[1]
ELSAID N, SAIED A, KANDIL H, et al. Impact of stress and hypertension on the cerebrovasculature[J]. Front Biosci (Landmark Ed), 2021, 26(12): 1643-1652.
[本文引用: 1]
[2]
KEMPURAJ D, AHMED M E, SELVAKUMAR G P, et al. Psychological stress-induced immune response and risk of Alzheimer' s disease in veterans from operation enduring freedom and operation Iraqi freedom[J]. Clin Ther, 2020, 42(6): 974-982.
[本文引用: 1]
[3]
VOLARIĆ M, ŠOJAT D, MAJNARIĆ L T, et al. The association between functional dyspepsia and metabolic syndrome-the state of the art[J]. Int J Environ Res Public Health, 2024, 21(2): 237.
[本文引用: 1]
[4]
ZHANG H Y, WANG M, ZHAO X, et al. Role of stress in skin diseases: a neuroendocrine-immune interaction view[J]. Brain Behav Immun, 2024, 116: 286-302.
[本文引用: 1]
[5]
中华医学会妇产科学分会绝经学组. 早发性卵巢功能不全的临床诊疗专家共识(2023版)[J]. 中华妇产科杂志, 2023, 58(10): 721-728.
[本文引用: 2]
Menopause Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. Consensus of clinical diagnosis and treatment of premature ovarian insufficiency (2023)[J]. Chinese Journal of Obstetrics and Gynecology, 2023, 58(10): 721-728.
[本文引用: 2]
[6]
European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI, WEBBER L, DAVIES M, et al. ESHRE Guideline: management of women with premature ovarian insufficiency[J]. Hum Reprod, 2016, 31(5): 926-937.
[本文引用: 1]
[7]
LI M, ZHU Y, WEI J, et al. The global prevalence of premature ovarian insufficiency: a systematic review and meta-analysis[J]. Climacteric, 2023, 26(2): 95-102.
[本文引用: 1]
[8]
中华医学会妇产科学分会绝经学组. 早发性卵巢功能不全的激素补充治疗专家共识[J]. 中华妇产科杂志, 2016, 51(12): 881-886.
[本文引用: 1]
Menopause Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. Consensus of hormone replacement therapy for premature ovarian insufficiency[J]. Chinese Journal of Obstetrics and Gynecology, 2016, 51(12): 881-886.
[本文引用: 1]
[9]
BRYDGES N M, BEST C, THOMAS K L. Female HPA axis displays heightened sensitivity to pre-pubertal stress[J]. Stress, 2020, 23(2): 190-200.
[本文引用: 1]
[10]
SUN J Y, GUO Y, FAN Y H, et al. Decreased expression of IDH1 by chronic unpredictable stress suppresses proliferation and accelerates senescence of granulosa cells through ROS activated MAPK signaling pathways[J]. Free Radic Biol Med, 2021, 169: 122-136.
[本文引用: 4]
[11]
TAL R, SEIFER D B. Ovarian reserve testing: a user' s guide[J]. Am J Obstet Gynecol, 2017, 217(2): 129-140.
[本文引用: 1]
[12]
VEGETTI W, ALAGNA F. FSH and folliculogenesis: from physiology to ovarian stimulation[J]. Reprod Biomed Online, 2006, 12(6): 684-694.
[本文引用: 1]
[13]
谢幸, 孔北华, 段涛. 妇产科学[M]. 9版. 北京: 人民卫生出版社, 2018: 20-28.
[本文引用: 1]
XIE X, KONG B H, DUAN T. Obstetrics and gynecology[M]. 9th ed. Beijing: People' s Medical Publishing House, 2018: 20-28.
[本文引用: 1]
[14]
KRUSZYŃSKA A, SŁOWIŃSKA-SRZEDNICKA J. Anti-Müllerian hormone (AMH) as a good predictor of time of menopause[J]. Prz Menopauzalny, 2017, 16(2): 47-50.
[本文引用: 1]
[15]
SUN J Y, FAN Y H, GUO Y, et al. Chronic and cumulative adverse life events in women with primary ovarian insufficiency: an exploratory qualitative study[J]. Front Endocrinol (Lausanne), 2022, 13: 856044.
[本文引用: 1]
[16]
YEĞIN G F, DESDICIOĞLU R, SEÇEN E İ, et al. Low anti-Mullerian hormone levels are associated with the severity of anxiety experienced by healthcare professionals during the COVID-19 pandemic[J]. Reprod Sci, 2022, 29(2): 627-632.
[本文引用: 1]
[17]
DONG Y Z, ZHOU F J, SUN Y P. Psychological stress is related to a decrease of serum anti-Müllerian hormone level in infertile women[J]. Reprod Biol Endocrinol, 2017, 15(1): 51.
[本文引用: 1]
[18]
PAL L, BEVILACQUA K, SANTORO N F. Chronic psychosocial stressors are detrimental to ovarian reserve: a study of infertile women[J]. J Psychosom Obstet Gynaecol, 2010, 31(3): 130-139.
[本文引用: 1]
[19]
XI W Y, MAO H, CUI Z W, et al. Scream sound-induced chronic psychological stress results in diminished ovarian reserve in adult female rat[J]. Endocrinology, 2022, 163(6): bqac042.
[本文引用: 3]
[20]
KIM J, YOU S. High housing density-induced chronic stress diminishes ovarian reserve via granulosa cell apoptosis by angiotensin Ⅱ overexpression in mice[J]. Int J Mol Sci, 2022, 23(15): 8614.
[本文引用: 4]
[21]
JIANG Y W, XU J, TAO C Q, et al. Chronic stress induces meiotic arrest failure and ovarian reserve decline via the cAMP signaling pathway[J]. Front Endocrinol (Lausanne), 2023, 14: 1177061.
[本文引用: 3]
[22]
XU M H, SUN J Y, WANG Q, et al. Chronic restraint stress induces excessive activation of primordial follicles in mice ovaries[J]. PLoS One, 2018, 13(3): e0194894.
[本文引用: 3]
[23]
FU X Y, ZHENG Q, ZHANG N, et al. CUMS promotes the development of premature ovarian insufficiency mediated by nerve growth factor and its receptor in rats[J]. Biomed Res Int, 2020, 2020: 1946853.
[本文引用: 2]
[24]
NAIR B B, KHANT AUNG Z, PORTEOUS R, et al. Impact of chronic variable stress on neuroendocrine hypothalamus and pituitary in male and female C57BL/6J mice[J]. J Neuroendocrinol, 2021, 33(5): e12972.
[本文引用: 2]
[25]
SUN J Y, GUO Y, ZHANG Q W, et al. Chronic restraint stress disturbs meiotic resumption through APC/C-mediated cyclin B1 excessive degradation in mouse oocytes[J]. Cell Cycle, 2018, 17(13): 1591-1601.
[本文引用: 1]
[26]
GUO Y, SUN J Y, BU S X, et al. Melatonin protects against chronic stress-induced oxidative meiotic defects in mice MII oocytes by regulating SIRT1[J]. Cell Cycle, 2020, 19(13): 1677-1695.
