合成,而且还抑制了细胞周期蛋白D3(cyclin D3,CCND3)和细胞周期依赖性激酶4(cyclin-dependent kinase 4,CDK4)的表达,导致T细胞停留在G0—G1期,最终抑制T细胞的增殖[26 -27 ] . ...
1
... 研究[8 ] 发现胰腺癌患者体内循环的MDSC(主要为G-MDSC)可高度表达Arg-1并抑制T细胞反应,这证实了Arg-1是胰腺癌中G-MDSC的特征性表达.当然,MDSC也可通过分泌活性氧(reactive oxygen species,ROS)来抑制T细胞免疫应答反应.与MDSC共培养之后,T细胞的功能和增殖明显受到抑制[28 ] .不同亚型的MDSC,通过不同的机制抑制T细胞:如M-MDSC主要通过STAT1途径,上调Arg-1、iNOS和TGF-β的表达等,实现非特异性T细胞失活;相对而言,G-MDSC则会通过STAT3信号通路产生较高水平的ROS,并通过与T淋巴细胞的接触发挥免疫抑制功能,使T细胞表现出对抗原特异性刺激的不敏感性,但对非特异性刺激有反应的现象[16 ,29 ,30 ] . ...
1
... 研究[8 ] 发现胰腺癌患者体内循环的MDSC(主要为G-MDSC)可高度表达Arg-1并抑制T细胞反应,这证实了Arg-1是胰腺癌中G-MDSC的特征性表达.当然,MDSC也可通过分泌活性氧(reactive oxygen species,ROS)来抑制T细胞免疫应答反应.与MDSC共培养之后,T细胞的功能和增殖明显受到抑制[28 ] .不同亚型的MDSC,通过不同的机制抑制T细胞:如M-MDSC主要通过STAT1途径,上调Arg-1、iNOS和TGF-β的表达等,实现非特异性T细胞失活;相对而言,G-MDSC则会通过STAT3信号通路产生较高水平的ROS,并通过与T淋巴细胞的接触发挥免疫抑制功能,使T细胞表现出对抗原特异性刺激的不敏感性,但对非特异性刺激有反应的现象[16 ,29 ,30 ] . ...
1
... 研究[8 ] 发现胰腺癌患者体内循环的MDSC(主要为G-MDSC)可高度表达Arg-1并抑制T细胞反应,这证实了Arg-1是胰腺癌中G-MDSC的特征性表达.当然,MDSC也可通过分泌活性氧(reactive oxygen species,ROS)来抑制T细胞免疫应答反应.与MDSC共培养之后,T细胞的功能和增殖明显受到抑制[28 ] .不同亚型的MDSC,通过不同的机制抑制T细胞:如M-MDSC主要通过STAT1途径,上调Arg-1、iNOS和TGF-β的表达等,实现非特异性T细胞失活;相对而言,G-MDSC则会通过STAT3信号通路产生较高水平的ROS,并通过与T淋巴细胞的接触发挥免疫抑制功能,使T细胞表现出对抗原特异性刺激的不敏感性,但对非特异性刺激有反应的现象[16 ,29 ,30 ] . ...
2
... 总的来说,M-MDSC和G-MDSC均具有免疫抑制作用.但在具体分析MDSC用于消除免疫抑制的主要机制时,要着重注意M-MDSC和G-MDSC的所占比例.在胰腺癌中,肿瘤组织中浸润的MDSC主要是G-MDSC,以其升高为主[8 ] .在胰腺癌TME中,MDSC可参与嗜中性粒细胞形成和进化[31 ] .MDSC也可促进M1型巨噬细胞(M1 type macrophage,M1)向M2型转化,从而加快肿瘤生长,促进肿瘤转移[32 ] .研究表明,在肿瘤环境下缺氧,特别是缺氧诱导因子(hypoxia inducible factor-1α,HIF-1α)可驱动MDSC分化为肿瘤相关巨噬细胞(tumor-associated macrophage,TAM)[33 ] 参与胰腺导管腺癌(pancreatic ductaladenocarcinoma,PDAC)中嗜神经侵袭(perineural invasion,PNI)的形成,其增多会导致PDAC预后不良[34 ] .在胰腺癌组织中,可观察到丰富的M2型TAM,与肿瘤的TNM分期密切相关,可用来评估患者的预后以及肿瘤的进展情况[35 ] . ...
... 除免疫抑制作用外,MDSC也可通过促进肿瘤血管生成以及肿瘤侵袭转移等机制参与肿瘤的发生发展[36 ] .MDSC可通过下调γ干扰素(interferon-γ,IFN-γ)、过表达炎症因子、表达基质金属蛋白酶9(matrix metalloproteinase-9,MMP9)和其他重塑因子降低细胞外基质(extracellular matrix,ECM)和基底膜的完整性,诱导血管渗漏而促进胰腺癌在微环境中的发展和转移[31 ] .上皮-间充质转化(epithelial-mesenchymal transition,EMT)是一种可逆的细胞过程,上皮细胞瞬间进入准间充质细胞状态,可使肿瘤细胞具有更大的侵袭和转移潜力,并产生更大的治疗耐药性.EMT的增加被认为是肿瘤转移的主要标志之一.在肿瘤形成的早期M-MDSC可通过IL-6介导的STAT3磷酸化诱导EMT,EMT和转移也会随着G-MDSC的增多而增多.在胰腺癌患者中可根据EMT标志物与MDSC亚群频率的比较确定这些MDSC在胰腺癌组织中的作用[4 ,37 ] . ...
1
... 总的来说,M-MDSC和G-MDSC均具有免疫抑制作用.但在具体分析MDSC用于消除免疫抑制的主要机制时,要着重注意M-MDSC和G-MDSC的所占比例.在胰腺癌中,肿瘤组织中浸润的MDSC主要是G-MDSC,以其升高为主[8 ] .在胰腺癌TME中,MDSC可参与嗜中性粒细胞形成和进化[31 ] .MDSC也可促进M1型巨噬细胞(M1 type macrophage,M1)向M2型转化,从而加快肿瘤生长,促进肿瘤转移[32 ] .研究表明,在肿瘤环境下缺氧,特别是缺氧诱导因子(hypoxia inducible factor-1α,HIF-1α)可驱动MDSC分化为肿瘤相关巨噬细胞(tumor-associated macrophage,TAM)[33 ] 参与胰腺导管腺癌(pancreatic ductaladenocarcinoma,PDAC)中嗜神经侵袭(perineural invasion,PNI)的形成,其增多会导致PDAC预后不良[34 ] .在胰腺癌组织中,可观察到丰富的M2型TAM,与肿瘤的TNM分期密切相关,可用来评估患者的预后以及肿瘤的进展情况[35 ] . ...
1
... 总的来说,M-MDSC和G-MDSC均具有免疫抑制作用.但在具体分析MDSC用于消除免疫抑制的主要机制时,要着重注意M-MDSC和G-MDSC的所占比例.在胰腺癌中,肿瘤组织中浸润的MDSC主要是G-MDSC,以其升高为主[8 ] .在胰腺癌TME中,MDSC可参与嗜中性粒细胞形成和进化[31 ] .MDSC也可促进M1型巨噬细胞(M1 type macrophage,M1)向M2型转化,从而加快肿瘤生长,促进肿瘤转移[32 ] .研究表明,在肿瘤环境下缺氧,特别是缺氧诱导因子(hypoxia inducible factor-1α,HIF-1α)可驱动MDSC分化为肿瘤相关巨噬细胞(tumor-associated macrophage,TAM)[33 ] 参与胰腺导管腺癌(pancreatic ductaladenocarcinoma,PDAC)中嗜神经侵袭(perineural invasion,PNI)的形成,其增多会导致PDAC预后不良[34 ] .在胰腺癌组织中,可观察到丰富的M2型TAM,与肿瘤的TNM分期密切相关,可用来评估患者的预后以及肿瘤的进展情况[35 ] . ...
1
... 总的来说,M-MDSC和G-MDSC均具有免疫抑制作用.但在具体分析MDSC用于消除免疫抑制的主要机制时,要着重注意M-MDSC和G-MDSC的所占比例.在胰腺癌中,肿瘤组织中浸润的MDSC主要是G-MDSC,以其升高为主[8 ] .在胰腺癌TME中,MDSC可参与嗜中性粒细胞形成和进化[31 ] .MDSC也可促进M1型巨噬细胞(M1 type macrophage,M1)向M2型转化,从而加快肿瘤生长,促进肿瘤转移[32 ] .研究表明,在肿瘤环境下缺氧,特别是缺氧诱导因子(hypoxia inducible factor-1α,HIF-1α)可驱动MDSC分化为肿瘤相关巨噬细胞(tumor-associated macrophage,TAM)[33 ] 参与胰腺导管腺癌(pancreatic ductaladenocarcinoma,PDAC)中嗜神经侵袭(perineural invasion,PNI)的形成,其增多会导致PDAC预后不良[34 ] .在胰腺癌组织中,可观察到丰富的M2型TAM,与肿瘤的TNM分期密切相关,可用来评估患者的预后以及肿瘤的进展情况[35 ] . ...
1
... 总的来说,M-MDSC和G-MDSC均具有免疫抑制作用.但在具体分析MDSC用于消除免疫抑制的主要机制时,要着重注意M-MDSC和G-MDSC的所占比例.在胰腺癌中,肿瘤组织中浸润的MDSC主要是G-MDSC,以其升高为主[8 ] .在胰腺癌TME中,MDSC可参与嗜中性粒细胞形成和进化[31 ] .MDSC也可促进M1型巨噬细胞(M1 type macrophage,M1)向M2型转化,从而加快肿瘤生长,促进肿瘤转移[32 ] .研究表明,在肿瘤环境下缺氧,特别是缺氧诱导因子(hypoxia inducible factor-1α,HIF-1α)可驱动MDSC分化为肿瘤相关巨噬细胞(tumor-associated macrophage,TAM)[33 ] 参与胰腺导管腺癌(pancreatic ductaladenocarcinoma,PDAC)中嗜神经侵袭(perineural invasion,PNI)的形成,其增多会导致PDAC预后不良[34 ] .在胰腺癌组织中,可观察到丰富的M2型TAM,与肿瘤的TNM分期密切相关,可用来评估患者的预后以及肿瘤的进展情况[35 ] . ...
1
... 除免疫抑制作用外,MDSC也可通过促进肿瘤血管生成以及肿瘤侵袭转移等机制参与肿瘤的发生发展[36 ] .MDSC可通过下调γ干扰素(interferon-γ,IFN-γ)、过表达炎症因子、表达基质金属蛋白酶9(matrix metalloproteinase-9,MMP9)和其他重塑因子降低细胞外基质(extracellular matrix,ECM)和基底膜的完整性,诱导血管渗漏而促进胰腺癌在微环境中的发展和转移[31 ] .上皮-间充质转化(epithelial-mesenchymal transition,EMT)是一种可逆的细胞过程,上皮细胞瞬间进入准间充质细胞状态,可使肿瘤细胞具有更大的侵袭和转移潜力,并产生更大的治疗耐药性.EMT的增加被认为是肿瘤转移的主要标志之一.在肿瘤形成的早期M-MDSC可通过IL-6介导的STAT3磷酸化诱导EMT,EMT和转移也会随着G-MDSC的增多而增多.在胰腺癌患者中可根据EMT标志物与MDSC亚群频率的比较确定这些MDSC在胰腺癌组织中的作用[4 ,37 ] . ...