[本文引用: 1]
[27]
ZHAO X L, MA R H, ZHANG X Y, et al. Transcriptomic study of the mechanism by which the Kai Yu Zhong Yu recipe improves oocyte quality in a stressed mouse model[J]. J Ethnopharmacol, 2021, 278: 114298.
[本文引用: 1]
[28]
SEN A, CAIAZZA F. Oocyte maturation: a story of arrest and release[J]. Front Biosci (Schol Ed), 2013, 5(2): 451-477.
[本文引用: 2]
[29]
ZHAO X L, MA R H, ZHANG X Y, et al. Reduced growth capacity of preimplantation mouse embryos in chronic unpredictable stress model[J]. Mol Reprod Dev, 2021, 88(1): 80-95.
[本文引用: 1]
[30]
CASILLAS F, BETANCOURT M, JUÁREZ-ROJAS L, et al. Chronic stress detrimentally affects in vivo maturation in rat oocytes and oocyte viability at all phases of the estrous cycle[J]. Animals (Basel), 2021, 11(9): 2478.
[本文引用: 1]
[31]
GAO Y, CHEN F, KONG Q Q, et al. Stresses on female mice impair oocyte developmental potential: effects of stress severity and duration on oocytes at the growing follicle stage[J]. Reprod Sci, 2016, 23(9): 1148-1157.
[本文引用: 1]
[32]
CHROUSOS G P, GOLD P W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis[J]. JAMA, 1992, 267(9): 1244-1252.
[本文引用: 1]
[33]
GLASER R, KIECOLT-GLASER J K. Stress-induced immune dysfunction: implications for health[J]. Nat Rev Immunol, 2005, 5(3): 243-251.
[本文引用: 2]
[34]
KIAPEKOU E, ZAPANTI E, MASTORAKOS G, et al. Update on the role of ovarian corticotropin-releasing hormone[J]. Ann N Y Acad Sci, 2010, 1205: 225-229.
[本文引用: 1]
[35]
LI C Y, LI Z B, KONG Q Q, et al. Restraint-induced corticotrophin-releasing hormone elevation triggers apoptosis of ovarian cells and impairs oocyte competence via activation of the Fas/FasL system[J]. Biol Reprod, 2018, 99(4): 828-837.
[本文引用: 1]
[36]
DI NATALE M R, SOCH A, ZIKO I, et al. Chronic predator stress in female mice reduces primordial follicle numbers: implications for the role of ghrelin[J]. J Endocrinol, 2019, 241(3): 201-219.
[本文引用: 1]
[37]
BENITEZ A, RIQUELME R, DEL CAMPO M, et al. Nerve growth factor: a dual activator of noradrenergic and cholinergic systems of the rat ovary[J]. Front Endocrinol (Lausanne), 2021, 12: 636600.
[本文引用: 1]
[38]
RIQUELME R, RUZ F, MAYERHOFER A, et al. Role of ovarian sympathetic nerves and cholinergic local system during cold stress[J]. J Endocrinol, 2019, 242(2): 115-124.
[本文引用: 1]
[39]
LI M, XUE L, XU W B, et al. Rno-miR-128-3p promotes apoptosis in rat granulosa cells (GCs) induced by norepinephrine through Wilms tumor 1 (WT1)[J]. In Vitro Cell Dev Biol Anim, 2021, 57(8): 775-785.
[本文引用: 1]
[40]
RETANA-MÁRQUEZ S, JUÁREZ-ROJAS L, ÁVILA-QUINTERO A, et al. Neuroendocrine disruption is associated to infertility in chronically stressed female rats[J]. Reprod Biol, 2020, 20(4): 474-483.
[本文引用: 1]
[41]
HUANG Y Q, LIU Q Y, HUANG G F, et al. Hypothalamic kisspeptin neurons regulates energy metabolism and reproduction under chronic stress[J]. Front Endocrinol (Lausanne), 2022, 13: 844397.
[本文引用: 2]
[42]
HARTER C J L, KAVANAGH G S, SMITH J T. The role of kisspeptin neurons in reproduction and metabolism[J]. J Endocrinol, 2018, 238(3): R173-R183.
[本文引用: 1]
[43]
SAHIN Z, OZKURKCULER A, KALKAN O F, et al. Gonadotropin levels reduced in seven days immobilization stress-induced depressive-like behavior in female rats[J]. J Basic Clin Physiol Pharmacol, 2021, 33(2): 199-206.
[本文引用: 1]
[44]
RUSSELL G, LIGHTMAN S. The human stress response[J]. Nat Rev Endocrinol, 2019, 15(9): 525-534.
[本文引用: 1]
[45]
OSTER H, CHALLET E, OTT V, et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids[J]. Endocr Rev, 2017, 38(1): 3-45.
[本文引用: 1]
[46]
LUO E, STEPHENS S B, CHAING S, et al. Corticosterone blocks ovarian cyclicity and the LH surge via decreased kisspeptin neuron activation in female mice[J]. Endocrinology, 2016, 157(3): 1187-1199.
[本文引用: 1]
[47]
WANG Y, LIU W J, DU J, et al. NGF promotes mouse granulosa cell proliferation by inhibiting ESR2 mediated down-regulation of CDKN1A[J]. Mol Cell Endocrinol, 2015, 406: 68-77.
[本文引用: 1]
[48]
GHATEBI M, ZAVAREH S, LASHKARBOLOUKI T, et al. Implications from early life stress on the development of mouse ovarian follicles: focus on oxidative stress[J]. J Obstet Gynaecol Res, 2019, 45(8): 1506-1514.
[本文引用: 1]
1
... 应激是机体在内、外环境因素或社会心理因素刺激时出现的全身性、非特异性适应反应,又称为应激反应.在现代社会中,人们普遍存在着工作、学习、家庭和社会来源的心理应激,导致机体长期暴露于应激源中.既往研究表明,长期经历应激性事件与心脑血管疾病[1 ] 、阿尔茨海默病[2 ] 、肥胖、功能性胃肠疾病[3 ] 、皮肤病[4 ] 等的发生存在关联. ...
1
... 应激是机体在内、外环境因素或社会心理因素刺激时出现的全身性、非特异性适应反应,又称为应激反应.在现代社会中,人们普遍存在着工作、学习、家庭和社会来源的心理应激,导致机体长期暴露于应激源中.既往研究表明,长期经历应激性事件与心脑血管疾病[1 ] 、阿尔茨海默病[2 ] 、肥胖、功能性胃肠疾病[3 ] 、皮肤病[4 ] 等的发生存在关联. ...
1
... 应激是机体在内、外环境因素或社会心理因素刺激时出现的全身性、非特异性适应反应,又称为应激反应.在现代社会中,人们普遍存在着工作、学习、家庭和社会来源的心理应激,导致机体长期暴露于应激源中.既往研究表明,长期经历应激性事件与心脑血管疾病[1 ] 、阿尔茨海默病[2 ] 、肥胖、功能性胃肠疾病[3 ] 、皮肤病[4 ] 等的发生存在关联. ...