1
... 除免疫抑制作用外,MDSC也可通过促进肿瘤血管生成以及肿瘤侵袭转移等机制参与肿瘤的发生发展[36 ] .MDSC可通过下调γ干扰素(interferon-γ,IFN-γ)、过表达炎症因子、表达基质金属蛋白酶9(matrix metalloproteinase-9,MMP9)和其他重塑因子降低细胞外基质(extracellular matrix,ECM)和基底膜的完整性,诱导血管渗漏而促进胰腺癌在微环境中的发展和转移[31 ] .上皮-间充质转化(epithelial-mesenchymal transition,EMT)是一种可逆的细胞过程,上皮细胞瞬间进入准间充质细胞状态,可使肿瘤细胞具有更大的侵袭和转移潜力,并产生更大的治疗耐药性.EMT的增加被认为是肿瘤转移的主要标志之一.在肿瘤形成的早期M-MDSC可通过IL-6介导的STAT3磷酸化诱导EMT,EMT和转移也会随着G-MDSC的增多而增多.在胰腺癌患者中可根据EMT标志物与MDSC亚群频率的比较确定这些MDSC在胰腺癌组织中的作用[4 ,37 ] . ...
1
... 目前,胰腺癌的免疫治疗越来越受到重视.鉴于MDSC对抗肿瘤免疫反应的重要性,寻找并确定靶向MDSC的药物和方法十分重要.通过针对MDSC的靶向治疗,可减弱其在胰腺癌TME中的免疫抑制能力,从而破坏免疫逃逸,提高胰腺癌的治愈率并改善患者的预后[38 ] .目前,实现靶向MDSC抗肿瘤治疗的方法主要为清除MDSC、抑制MDSC免疫功能以及抑制MDSC迁移和募集等.此外,胰腺癌患者的现有临床研究中,研究者们也在积极探索一些针对MDSC的相关药物以及免疫治疗方法. ...
2
... 现已证实一些低剂量化疗药物可以治疗胰腺癌,如吉西他滨(Gemcitabine,GEM)、5-氟尿嘧啶(5-fluorouracil,5-FU)等[39 ] .GEM是一种嘧啶类抗代谢药物,具有良好的免疫效应[40 ] .研究[41 ] 发现,接种4T1乳腺癌细胞的BALB/c小鼠经GEM处理后,脾脏内MDSC数量明显减少,但T、B细胞以及NK细胞等并无变化.这说明GEM对MDSC有着独特的杀伤作用,可以通过诱导MDSC凋亡和坏死清除MDSC,降低脾脏内MDSC的数量.对胰腺癌来说,虽然GEM可以用于治疗,但效果并不明显,对延缓患者的生存期几乎不起作用[42 ] .另外,与GEM一样,唑来膦酸(zoledronic acid,ZOL)也是胰腺癌的治疗药物,可优先作用于MDSC.其已被证明可以抑制肿瘤介导的造血增加,消除MDSC.使用ZOL对MDSC进行药理靶向,可明显改善抗肿瘤效果,减少肿瘤血管生成并增加了T细胞向肿瘤的募集[43 ] .ZOL对MDSC的作用在胰腺癌中特别有意义.因此,这两种药物的联合使用是克服肿瘤诱导的胰腺癌免疫抑制的潜在方法[44 ] . ...
... MDSC向肿瘤组织募集是由肿瘤相关的一些趋化因子介导的.因此,可通过趋化因子拮抗剂来阻断MDSC与趋化因子的反应从而阻止MDSC向肿瘤部位的迁移[56 ] .C-C基序趋化因子受体5(C-C chemokine receptor 5,CCR5)与其配体(C-C chemokine ligand 5,CCL5)的相互作用可促进肿瘤生长以及MDSC向肿瘤部位的迁移.通过靶向CCR5∕CCL5,不仅可以防止MDSC在TME中的迁移和积聚,还可以抑制胰腺癌、乳腺癌等癌症患者的肿瘤生长[57 ] .集落刺激因子1受体(colony-stimulating factor 1 receptor,CSF-1R)是一种酪氨酸激酶受体,也是抑制MDSC向肿瘤部位募集的主要靶点,在胰腺癌和乳腺癌等癌症中CSF-1R均可上调.当与其配体CSF-1结合时,可促进髓细胞向MDSC分化和扩张.靶向CSF-1R/CSF-1的治疗可改善抗肿瘤作用[39 ] .CCR2以及MDSC的浸润数量与胰腺癌患者的生存率显著相关.通过构建小鼠胰腺癌原位模型,利用CCR2阻断剂(PF04136309),可通过阻断CCL2/CCR2通路,减少肿瘤组织MDSC的浸润,从而减缓胰腺癌的生长和转移[58 ] . ...
1
... 现已证实一些低剂量化疗药物可以治疗胰腺癌,如吉西他滨(Gemcitabine,GEM)、5-氟尿嘧啶(5-fluorouracil,5-FU)等[39 ] .GEM是一种嘧啶类抗代谢药物,具有良好的免疫效应[40 ] .研究[41 ] 发现,接种4T1乳腺癌细胞的BALB/c小鼠经GEM处理后,脾脏内MDSC数量明显减少,但T、B细胞以及NK细胞等并无变化.这说明GEM对MDSC有着独特的杀伤作用,可以通过诱导MDSC凋亡和坏死清除MDSC,降低脾脏内MDSC的数量.对胰腺癌来说,虽然GEM可以用于治疗,但效果并不明显,对延缓患者的生存期几乎不起作用[42 ] .另外,与GEM一样,唑来膦酸(zoledronic acid,ZOL)也是胰腺癌的治疗药物,可优先作用于MDSC.其已被证明可以抑制肿瘤介导的造血增加,消除MDSC.使用ZOL对MDSC进行药理靶向,可明显改善抗肿瘤效果,减少肿瘤血管生成并增加了T细胞向肿瘤的募集[43 ] .ZOL对MDSC的作用在胰腺癌中特别有意义.因此,这两种药物的联合使用是克服肿瘤诱导的胰腺癌免疫抑制的潜在方法[44 ] . ...