1
... 应激是机体在内、外环境因素或社会心理因素刺激时出现的全身性、非特异性适应反应,又称为应激反应.在现代社会中,人们普遍存在着工作、学习、家庭和社会来源的心理应激,导致机体长期暴露于应激源中.既往研究表明,长期经历应激性事件与心脑血管疾病[1 ] 、阿尔茨海默病[2 ] 、肥胖、功能性胃肠疾病[3 ] 、皮肤病[4 ] 等的发生存在关联. ...
2
... 早发性卵巢功能不全(premature ovarian insufficiency,POI)是导致女性不孕的常见原因之一,指女性40岁之前发生卵巢功能减退,以月经异常(闭经、月经稀发)、促性腺激素水平升高和低雌激素水平为特征;其诊断标准为停经或月经稀发>4个月且间隔>4周连续2次血清基础卵泡刺激素(follicle stimulating hormone,FSH)>25 IU/L;而在此基础上出现2次FSH>40 IU/L时则被诊断为卵巢早衰(premature ovarian failure,POF),即为卵巢功能减退的终末阶段.研究[5 ] 发现,POI不仅会损害患者的生育能力,还会导致雌激素水平降低,从而使患者出现潮热、出汗和情绪改变等症状.此外,POI还会增加患者发生骨质疏松及心脑血管疾病的风险甚至缩短寿命[6 ] ,给其身心健康、经济状况及家庭关系等带来沉重的负担与困扰.目前,全球POI的总体患病率为3.5%,近20年内呈上升趋势[7 ] .POI的已知病因包括遗传学因素、免疫性因素及医源性因素等.而有超过50%的POI患者病因不明,这类POI即称为特发性POI[5 ] .随着病因研究的深入和临床病例的积累,人们逐渐发现卵巢功能减退是一类临床表现多样、病因复杂且呈进行性发展的疾病[8 ] ;同时,由于POI的防控相当困难,该疾病已成为影响年轻育龄女性健康的复杂性且难治性疾病.因此,早期识别POI的高危因素是十分必要的. ...
... [5 ].随着病因研究的深入和临床病例的积累,人们逐渐发现卵巢功能减退是一类临床表现多样、病因复杂且呈进行性发展的疾病[8 ] ;同时,由于POI的防控相当困难,该疾病已成为影响年轻育龄女性健康的复杂性且难治性疾病.因此,早期识别POI的高危因素是十分必要的. ...
2
... 早发性卵巢功能不全(premature ovarian insufficiency,POI)是导致女性不孕的常见原因之一,指女性40岁之前发生卵巢功能减退,以月经异常(闭经、月经稀发)、促性腺激素水平升高和低雌激素水平为特征;其诊断标准为停经或月经稀发>4个月且间隔>4周连续2次血清基础卵泡刺激素(follicle stimulating hormone,FSH)>25 IU/L;而在此基础上出现2次FSH>40 IU/L时则被诊断为卵巢早衰(premature ovarian failure,POF),即为卵巢功能减退的终末阶段.研究[5 ] 发现,POI不仅会损害患者的生育能力,还会导致雌激素水平降低,从而使患者出现潮热、出汗和情绪改变等症状.此外,POI还会增加患者发生骨质疏松及心脑血管疾病的风险甚至缩短寿命[6 ] ,给其身心健康、经济状况及家庭关系等带来沉重的负担与困扰.目前,全球POI的总体患病率为3.5%,近20年内呈上升趋势[7 ] .POI的已知病因包括遗传学因素、免疫性因素及医源性因素等.而有超过50%的POI患者病因不明,这类POI即称为特发性POI[5 ] .随着病因研究的深入和临床病例的积累,人们逐渐发现卵巢功能减退是一类临床表现多样、病因复杂且呈进行性发展的疾病[8 ] ;同时,由于POI的防控相当困难,该疾病已成为影响年轻育龄女性健康的复杂性且难治性疾病.因此,早期识别POI的高危因素是十分必要的. ...
... [5 ].随着病因研究的深入和临床病例的积累,人们逐渐发现卵巢功能减退是一类临床表现多样、病因复杂且呈进行性发展的疾病[8 ] ;同时,由于POI的防控相当困难,该疾病已成为影响年轻育龄女性健康的复杂性且难治性疾病.因此,早期识别POI的高危因素是十分必要的. ...
1
... 早发性卵巢功能不全(premature ovarian insufficiency,POI)是导致女性不孕的常见原因之一,指女性40岁之前发生卵巢功能减退,以月经异常(闭经、月经稀发)、促性腺激素水平升高和低雌激素水平为特征;其诊断标准为停经或月经稀发>4个月且间隔>4周连续2次血清基础卵泡刺激素(follicle stimulating hormone,FSH)>25 IU/L;而在此基础上出现2次FSH>40 IU/L时则被诊断为卵巢早衰(premature ovarian failure,POF),即为卵巢功能减退的终末阶段.研究[5 ] 发现,POI不仅会损害患者的生育能力,还会导致雌激素水平降低,从而使患者出现潮热、出汗和情绪改变等症状.此外,POI还会增加患者发生骨质疏松及心脑血管疾病的风险甚至缩短寿命[6 ] ,给其身心健康、经济状况及家庭关系等带来沉重的负担与困扰.目前,全球POI的总体患病率为3.5%,近20年内呈上升趋势[7 ] .POI的已知病因包括遗传学因素、免疫性因素及医源性因素等.而有超过50%的POI患者病因不明,这类POI即称为特发性POI[5 ] .随着病因研究的深入和临床病例的积累,人们逐渐发现卵巢功能减退是一类临床表现多样、病因复杂且呈进行性发展的疾病[8 ] ;同时,由于POI的防控相当困难,该疾病已成为影响年轻育龄女性健康的复杂性且难治性疾病.因此,早期识别POI的高危因素是十分必要的. ...
1
... 早发性卵巢功能不全(premature ovarian insufficiency,POI)是导致女性不孕的常见原因之一,指女性40岁之前发生卵巢功能减退,以月经异常(闭经、月经稀发)、促性腺激素水平升高和低雌激素水平为特征;其诊断标准为停经或月经稀发>4个月且间隔>4周连续2次血清基础卵泡刺激素(follicle stimulating hormone,FSH)>25 IU/L;而在此基础上出现2次FSH>40 IU/L时则被诊断为卵巢早衰(premature ovarian failure,POF),即为卵巢功能减退的终末阶段.研究[5 ] 发现,POI不仅会损害患者的生育能力,还会导致雌激素水平降低,从而使患者出现潮热、出汗和情绪改变等症状.此外,POI还会增加患者发生骨质疏松及心脑血管疾病的风险甚至缩短寿命[6 ] ,给其身心健康、经济状况及家庭关系等带来沉重的负担与困扰.目前,全球POI的总体患病率为3.5%,近20年内呈上升趋势[7 ] .POI的已知病因包括遗传学因素、免疫性因素及医源性因素等.而有超过50%的POI患者病因不明,这类POI即称为特发性POI[5 ] .随着病因研究的深入和临床病例的积累,人们逐渐发现卵巢功能减退是一类临床表现多样、病因复杂且呈进行性发展的疾病[8 ] ;同时,由于POI的防控相当困难,该疾病已成为影响年轻育龄女性健康的复杂性且难治性疾病.因此,早期识别POI的高危因素是十分必要的. ...
1
... 早发性卵巢功能不全(premature ovarian insufficiency,POI)是导致女性不孕的常见原因之一,指女性40岁之前发生卵巢功能减退,以月经异常(闭经、月经稀发)、促性腺激素水平升高和低雌激素水平为特征;其诊断标准为停经或月经稀发>4个月且间隔>4周连续2次血清基础卵泡刺激素(follicle stimulating hormone,FSH)>25 IU/L;而在此基础上出现2次FSH>40 IU/L时则被诊断为卵巢早衰(premature ovarian failure,POF),即为卵巢功能减退的终末阶段.研究[5 ] 发现,POI不仅会损害患者的生育能力,还会导致雌激素水平降低,从而使患者出现潮热、出汗和情绪改变等症状.此外,POI还会增加患者发生骨质疏松及心脑血管疾病的风险甚至缩短寿命[6 ] ,给其身心健康、经济状况及家庭关系等带来沉重的负担与困扰.目前,全球POI的总体患病率为3.5%,近20年内呈上升趋势[7 ] .POI的已知病因包括遗传学因素、免疫性因素及医源性因素等.而有超过50%的POI患者病因不明,这类POI即称为特发性POI[5 ] .随着病因研究的深入和临床病例的积累,人们逐渐发现卵巢功能减退是一类临床表现多样、病因复杂且呈进行性发展的疾病[8 ] ;同时,由于POI的防控相当困难,该疾病已成为影响年轻育龄女性健康的复杂性且难治性疾病.因此,早期识别POI的高危因素是十分必要的. ...
1
... 早发性卵巢功能不全(premature ovarian insufficiency,POI)是导致女性不孕的常见原因之一,指女性40岁之前发生卵巢功能减退,以月经异常(闭经、月经稀发)、促性腺激素水平升高和低雌激素水平为特征;其诊断标准为停经或月经稀发>4个月且间隔>4周连续2次血清基础卵泡刺激素(follicle stimulating hormone,FSH)>25 IU/L;而在此基础上出现2次FSH>40 IU/L时则被诊断为卵巢早衰(premature ovarian failure,POF),即为卵巢功能减退的终末阶段.研究[5 ] 发现,POI不仅会损害患者的生育能力,还会导致雌激素水平降低,从而使患者出现潮热、出汗和情绪改变等症状.此外,POI还会增加患者发生骨质疏松及心脑血管疾病的风险甚至缩短寿命[6 ] ,给其身心健康、经济状况及家庭关系等带来沉重的负担与困扰.目前,全球POI的总体患病率为3.5%,近20年内呈上升趋势[7 ] .POI的已知病因包括遗传学因素、免疫性因素及医源性因素等.而有超过50%的POI患者病因不明,这类POI即称为特发性POI[5 ] .随着病因研究的深入和临床病例的积累,人们逐渐发现卵巢功能减退是一类临床表现多样、病因复杂且呈进行性发展的疾病[8 ] ;同时,由于POI的防控相当困难,该疾病已成为影响年轻育龄女性健康的复杂性且难治性疾病.因此,早期识别POI的高危因素是十分必要的. ...
1
... 在现代社会中,随着生活节奏的加快和竞争的日益激烈,社会身份的多重性使女性持续承受着来自工作和家庭的压力,也导致其长期暴露于应激源中且逐渐形成慢性应激状态.同时,女性对与应激相关的精神病理会表现出更大的易感性[9 ] .近年来,已有研究[10 ] 表明慢性应激可能是导致卵巢功能减退的高危因素,但临床上无论是医师还是患者对于该疾病的认识普遍不足.基于此,本文就慢性应激与卵巢功能减退的相关性研究进展进行综述,以期引起社会的重视,为早期预防及干预卵巢功能减退、保护女性的生殖健康提供参考. ...
4
... 在现代社会中,随着生活节奏的加快和竞争的日益激烈,社会身份的多重性使女性持续承受着来自工作和家庭的压力,也导致其长期暴露于应激源中且逐渐形成慢性应激状态.同时,女性对与应激相关的精神病理会表现出更大的易感性[9 ] .近年来,已有研究[10 ] 表明慢性应激可能是导致卵巢功能减退的高危因素,但临床上无论是医师还是患者对于该疾病的认识普遍不足.基于此,本文就慢性应激与卵巢功能减退的相关性研究进展进行综述,以期引起社会的重视,为早期预防及干预卵巢功能减退、保护女性的生殖健康提供参考. ...
... GC是卵泡的重要组成部分,参与雌激素的生成与分泌,在卵泡发育中发挥关键作用.研究显示,尖叫声应激[19 ] 、高密度饲养应激[20 ] 及慢性不可预测应激[10 ] 等均会增加卵巢中GC的凋亡或衰老.本课题组的前期研究[10 ] 发现,在慢性不可预测应激中,小鼠窦状卵泡GC中异柠檬酸脱氢酶1(isocitrate dehydrogenase 1,IDH1)显著降低,导致该细胞中的活性氧含量增加,并上调自噬和丝裂原激活蛋白激酶信号通路,从而抑制该细胞的增殖并加速衰老.在高密度饲养应激下,小鼠卵巢组织中的血管紧张素Ⅱ(angiotensin Ⅱ,Ang Ⅱ)显著增加,从而可加速GC的凋亡[20 ] .这些结果均表明,长期暴露于应激源可引起卵巢GC凋亡,造成卵巢功能减退. ...
... [10 ]发现,在慢性不可预测应激中,小鼠窦状卵泡GC中异柠檬酸脱氢酶1(isocitrate dehydrogenase 1,IDH1)显著降低,导致该细胞中的活性氧含量增加,并上调自噬和丝裂原激活蛋白激酶信号通路,从而抑制该细胞的增殖并加速衰老.在高密度饲养应激下,小鼠卵巢组织中的血管紧张素Ⅱ(angiotensin Ⅱ,Ang Ⅱ)显著增加,从而可加速GC的凋亡[20 ] .这些结果均表明,长期暴露于应激源可引起卵巢GC凋亡,造成卵巢功能减退. ...