1
... 现已证实一些低剂量化疗药物可以治疗胰腺癌,如吉西他滨(Gemcitabine,GEM)、5-氟尿嘧啶(5-fluorouracil,5-FU)等[39 ] .GEM是一种嘧啶类抗代谢药物,具有良好的免疫效应[40 ] .研究[41 ] 发现,接种4T1乳腺癌细胞的BALB/c小鼠经GEM处理后,脾脏内MDSC数量明显减少,但T、B细胞以及NK细胞等并无变化.这说明GEM对MDSC有着独特的杀伤作用,可以通过诱导MDSC凋亡和坏死清除MDSC,降低脾脏内MDSC的数量.对胰腺癌来说,虽然GEM可以用于治疗,但效果并不明显,对延缓患者的生存期几乎不起作用[42 ] .另外,与GEM一样,唑来膦酸(zoledronic acid,ZOL)也是胰腺癌的治疗药物,可优先作用于MDSC.其已被证明可以抑制肿瘤介导的造血增加,消除MDSC.使用ZOL对MDSC进行药理靶向,可明显改善抗肿瘤效果,减少肿瘤血管生成并增加了T细胞向肿瘤的募集[43 ] .ZOL对MDSC的作用在胰腺癌中特别有意义.因此,这两种药物的联合使用是克服肿瘤诱导的胰腺癌免疫抑制的潜在方法[44 ] . ...
2
... 现已证实一些低剂量化疗药物可以治疗胰腺癌,如吉西他滨(Gemcitabine,GEM)、5-氟尿嘧啶(5-fluorouracil,5-FU)等[39 ] .GEM是一种嘧啶类抗代谢药物,具有良好的免疫效应[40 ] .研究[41 ] 发现,接种4T1乳腺癌细胞的BALB/c小鼠经GEM处理后,脾脏内MDSC数量明显减少,但T、B细胞以及NK细胞等并无变化.这说明GEM对MDSC有着独特的杀伤作用,可以通过诱导MDSC凋亡和坏死清除MDSC,降低脾脏内MDSC的数量.对胰腺癌来说,虽然GEM可以用于治疗,但效果并不明显,对延缓患者的生存期几乎不起作用[42 ] .另外,与GEM一样,唑来膦酸(zoledronic acid,ZOL)也是胰腺癌的治疗药物,可优先作用于MDSC.其已被证明可以抑制肿瘤介导的造血增加,消除MDSC.使用ZOL对MDSC进行药理靶向,可明显改善抗肿瘤效果,减少肿瘤血管生成并增加了T细胞向肿瘤的募集[43 ] .ZOL对MDSC的作用在胰腺癌中特别有意义.因此,这两种药物的联合使用是克服肿瘤诱导的胰腺癌免疫抑制的潜在方法[44 ] . ...
... 但值得关注的是,联合GEM靶向MDSC治疗胰腺癌这种策略正在被探索[6 ] .GHANSAH等[42 ] 在同源小鼠胰腺癌细胞Panc02种植模型中发现,GEM化疗可以明显缩小肿瘤,但并没有提高其生存期;树突细胞治疗对肿瘤大小及生存期更是无明显影响,但将GEM与树突细胞结合治疗不但可以明显缩小肿瘤体积,明显降低脾脏中的MDSC水平,还可显著提高总体生存期,所以考虑树突状细胞免疫治疗联合GEM化疗可提高胰腺癌小鼠的生存.在一项GEM化疗联合静脉注射ω-3脂肪酸(ω-3 fatty acids,ω-3FAs)与单独GEM化疗对胰腺癌患者免疫细胞的影响研究中发现,ω-3FAs脂肪酸可以抑制胰腺癌的生长,增强GEM的治疗作用.GEM联合应用ω-3FAs治疗胰腺癌明显减少了患者MDSC的数量,从而验证了ω-3FAs的积极作用[45 ] .5-FU是一种嘧啶类似物,研究发现它可强效抑制体内MDSC的细胞毒性[46 ] ,可选择性地杀死MDSC,从而促进抗肿瘤免疫反应[47 ] .值得注意的是,在GEM联合卡培他滨(一种5-氟尿嘧啶前药)对胰腺癌患者MDSC的影响研究中发现,GEM和卡培他滨联合治疗并没有一致的降低胰腺癌患者的MDSC[48 ] .也就是说,虽然这两种药物都可以治疗胰腺癌,但随着患者数量以及患病程度的增加联合效果并不理想.因此联合治疗也并不是最佳的治疗手段,也存在一定局限性.正是由于癌症治疗的复杂化,所以未来更应该注重联合治疗的科学合理性. ...
1
... 现已证实一些低剂量化疗药物可以治疗胰腺癌,如吉西他滨(Gemcitabine,GEM)、5-氟尿嘧啶(5-fluorouracil,5-FU)等[39 ] .GEM是一种嘧啶类抗代谢药物,具有良好的免疫效应[40 ] .研究[41 ] 发现,接种4T1乳腺癌细胞的BALB/c小鼠经GEM处理后,脾脏内MDSC数量明显减少,但T、B细胞以及NK细胞等并无变化.这说明GEM对MDSC有着独特的杀伤作用,可以通过诱导MDSC凋亡和坏死清除MDSC,降低脾脏内MDSC的数量.对胰腺癌来说,虽然GEM可以用于治疗,但效果并不明显,对延缓患者的生存期几乎不起作用[42 ] .另外,与GEM一样,唑来膦酸(zoledronic acid,ZOL)也是胰腺癌的治疗药物,可优先作用于MDSC.其已被证明可以抑制肿瘤介导的造血增加,消除MDSC.使用ZOL对MDSC进行药理靶向,可明显改善抗肿瘤效果,减少肿瘤血管生成并增加了T细胞向肿瘤的募集[43 ] .ZOL对MDSC的作用在胰腺癌中特别有意义.因此,这两种药物的联合使用是克服肿瘤诱导的胰腺癌免疫抑制的潜在方法[44 ] . ...
1
... 现已证实一些低剂量化疗药物可以治疗胰腺癌,如吉西他滨(Gemcitabine,GEM)、5-氟尿嘧啶(5-fluorouracil,5-FU)等[39 ] .GEM是一种嘧啶类抗代谢药物,具有良好的免疫效应[40 ] .研究[41 ] 发现,接种4T1乳腺癌细胞的BALB/c小鼠经GEM处理后,脾脏内MDSC数量明显减少,但T、B细胞以及NK细胞等并无变化.这说明GEM对MDSC有着独特的杀伤作用,可以通过诱导MDSC凋亡和坏死清除MDSC,降低脾脏内MDSC的数量.对胰腺癌来说,虽然GEM可以用于治疗,但效果并不明显,对延缓患者的生存期几乎不起作用[42 ] .另外,与GEM一样,唑来膦酸(zoledronic acid,ZOL)也是胰腺癌的治疗药物,可优先作用于MDSC.其已被证明可以抑制肿瘤介导的造血增加,消除MDSC.使用ZOL对MDSC进行药理靶向,可明显改善抗肿瘤效果,减少肿瘤血管生成并增加了T细胞向肿瘤的募集[43 ] .ZOL对MDSC的作用在胰腺癌中特别有意义.因此,这两种药物的联合使用是克服肿瘤诱导的胰腺癌免疫抑制的潜在方法[44 ] . ...
1
... 但值得关注的是,联合GEM靶向MDSC治疗胰腺癌这种策略正在被探索[6 ] .GHANSAH等[42 ] 在同源小鼠胰腺癌细胞Panc02种植模型中发现,GEM化疗可以明显缩小肿瘤,但并没有提高其生存期;树突细胞治疗对肿瘤大小及生存期更是无明显影响,但将GEM与树突细胞结合治疗不但可以明显缩小肿瘤体积,明显降低脾脏中的MDSC水平,还可显著提高总体生存期,所以考虑树突状细胞免疫治疗联合GEM化疗可提高胰腺癌小鼠的生存.在一项GEM化疗联合静脉注射ω-3脂肪酸(ω-3 fatty acids,ω-3FAs)与单独GEM化疗对胰腺癌患者免疫细胞的影响研究中发现,ω-3FAs脂肪酸可以抑制胰腺癌的生长,增强GEM的治疗作用.GEM联合应用ω-3FAs治疗胰腺癌明显减少了患者MDSC的数量,从而验证了ω-3FAs的积极作用[45 ] .5-FU是一种嘧啶类似物,研究发现它可强效抑制体内MDSC的细胞毒性[46 ] ,可选择性地杀死MDSC,从而促进抗肿瘤免疫反应[47 ] .值得注意的是,在GEM联合卡培他滨(一种5-氟尿嘧啶前药)对胰腺癌患者MDSC的影响研究中发现,GEM和卡培他滨联合治疗并没有一致的降低胰腺癌患者的MDSC[48 ] .也就是说,虽然这两种药物都可以治疗胰腺癌,但随着患者数量以及患病程度的增加联合效果并不理想.因此联合治疗也并不是最佳的治疗手段,也存在一定局限性.正是由于癌症治疗的复杂化,所以未来更应该注重联合治疗的科学合理性. ...
1
... 但值得关注的是,联合GEM靶向MDSC治疗胰腺癌这种策略正在被探索[6 ] .GHANSAH等[42 ] 在同源小鼠胰腺癌细胞Panc02种植模型中发现,GEM化疗可以明显缩小肿瘤,但并没有提高其生存期;树突细胞治疗对肿瘤大小及生存期更是无明显影响,但将GEM与树突细胞结合治疗不但可以明显缩小肿瘤体积,明显降低脾脏中的MDSC水平,还可显著提高总体生存期,所以考虑树突状细胞免疫治疗联合GEM化疗可提高胰腺癌小鼠的生存.在一项GEM化疗联合静脉注射ω-3脂肪酸(ω-3 fatty acids,ω-3FAs)与单独GEM化疗对胰腺癌患者免疫细胞的影响研究中发现,ω-3FAs脂肪酸可以抑制胰腺癌的生长,增强GEM的治疗作用.GEM联合应用ω-3FAs治疗胰腺癌明显减少了患者MDSC的数量,从而验证了ω-3FAs的积极作用[45 ] .5-FU是一种嘧啶类似物,研究发现它可强效抑制体内MDSC的细胞毒性[46 ] ,可选择性地杀死MDSC,从而促进抗肿瘤免疫反应[47 ] .值得注意的是,在GEM联合卡培他滨(一种5-氟尿嘧啶前药)对胰腺癌患者MDSC的影响研究中发现,GEM和卡培他滨联合治疗并没有一致的降低胰腺癌患者的MDSC[48 ] .也就是说,虽然这两种药物都可以治疗胰腺癌,但随着患者数量以及患病程度的增加联合效果并不理想.因此联合治疗也并不是最佳的治疗手段,也存在一定局限性.正是由于癌症治疗的复杂化,所以未来更应该注重联合治疗的科学合理性. ...