... 慢性应激可作用于下丘脑和垂体,导致其激素分泌出现异常,从而影响卵巢功能.在HPO轴中,由下丘脑分泌的KISSPEPTIN、GnRH及由垂体分泌的FSH、促黄体素(luteinizing hormone,LH)均发挥着重要作用.研究[40 -41 ] 发现,长期经冷水或束缚应激刺激可导致个体的下丘脑KISSPEPTIN分泌减少,从而抑制GnRH神经元(即调控生殖的关键神经元).KISSPEPTIN是一种由KISSPEPTIN神经元分泌的神经肽,常通过作用于下丘脑的GnRH神经元参与卵巢功能的调控[42 ] .HUANG等[41 ] 发现,慢性束缚应激后的雌性小鼠下丘脑中的Kisspeptin 下调可能是受KISSPEPTIN神经元上的糖皮质激素受体调控.同时,对不同的应激模型开展研究[10 ,24 ,43 ] 也发现,由垂体分泌的FSH、LH水平会出现不同程度的波动,进而影响小鼠的卵泡发育和排卵等功能. ...
1
... 目前,常见的卵巢功能评估指标有血清FSH和抗米勒管激素(anti-Müllerian hormone,AMH)[11 ] .FSH是一种由2个肽亚基组成的糖蛋白激素[12 ] ,在下丘脑分泌的促性腺激素释放激素(gonadotropin releasing hormone,GnRH)作用下由垂体前叶分泌,是用来评估卵巢功能的常用指标.FSH在女性生殖系统中发挥了较重要的作用,如在下丘脑-垂体-卵巢(hypothalamic-pituitary-ovarian,HPO)轴中,FSH可负反馈调节GnRH的分泌,同时自身受卵巢分泌的雌激素、孕激素和抑制素的负反馈调节[13 ] .在女性中,AMH主要由初级卵泡、窦前卵泡和小窦卵泡的颗粒细胞(granulosa cell,GC)产生[14 ] ,是评估卵巢功能的一个良好指标.随着卵巢功能的下降,育龄女性的AMH也会逐渐下降. ...
1
... 目前,常见的卵巢功能评估指标有血清FSH和抗米勒管激素(anti-Müllerian hormone,AMH)[11 ] .FSH是一种由2个肽亚基组成的糖蛋白激素[12 ] ,在下丘脑分泌的促性腺激素释放激素(gonadotropin releasing hormone,GnRH)作用下由垂体前叶分泌,是用来评估卵巢功能的常用指标.FSH在女性生殖系统中发挥了较重要的作用,如在下丘脑-垂体-卵巢(hypothalamic-pituitary-ovarian,HPO)轴中,FSH可负反馈调节GnRH的分泌,同时自身受卵巢分泌的雌激素、孕激素和抑制素的负反馈调节[13 ] .在女性中,AMH主要由初级卵泡、窦前卵泡和小窦卵泡的颗粒细胞(granulosa cell,GC)产生[14 ] ,是评估卵巢功能的一个良好指标.随着卵巢功能的下降,育龄女性的AMH也会逐渐下降. ...
1
... 目前,常见的卵巢功能评估指标有血清FSH和抗米勒管激素(anti-Müllerian hormone,AMH)[11 ] .FSH是一种由2个肽亚基组成的糖蛋白激素[12 ] ,在下丘脑分泌的促性腺激素释放激素(gonadotropin releasing hormone,GnRH)作用下由垂体前叶分泌,是用来评估卵巢功能的常用指标.FSH在女性生殖系统中发挥了较重要的作用,如在下丘脑-垂体-卵巢(hypothalamic-pituitary-ovarian,HPO)轴中,FSH可负反馈调节GnRH的分泌,同时自身受卵巢分泌的雌激素、孕激素和抑制素的负反馈调节[13 ] .在女性中,AMH主要由初级卵泡、窦前卵泡和小窦卵泡的颗粒细胞(granulosa cell,GC)产生[14 ] ,是评估卵巢功能的一个良好指标.随着卵巢功能的下降,育龄女性的AMH也会逐渐下降. ...
1
... 目前,常见的卵巢功能评估指标有血清FSH和抗米勒管激素(anti-Müllerian hormone,AMH)[11 ] .FSH是一种由2个肽亚基组成的糖蛋白激素[12 ] ,在下丘脑分泌的促性腺激素释放激素(gonadotropin releasing hormone,GnRH)作用下由垂体前叶分泌,是用来评估卵巢功能的常用指标.FSH在女性生殖系统中发挥了较重要的作用,如在下丘脑-垂体-卵巢(hypothalamic-pituitary-ovarian,HPO)轴中,FSH可负反馈调节GnRH的分泌,同时自身受卵巢分泌的雌激素、孕激素和抑制素的负反馈调节[13 ] .在女性中,AMH主要由初级卵泡、窦前卵泡和小窦卵泡的颗粒细胞(granulosa cell,GC)产生[14 ] ,是评估卵巢功能的一个良好指标.随着卵巢功能的下降,育龄女性的AMH也会逐渐下降. ...
1
... 目前,常见的卵巢功能评估指标有血清FSH和抗米勒管激素(anti-Müllerian hormone,AMH)[11 ] .FSH是一种由2个肽亚基组成的糖蛋白激素[12 ] ,在下丘脑分泌的促性腺激素释放激素(gonadotropin releasing hormone,GnRH)作用下由垂体前叶分泌,是用来评估卵巢功能的常用指标.FSH在女性生殖系统中发挥了较重要的作用,如在下丘脑-垂体-卵巢(hypothalamic-pituitary-ovarian,HPO)轴中,FSH可负反馈调节GnRH的分泌,同时自身受卵巢分泌的雌激素、孕激素和抑制素的负反馈调节[13 ] .在女性中,AMH主要由初级卵泡、窦前卵泡和小窦卵泡的颗粒细胞(granulosa cell,GC)产生[14 ] ,是评估卵巢功能的一个良好指标.随着卵巢功能的下降,育龄女性的AMH也会逐渐下降. ...
1
... 一些基于人群的研究认为,长期暴露于慢性应激与卵巢功能减退的发生存在关联,并可能是卵巢功能减退的高危因素.本课题组对43名被诊断为特发性POF的女性进行半结构化访谈后发现,受访者在诊断前曾持续暴露于工作压力、家庭压力和睡眠问题相关的不良生活事件中[15 ] .同时,慢性应激与AMH水平降低也存在关联.在新型冠状病毒肺炎大流行期间,直接向患者提供医疗服务的医疗保健专业人员的AMH水平与所其经历的焦虑程度呈负相关[16 ] .相似的是,不孕女性的AMH水平与其心理压力水平呈显著负相关[17 ] .此外,有研究[18 ] 显示慢性应激源与血清FSH水平独立相关,是卵巢储备功能减退发生的预测因素之一.目前,越来越多的基础研究从慢性应激影响卵泡、卵母细胞或GC的角度,对慢性应激损伤卵巢功能的机制进行探索,具体介绍如下. ...