1
... 但值得关注的是,联合GEM靶向MDSC治疗胰腺癌这种策略正在被探索[6 ] .GHANSAH等[42 ] 在同源小鼠胰腺癌细胞Panc02种植模型中发现,GEM化疗可以明显缩小肿瘤,但并没有提高其生存期;树突细胞治疗对肿瘤大小及生存期更是无明显影响,但将GEM与树突细胞结合治疗不但可以明显缩小肿瘤体积,明显降低脾脏中的MDSC水平,还可显著提高总体生存期,所以考虑树突状细胞免疫治疗联合GEM化疗可提高胰腺癌小鼠的生存.在一项GEM化疗联合静脉注射ω-3脂肪酸(ω-3 fatty acids,ω-3FAs)与单独GEM化疗对胰腺癌患者免疫细胞的影响研究中发现,ω-3FAs脂肪酸可以抑制胰腺癌的生长,增强GEM的治疗作用.GEM联合应用ω-3FAs治疗胰腺癌明显减少了患者MDSC的数量,从而验证了ω-3FAs的积极作用[45 ] .5-FU是一种嘧啶类似物,研究发现它可强效抑制体内MDSC的细胞毒性[46 ] ,可选择性地杀死MDSC,从而促进抗肿瘤免疫反应[47 ] .值得注意的是,在GEM联合卡培他滨(一种5-氟尿嘧啶前药)对胰腺癌患者MDSC的影响研究中发现,GEM和卡培他滨联合治疗并没有一致的降低胰腺癌患者的MDSC[48 ] .也就是说,虽然这两种药物都可以治疗胰腺癌,但随着患者数量以及患病程度的增加联合效果并不理想.因此联合治疗也并不是最佳的治疗手段,也存在一定局限性.正是由于癌症治疗的复杂化,所以未来更应该注重联合治疗的科学合理性. ...
1
... 但值得关注的是,联合GEM靶向MDSC治疗胰腺癌这种策略正在被探索[6 ] .GHANSAH等[42 ] 在同源小鼠胰腺癌细胞Panc02种植模型中发现,GEM化疗可以明显缩小肿瘤,但并没有提高其生存期;树突细胞治疗对肿瘤大小及生存期更是无明显影响,但将GEM与树突细胞结合治疗不但可以明显缩小肿瘤体积,明显降低脾脏中的MDSC水平,还可显著提高总体生存期,所以考虑树突状细胞免疫治疗联合GEM化疗可提高胰腺癌小鼠的生存.在一项GEM化疗联合静脉注射ω-3脂肪酸(ω-3 fatty acids,ω-3FAs)与单独GEM化疗对胰腺癌患者免疫细胞的影响研究中发现,ω-3FAs脂肪酸可以抑制胰腺癌的生长,增强GEM的治疗作用.GEM联合应用ω-3FAs治疗胰腺癌明显减少了患者MDSC的数量,从而验证了ω-3FAs的积极作用[45 ] .5-FU是一种嘧啶类似物,研究发现它可强效抑制体内MDSC的细胞毒性[46 ] ,可选择性地杀死MDSC,从而促进抗肿瘤免疫反应[47 ] .值得注意的是,在GEM联合卡培他滨(一种5-氟尿嘧啶前药)对胰腺癌患者MDSC的影响研究中发现,GEM和卡培他滨联合治疗并没有一致的降低胰腺癌患者的MDSC[48 ] .也就是说,虽然这两种药物都可以治疗胰腺癌,但随着患者数量以及患病程度的增加联合效果并不理想.因此联合治疗也并不是最佳的治疗手段,也存在一定局限性.正是由于癌症治疗的复杂化,所以未来更应该注重联合治疗的科学合理性. ...
1
... 除以上一些抗癌药物之外,研究发现通过肿瘤坏死因子(tumor necrosis factor,TNF)相关的凋亡诱导配体受体(TNF-related apoptosis-inducing ligand receptor,TRAIL-R)靶向抗体,也可清除癌症患者的MDSC[49 ] .使用激动性TRAIL-R抗体DS-8273a治疗后,包括胰腺癌患者在内的多数受试者外周血以及肿瘤组织内的MDSC数量可减少到正常水平,同时DS-8273a并不影响其他免疫细胞的数量,这也表明了DS-827a对MDSC的特异性[50 ] .此外,我们前期的研究发现信筒子醌也是治疗胰腺癌的有效抗肿瘤候选药物.其可通过抑制IL-6分泌,减少免疫抑制细胞MDSC和Treg细胞浸润,显著降低体内胰腺癌细胞的致瘤性[51 ] .Src同源肌醇磷酸酶(Src homology2-containing inositol-5′-phosphatase1,SHIP-1)也可能是一个潜在治疗MDSC相关恶性肿瘤的靶点[52 ] ,当敲除胰腺癌小鼠模型中的Ship-1 基因后,通过负性调节磷脂酰肌醇3-激酶/蛋白激酶B信号通路明显降低了MDSC的扩增,进而延缓胰腺癌的进展. ...
1
... 除以上一些抗癌药物之外,研究发现通过肿瘤坏死因子(tumor necrosis factor,TNF)相关的凋亡诱导配体受体(TNF-related apoptosis-inducing ligand receptor,TRAIL-R)靶向抗体,也可清除癌症患者的MDSC[49 ] .使用激动性TRAIL-R抗体DS-8273a治疗后,包括胰腺癌患者在内的多数受试者外周血以及肿瘤组织内的MDSC数量可减少到正常水平,同时DS-8273a并不影响其他免疫细胞的数量,这也表明了DS-827a对MDSC的特异性[50 ] .此外,我们前期的研究发现信筒子醌也是治疗胰腺癌的有效抗肿瘤候选药物.其可通过抑制IL-6分泌,减少免疫抑制细胞MDSC和Treg细胞浸润,显著降低体内胰腺癌细胞的致瘤性[51 ] .Src同源肌醇磷酸酶(Src homology2-containing inositol-5′-phosphatase1,SHIP-1)也可能是一个潜在治疗MDSC相关恶性肿瘤的靶点[52 ] ,当敲除胰腺癌小鼠模型中的Ship-1 基因后,通过负性调节磷脂酰肌醇3-激酶/蛋白激酶B信号通路明显降低了MDSC的扩增,进而延缓胰腺癌的进展. ...