1
... 一些基于人群的研究认为,长期暴露于慢性应激与卵巢功能减退的发生存在关联,并可能是卵巢功能减退的高危因素.本课题组对43名被诊断为特发性POF的女性进行半结构化访谈后发现,受访者在诊断前曾持续暴露于工作压力、家庭压力和睡眠问题相关的不良生活事件中[15 ] .同时,慢性应激与AMH水平降低也存在关联.在新型冠状病毒肺炎大流行期间,直接向患者提供医疗服务的医疗保健专业人员的AMH水平与所其经历的焦虑程度呈负相关[16 ] .相似的是,不孕女性的AMH水平与其心理压力水平呈显著负相关[17 ] .此外,有研究[18 ] 显示慢性应激源与血清FSH水平独立相关,是卵巢储备功能减退发生的预测因素之一.目前,越来越多的基础研究从慢性应激影响卵泡、卵母细胞或GC的角度,对慢性应激损伤卵巢功能的机制进行探索,具体介绍如下. ...
1
... 一些基于人群的研究认为,长期暴露于慢性应激与卵巢功能减退的发生存在关联,并可能是卵巢功能减退的高危因素.本课题组对43名被诊断为特发性POF的女性进行半结构化访谈后发现,受访者在诊断前曾持续暴露于工作压力、家庭压力和睡眠问题相关的不良生活事件中[15 ] .同时,慢性应激与AMH水平降低也存在关联.在新型冠状病毒肺炎大流行期间,直接向患者提供医疗服务的医疗保健专业人员的AMH水平与所其经历的焦虑程度呈负相关[16 ] .相似的是,不孕女性的AMH水平与其心理压力水平呈显著负相关[17 ] .此外,有研究[18 ] 显示慢性应激源与血清FSH水平独立相关,是卵巢储备功能减退发生的预测因素之一.目前,越来越多的基础研究从慢性应激影响卵泡、卵母细胞或GC的角度,对慢性应激损伤卵巢功能的机制进行探索,具体介绍如下. ...
1
... 一些基于人群的研究认为,长期暴露于慢性应激与卵巢功能减退的发生存在关联,并可能是卵巢功能减退的高危因素.本课题组对43名被诊断为特发性POF的女性进行半结构化访谈后发现,受访者在诊断前曾持续暴露于工作压力、家庭压力和睡眠问题相关的不良生活事件中[15 ] .同时,慢性应激与AMH水平降低也存在关联.在新型冠状病毒肺炎大流行期间,直接向患者提供医疗服务的医疗保健专业人员的AMH水平与所其经历的焦虑程度呈负相关[16 ] .相似的是,不孕女性的AMH水平与其心理压力水平呈显著负相关[17 ] .此外,有研究[18 ] 显示慢性应激源与血清FSH水平独立相关,是卵巢储备功能减退发生的预测因素之一.目前,越来越多的基础研究从慢性应激影响卵泡、卵母细胞或GC的角度,对慢性应激损伤卵巢功能的机制进行探索,具体介绍如下. ...
3
... 卵巢原始卵泡作为卵泡发育的起点和卵泡的储备形式,在雌性生殖寿命的正常维持中具有重要作用.研究发现,原始卵泡的过度激活会导致卵巢储备功能下降,与POF的发生密切相关;尖叫声应激[19 ] 、高密度饲养应激[20 ] 、束缚应激[21 -22 ] 及慢性不可预测应激[23 -24 ] 等均可加速原始卵泡的过度激活,增加闭锁卵泡数量,从而使卵泡池健康卵泡数量减少.以慢性束缚应激为例,本课题组的前期研究[22 ] 发现,慢性束缚应激可导致Kit配体及其受体表达水平升高,磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/磷酸酯酶和张力蛋白同源物(phosphatase and tensin homolog,PTEN)/蛋白激酶B(protein kinase B,AKT)通路的激活,从而诱导小鼠卵巢原始卵泡过度激活.综上,慢性应激会对各级卵泡产生较负面的影响,从而导致卵巢功能减退. ...
... GC是卵泡的重要组成部分,参与雌激素的生成与分泌,在卵泡发育中发挥关键作用.研究显示,尖叫声应激[19 ] 、高密度饲养应激[20 ] 及慢性不可预测应激[10 ] 等均会增加卵巢中GC的凋亡或衰老.本课题组的前期研究[10 ] 发现,在慢性不可预测应激中,小鼠窦状卵泡GC中异柠檬酸脱氢酶1(isocitrate dehydrogenase 1,IDH1)显著降低,导致该细胞中的活性氧含量增加,并上调自噬和丝裂原激活蛋白激酶信号通路,从而抑制该细胞的增殖并加速衰老.在高密度饲养应激下,小鼠卵巢组织中的血管紧张素Ⅱ(angiotensin Ⅱ,Ang Ⅱ)显著增加,从而可加速GC的凋亡[20 ] .这些结果均表明,长期暴露于应激源可引起卵巢GC凋亡,造成卵巢功能减退. ...
... 皮质酮是啮齿类动物肾上腺皮质中产生的一种类固醇激素,也是HPA轴调节的重要激素[44 ] ,可调节体内能量代谢、应激反应、免疫和认知[45 ] ,在人体内对应的产物为皮质醇.既往多数研究[19 -20 ] 发现,慢性应激可导致动物体内皮质酮含量增加.LUO等[46 ] 发现皮质酮可强烈抑制雌性小鼠前腹侧脑室周围核和邻近四周核的KISSPEPTIN神经元活性,也可抑制垂体中GnRH受体和Lh 的表达.这些研究表明,慢性应激可能通过分泌的皮质酮/皮质醇影响HPO轴,进而影响卵巢功能;而有关皮质酮/皮质醇浓度的变化对卵巢功能的具体调控,尚有待进一步研究. ...
4
... 卵巢原始卵泡作为卵泡发育的起点和卵泡的储备形式,在雌性生殖寿命的正常维持中具有重要作用.研究发现,原始卵泡的过度激活会导致卵巢储备功能下降,与POF的发生密切相关;尖叫声应激[19 ] 、高密度饲养应激[20 ] 、束缚应激[21 -22 ] 及慢性不可预测应激[23 -24 ] 等均可加速原始卵泡的过度激活,增加闭锁卵泡数量,从而使卵泡池健康卵泡数量减少.以慢性束缚应激为例,本课题组的前期研究[22 ] 发现,慢性束缚应激可导致Kit配体及其受体表达水平升高,磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/磷酸酯酶和张力蛋白同源物(phosphatase and tensin homolog,PTEN)/蛋白激酶B(protein kinase B,AKT)通路的激活,从而诱导小鼠卵巢原始卵泡过度激活.综上,慢性应激会对各级卵泡产生较负面的影响,从而导致卵巢功能减退. ...
... GC是卵泡的重要组成部分,参与雌激素的生成与分泌,在卵泡发育中发挥关键作用.研究显示,尖叫声应激[19 ] 、高密度饲养应激[20 ] 及慢性不可预测应激[10 ] 等均会增加卵巢中GC的凋亡或衰老.本课题组的前期研究[10 ] 发现,在慢性不可预测应激中,小鼠窦状卵泡GC中异柠檬酸脱氢酶1(isocitrate dehydrogenase 1,IDH1)显著降低,导致该细胞中的活性氧含量增加,并上调自噬和丝裂原激活蛋白激酶信号通路,从而抑制该细胞的增殖并加速衰老.在高密度饲养应激下,小鼠卵巢组织中的血管紧张素Ⅱ(angiotensin Ⅱ,Ang Ⅱ)显著增加,从而可加速GC的凋亡[20 ] .这些结果均表明,长期暴露于应激源可引起卵巢GC凋亡,造成卵巢功能减退. ...
... [20 ].这些结果均表明,长期暴露于应激源可引起卵巢GC凋亡,造成卵巢功能减退. ...
... 皮质酮是啮齿类动物肾上腺皮质中产生的一种类固醇激素,也是HPA轴调节的重要激素[44 ] ,可调节体内能量代谢、应激反应、免疫和认知[45 ] ,在人体内对应的产物为皮质醇.既往多数研究[19 -20 ] 发现,慢性应激可导致动物体内皮质酮含量增加.LUO等[46 ] 发现皮质酮可强烈抑制雌性小鼠前腹侧脑室周围核和邻近四周核的KISSPEPTIN神经元活性,也可抑制垂体中GnRH受体和Lh 的表达.这些研究表明,慢性应激可能通过分泌的皮质酮/皮质醇影响HPO轴,进而影响卵巢功能;而有关皮质酮/皮质醇浓度的变化对卵巢功能的具体调控,尚有待进一步研究. ...
3
... 卵巢原始卵泡作为卵泡发育的起点和卵泡的储备形式,在雌性生殖寿命的正常维持中具有重要作用.研究发现,原始卵泡的过度激活会导致卵巢储备功能下降,与POF的发生密切相关;尖叫声应激[19 ] 、高密度饲养应激[20 ] 、束缚应激[21 -22 ] 及慢性不可预测应激[23 -24 ] 等均可加速原始卵泡的过度激活,增加闭锁卵泡数量,从而使卵泡池健康卵泡数量减少.以慢性束缚应激为例,本课题组的前期研究[22 ] 发现,慢性束缚应激可导致Kit配体及其受体表达水平升高,磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/磷酸酯酶和张力蛋白同源物(phosphatase and tensin homolog,PTEN)/蛋白激酶B(protein kinase B,AKT)通路的激活,从而诱导小鼠卵巢原始卵泡过度激活.综上,慢性应激会对各级卵泡产生较负面的影响,从而导致卵巢功能减退. ...
... 卵母细胞的质量会直接影响生育结局.在动物实验中,本课题组发现慢性束缚应激会降低细胞周期蛋白B1的表达,增加卵母细胞异常双极纺锤体百分比,从而使生发囊泡破裂的百分比降低并延长其破裂时间[25 ] .本课题组还发现,慢性束缚应激会增加卵母细胞中活性氧水平,使卵母细胞中线粒体脱氧核糖核酸急剧减少,并导致其线粒体功能发生障碍、线粒体膜电位显著降低,致使卵母细胞中三磷酸腺苷含量降低,从而导致染色质错位[26 ] .ZHAO等[27 ] 的研究发现,长期经历慢性不可预测应激可干扰小鼠卵母细胞中的线粒体分布.在哺乳动物卵母细胞中,减数分裂停滞受细胞内高水平的环腺苷酸(cyclic adenylic acid,cAMP)调节[28 ] .JIANG等[21 ] 研究发现,慢性束缚应激能够显著下调小鼠卵母细胞中的G蛋白偶联受体3、壁颗粒细胞中的利钠肽C和卵丘细胞中的利钠肽受体2水平,从而干扰cAMP信号通路;此外,该团队还发现慢性束缚应激小鼠的减数分裂停滞失败(meiotic arrest failure,MAF)显著增加,卵母细胞的中期染色体发生浓缩、纺锤体组装或第一极体被释放,且MAF卵泡中大多数间隙连接被破坏[21 ] .由于卵泡中的间隙连接参与了cAMP的合成和扩散过程[28 ] ,继而推测慢性束缚应激通过干扰卵母细胞中cAMP的产生过程导致MAF.ZHAO等[29 ] 发现,强烈的慢性不可预测应激还会降低小鼠卵母细胞的发育潜力及受精率,并诱发囊胚出现显著的细胞凋亡.CASILLAS等[30 ] 的研究发现,经长期冷水应激的大鼠,其体内卵母细胞活力较低,异常卵母细胞的百分比较高.GAO等[31 ] 的研究显示,随着不可预测应激时间的延长,小鼠卵母细胞氧化应激和窦状卵泡凋亡逐渐增多.由此可见,长期暴露于应激源会降低卵母细胞质量,且随着暴露时间的延长和程度的增加,卵母细胞受到的损害随之加剧. ...
... [21 ].由于卵泡中的间隙连接参与了cAMP的合成和扩散过程[28 ] ,继而推测慢性束缚应激通过干扰卵母细胞中cAMP的产生过程导致MAF.ZHAO等[29 ] 发现,强烈的慢性不可预测应激还会降低小鼠卵母细胞的发育潜力及受精率,并诱发囊胚出现显著的细胞凋亡.CASILLAS等[30 ] 的研究发现,经长期冷水应激的大鼠,其体内卵母细胞活力较低,异常卵母细胞的百分比较高.GAO等[31 ] 的研究显示,随着不可预测应激时间的延长,小鼠卵母细胞氧化应激和窦状卵泡凋亡逐渐增多.由此可见,长期暴露于应激源会降低卵母细胞质量,且随着暴露时间的延长和程度的增加,卵母细胞受到的损害随之加剧. ...
3
... 卵巢原始卵泡作为卵泡发育的起点和卵泡的储备形式,在雌性生殖寿命的正常维持中具有重要作用.研究发现,原始卵泡的过度激活会导致卵巢储备功能下降,与POF的发生密切相关;尖叫声应激[19 ] 、高密度饲养应激[20 ] 、束缚应激[21 -22 ] 及慢性不可预测应激[23 -24 ] 等均可加速原始卵泡的过度激活,增加闭锁卵泡数量,从而使卵泡池健康卵泡数量减少.以慢性束缚应激为例,本课题组的前期研究[22 ] 发现,慢性束缚应激可导致Kit配体及其受体表达水平升高,磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/磷酸酯酶和张力蛋白同源物(phosphatase and tensin homolog,PTEN)/蛋白激酶B(protein kinase B,AKT)通路的激活,从而诱导小鼠卵巢原始卵泡过度激活.综上,慢性应激会对各级卵泡产生较负面的影响,从而导致卵巢功能减退. ...
... [22 ]发现,慢性束缚应激可导致Kit配体及其受体表达水平升高,磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/磷酸酯酶和张力蛋白同源物(phosphatase and tensin homolog,PTEN)/蛋白激酶B(protein kinase B,AKT)通路的激活,从而诱导小鼠卵巢原始卵泡过度激活.综上,慢性应激会对各级卵泡产生较负面的影响,从而导致卵巢功能减退. ...