1
... 除以上一些抗癌药物之外,研究发现通过肿瘤坏死因子(tumor necrosis factor,TNF)相关的凋亡诱导配体受体(TNF-related apoptosis-inducing ligand receptor,TRAIL-R)靶向抗体,也可清除癌症患者的MDSC[49 ] .使用激动性TRAIL-R抗体DS-8273a治疗后,包括胰腺癌患者在内的多数受试者外周血以及肿瘤组织内的MDSC数量可减少到正常水平,同时DS-8273a并不影响其他免疫细胞的数量,这也表明了DS-827a对MDSC的特异性[50 ] .此外,我们前期的研究发现信筒子醌也是治疗胰腺癌的有效抗肿瘤候选药物.其可通过抑制IL-6分泌,减少免疫抑制细胞MDSC和Treg细胞浸润,显著降低体内胰腺癌细胞的致瘤性[51 ] .Src同源肌醇磷酸酶(Src homology2-containing inositol-5′-phosphatase1,SHIP-1)也可能是一个潜在治疗MDSC相关恶性肿瘤的靶点[52 ] ,当敲除胰腺癌小鼠模型中的Ship-1 基因后,通过负性调节磷脂酰肌醇3-激酶/蛋白激酶B信号通路明显降低了MDSC的扩增,进而延缓胰腺癌的进展. ...
1
... 除以上一些抗癌药物之外,研究发现通过肿瘤坏死因子(tumor necrosis factor,TNF)相关的凋亡诱导配体受体(TNF-related apoptosis-inducing ligand receptor,TRAIL-R)靶向抗体,也可清除癌症患者的MDSC[49 ] .使用激动性TRAIL-R抗体DS-8273a治疗后,包括胰腺癌患者在内的多数受试者外周血以及肿瘤组织内的MDSC数量可减少到正常水平,同时DS-8273a并不影响其他免疫细胞的数量,这也表明了DS-827a对MDSC的特异性[50 ] .此外,我们前期的研究发现信筒子醌也是治疗胰腺癌的有效抗肿瘤候选药物.其可通过抑制IL-6分泌,减少免疫抑制细胞MDSC和Treg细胞浸润,显著降低体内胰腺癌细胞的致瘤性[51 ] .Src同源肌醇磷酸酶(Src homology2-containing inositol-5′-phosphatase1,SHIP-1)也可能是一个潜在治疗MDSC相关恶性肿瘤的靶点[52 ] ,当敲除胰腺癌小鼠模型中的Ship-1 基因后,通过负性调节磷脂酰肌醇3-激酶/蛋白激酶B信号通路明显降低了MDSC的扩增,进而延缓胰腺癌的进展. ...
1
... 目前,MDSC主要是通过分泌或表达一些抑制性因子发挥免疫抑制作用,比如,Arg-1、iNOS和ROS等[53 ] .因此,通过阻断这些抑制性因子的表达从而消除MDSC的免疫抑制功能不失为一种有效的手段.在胰腺癌小鼠模型中一种胰腺癌上调因子(pancreatic adenocarcinoma up-regulated factor,PAUF)可通过toll样受体4(Toll-like receptor 4,TLR4)信号转导参与MAPK/ERK信号通路升高Arg-1、NO和ROS的水平,从而增强MDSC的免疫抑制活性[54 ] .当使用人抗PAUF单克隆抗体PMAb83治疗后,明显降低了MDSC的数量以及免疫抑制活性[6 ,54 ] ,继而表明通过靶向PAUF治疗胰腺癌有巨大的潜力.三萜类人工合成物CDDO-Me也可抑制荷瘤小鼠以及癌症患者的MDSC活性,并改善抗肿瘤免疫[55 ] .但联合GEM治疗并没有明显影响MDSC,并未达到预期效果[6 ,55 ] .我们前期的研究[15 ] 发现用载脂蛋白AI(apolipoprotein A-I,ApoA-I)的模拟肽L-4F可以通过降低G-MDSC的数量和功能来抑制小鼠胰腺癌的发展. ...
2
... 目前,MDSC主要是通过分泌或表达一些抑制性因子发挥免疫抑制作用,比如,Arg-1、iNOS和ROS等[53 ] .因此,通过阻断这些抑制性因子的表达从而消除MDSC的免疫抑制功能不失为一种有效的手段.在胰腺癌小鼠模型中一种胰腺癌上调因子(pancreatic adenocarcinoma up-regulated factor,PAUF)可通过toll样受体4(Toll-like receptor 4,TLR4)信号转导参与MAPK/ERK信号通路升高Arg-1、NO和ROS的水平,从而增强MDSC的免疫抑制活性[54 ] .当使用人抗PAUF单克隆抗体PMAb83治疗后,明显降低了MDSC的数量以及免疫抑制活性[6 ,54 ] ,继而表明通过靶向PAUF治疗胰腺癌有巨大的潜力.三萜类人工合成物CDDO-Me也可抑制荷瘤小鼠以及癌症患者的MDSC活性,并改善抗肿瘤免疫[55 ] .但联合GEM治疗并没有明显影响MDSC,并未达到预期效果[6 ,55 ] .我们前期的研究[15 ] 发现用载脂蛋白AI(apolipoprotein A-I,ApoA-I)的模拟肽L-4F可以通过降低G-MDSC的数量和功能来抑制小鼠胰腺癌的发展. ...
... ,54 ],继而表明通过靶向PAUF治疗胰腺癌有巨大的潜力.三萜类人工合成物CDDO-Me也可抑制荷瘤小鼠以及癌症患者的MDSC活性,并改善抗肿瘤免疫[55 ] .但联合GEM治疗并没有明显影响MDSC,并未达到预期效果[6 ,55 ] .我们前期的研究[15 ] 发现用载脂蛋白AI(apolipoprotein A-I,ApoA-I)的模拟肽L-4F可以通过降低G-MDSC的数量和功能来抑制小鼠胰腺癌的发展. ...
2
... 目前,MDSC主要是通过分泌或表达一些抑制性因子发挥免疫抑制作用,比如,Arg-1、iNOS和ROS等[53 ] .因此,通过阻断这些抑制性因子的表达从而消除MDSC的免疫抑制功能不失为一种有效的手段.在胰腺癌小鼠模型中一种胰腺癌上调因子(pancreatic adenocarcinoma up-regulated factor,PAUF)可通过toll样受体4(Toll-like receptor 4,TLR4)信号转导参与MAPK/ERK信号通路升高Arg-1、NO和ROS的水平,从而增强MDSC的免疫抑制活性[54 ] .当使用人抗PAUF单克隆抗体PMAb83治疗后,明显降低了MDSC的数量以及免疫抑制活性[6 ,54 ] ,继而表明通过靶向PAUF治疗胰腺癌有巨大的潜力.三萜类人工合成物CDDO-Me也可抑制荷瘤小鼠以及癌症患者的MDSC活性,并改善抗肿瘤免疫[55 ] .但联合GEM治疗并没有明显影响MDSC,并未达到预期效果[6 ,55 ] .我们前期的研究[15 ] 发现用载脂蛋白AI(apolipoprotein A-I,ApoA-I)的模拟肽L-4F可以通过降低G-MDSC的数量和功能来抑制小鼠胰腺癌的发展. ...
... ,55 ].我们前期的研究[15 ] 发现用载脂蛋白AI(apolipoprotein A-I,ApoA-I)的模拟肽L-4F可以通过降低G-MDSC的数量和功能来抑制小鼠胰腺癌的发展. ...
1
... MDSC向肿瘤组织募集是由肿瘤相关的一些趋化因子介导的.因此,可通过趋化因子拮抗剂来阻断MDSC与趋化因子的反应从而阻止MDSC向肿瘤部位的迁移[56 ] .C-C基序趋化因子受体5(C-C chemokine receptor 5,CCR5)与其配体(C-C chemokine ligand 5,CCL5)的相互作用可促进肿瘤生长以及MDSC向肿瘤部位的迁移.通过靶向CCR5∕CCL5,不仅可以防止MDSC在TME中的迁移和积聚,还可以抑制胰腺癌、乳腺癌等癌症患者的肿瘤生长[57 ] .集落刺激因子1受体(colony-stimulating factor 1 receptor,CSF-1R)是一种酪氨酸激酶受体,也是抑制MDSC向肿瘤部位募集的主要靶点,在胰腺癌和乳腺癌等癌症中CSF-1R均可上调.当与其配体CSF-1结合时,可促进髓细胞向MDSC分化和扩张.靶向CSF-1R/CSF-1的治疗可改善抗肿瘤作用[39 ] .CCR2以及MDSC的浸润数量与胰腺癌患者的生存率显著相关.通过构建小鼠胰腺癌原位模型,利用CCR2阻断剂(PF04136309),可通过阻断CCL2/CCR2通路,减少肿瘤组织MDSC的浸润,从而减缓胰腺癌的生长和转移[58 ] . ...
1
... MDSC向肿瘤组织募集是由肿瘤相关的一些趋化因子介导的.因此,可通过趋化因子拮抗剂来阻断MDSC与趋化因子的反应从而阻止MDSC向肿瘤部位的迁移[56 ] .C-C基序趋化因子受体5(C-C chemokine receptor 5,CCR5)与其配体(C-C chemokine ligand 5,CCL5)的相互作用可促进肿瘤生长以及MDSC向肿瘤部位的迁移.通过靶向CCR5∕CCL5,不仅可以防止MDSC在TME中的迁移和积聚,还可以抑制胰腺癌、乳腺癌等癌症患者的肿瘤生长[57 ] .集落刺激因子1受体(colony-stimulating factor 1 receptor,CSF-1R)是一种酪氨酸激酶受体,也是抑制MDSC向肿瘤部位募集的主要靶点,在胰腺癌和乳腺癌等癌症中CSF-1R均可上调.当与其配体CSF-1结合时,可促进髓细胞向MDSC分化和扩张.靶向CSF-1R/CSF-1的治疗可改善抗肿瘤作用[39 ] .CCR2以及MDSC的浸润数量与胰腺癌患者的生存率显著相关.通过构建小鼠胰腺癌原位模型,利用CCR2阻断剂(PF04136309),可通过阻断CCL2/CCR2通路,减少肿瘤组织MDSC的浸润,从而减缓胰腺癌的生长和转移[58 ] . ...
1
... MDSC向肿瘤组织募集是由肿瘤相关的一些趋化因子介导的.因此,可通过趋化因子拮抗剂来阻断MDSC与趋化因子的反应从而阻止MDSC向肿瘤部位的迁移[56 ] .C-C基序趋化因子受体5(C-C chemokine receptor 5,CCR5)与其配体(C-C chemokine ligand 5,CCL5)的相互作用可促进肿瘤生长以及MDSC向肿瘤部位的迁移.通过靶向CCR5∕CCL5,不仅可以防止MDSC在TME中的迁移和积聚,还可以抑制胰腺癌、乳腺癌等癌症患者的肿瘤生长[57 ] .集落刺激因子1受体(colony-stimulating factor 1 receptor,CSF-1R)是一种酪氨酸激酶受体,也是抑制MDSC向肿瘤部位募集的主要靶点,在胰腺癌和乳腺癌等癌症中CSF-1R均可上调.当与其配体CSF-1结合时,可促进髓细胞向MDSC分化和扩张.靶向CSF-1R/CSF-1的治疗可改善抗肿瘤作用[39 ] .CCR2以及MDSC的浸润数量与胰腺癌患者的生存率显著相关.通过构建小鼠胰腺癌原位模型,利用CCR2阻断剂(PF04136309),可通过阻断CCL2/CCR2通路,减少肿瘤组织MDSC的浸润,从而减缓胰腺癌的生长和转移[58 ] . ...