... 在慢性应激诱导的卵巢功能减退过程中,CRH起到了重要作用.CRH是由下丘脑室旁核小细胞神经元分泌的一种41个氨基酸组成的多肽.在中枢神经系统中,CRH是应激系统的主要调节因子,可间接调节免疫系统和免疫反应[34 ] .本课题组的前期研究[22 ] 发现慢性束缚应激小鼠的血清CRH水平较高,而采用100 nmol/L CRH体外培养3日龄正常小鼠的卵巢后可显著提升卵巢中活化原始卵泡的数目,提示CRH是促进原始卵泡激活的重要内分泌因子.此外,束缚应激还能够增强小鼠卵巢中CRH及其受体的表达,激活GC和卵母细胞中的凋亡相关因子/凋亡相关因子配体系统,以诱导细胞凋亡的发生[35 ] .DI NATALE等[36 ] 研究发现,应激可引起CRH的释放和γ-氨基丁酸能信号的激活,并进一步抑制GnRH脉冲,从而影响排卵.上述结果表明,慢性应激可通过释放CRH来影响卵泡发育并抑制HPO轴,从而影响卵巢功能. ...
2
... 卵巢原始卵泡作为卵泡发育的起点和卵泡的储备形式,在雌性生殖寿命的正常维持中具有重要作用.研究发现,原始卵泡的过度激活会导致卵巢储备功能下降,与POF的发生密切相关;尖叫声应激[19 ] 、高密度饲养应激[20 ] 、束缚应激[21 -22 ] 及慢性不可预测应激[23 -24 ] 等均可加速原始卵泡的过度激活,增加闭锁卵泡数量,从而使卵泡池健康卵泡数量减少.以慢性束缚应激为例,本课题组的前期研究[22 ] 发现,慢性束缚应激可导致Kit配体及其受体表达水平升高,磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/磷酸酯酶和张力蛋白同源物(phosphatase and tensin homolog,PTEN)/蛋白激酶B(protein kinase B,AKT)通路的激活,从而诱导小鼠卵巢原始卵泡过度激活.综上,慢性应激会对各级卵泡产生较负面的影响,从而导致卵巢功能减退. ...
... 慢性应激还可通过降低神经生长因子(nerve growth factor,NGF)水平导致卵巢功能减退.研究[47 ] 显示NGF是一种多肽生长因子,可与高亲和力的NGF受体——酪氨酸激酶受体A(tyrosine kinase receptor A,TrkA)结合,影响卵泡的发育;慢性不可预测应激可下调大鼠NGF和TrkA的表达水平,进而促进POI的发生[23 ] .此外,小鼠在经历了母亲分离应激后,其窦前卵泡的抗氧化相关酶活性下降,导致其窦前卵泡的总抗氧化能力降低,从而抑制了卵泡发育[48 ] . ...
2
... 卵巢原始卵泡作为卵泡发育的起点和卵泡的储备形式,在雌性生殖寿命的正常维持中具有重要作用.研究发现,原始卵泡的过度激活会导致卵巢储备功能下降,与POF的发生密切相关;尖叫声应激[19 ] 、高密度饲养应激[20 ] 、束缚应激[21 -22 ] 及慢性不可预测应激[23 -24 ] 等均可加速原始卵泡的过度激活,增加闭锁卵泡数量,从而使卵泡池健康卵泡数量减少.以慢性束缚应激为例,本课题组的前期研究[22 ] 发现,慢性束缚应激可导致Kit配体及其受体表达水平升高,磷脂酰肌醇3-激酶(phosphoinositide 3-kinase,PI3K)/磷酸酯酶和张力蛋白同源物(phosphatase and tensin homolog,PTEN)/蛋白激酶B(protein kinase B,AKT)通路的激活,从而诱导小鼠卵巢原始卵泡过度激活.综上,慢性应激会对各级卵泡产生较负面的影响,从而导致卵巢功能减退. ...
... 慢性应激可作用于下丘脑和垂体,导致其激素分泌出现异常,从而影响卵巢功能.在HPO轴中,由下丘脑分泌的KISSPEPTIN、GnRH及由垂体分泌的FSH、促黄体素(luteinizing hormone,LH)均发挥着重要作用.研究[40 -41 ] 发现,长期经冷水或束缚应激刺激可导致个体的下丘脑KISSPEPTIN分泌减少,从而抑制GnRH神经元(即调控生殖的关键神经元).KISSPEPTIN是一种由KISSPEPTIN神经元分泌的神经肽,常通过作用于下丘脑的GnRH神经元参与卵巢功能的调控[42 ] .HUANG等[41 ] 发现,慢性束缚应激后的雌性小鼠下丘脑中的Kisspeptin 下调可能是受KISSPEPTIN神经元上的糖皮质激素受体调控.同时,对不同的应激模型开展研究[10 ,24 ,43 ] 也发现,由垂体分泌的FSH、LH水平会出现不同程度的波动,进而影响小鼠的卵泡发育和排卵等功能. ...
1
... 卵母细胞的质量会直接影响生育结局.在动物实验中,本课题组发现慢性束缚应激会降低细胞周期蛋白B1的表达,增加卵母细胞异常双极纺锤体百分比,从而使生发囊泡破裂的百分比降低并延长其破裂时间[25 ] .本课题组还发现,慢性束缚应激会增加卵母细胞中活性氧水平,使卵母细胞中线粒体脱氧核糖核酸急剧减少,并导致其线粒体功能发生障碍、线粒体膜电位显著降低,致使卵母细胞中三磷酸腺苷含量降低,从而导致染色质错位[26 ] .ZHAO等[27 ] 的研究发现,长期经历慢性不可预测应激可干扰小鼠卵母细胞中的线粒体分布.在哺乳动物卵母细胞中,减数分裂停滞受细胞内高水平的环腺苷酸(cyclic adenylic acid,cAMP)调节[28 ] .JIANG等[21 ] 研究发现,慢性束缚应激能够显著下调小鼠卵母细胞中的G蛋白偶联受体3、壁颗粒细胞中的利钠肽C和卵丘细胞中的利钠肽受体2水平,从而干扰cAMP信号通路;此外,该团队还发现慢性束缚应激小鼠的减数分裂停滞失败(meiotic arrest failure,MAF)显著增加,卵母细胞的中期染色体发生浓缩、纺锤体组装或第一极体被释放,且MAF卵泡中大多数间隙连接被破坏[21 ] .由于卵泡中的间隙连接参与了cAMP的合成和扩散过程[28 ] ,继而推测慢性束缚应激通过干扰卵母细胞中cAMP的产生过程导致MAF.ZHAO等[29 ] 发现,强烈的慢性不可预测应激还会降低小鼠卵母细胞的发育潜力及受精率,并诱发囊胚出现显著的细胞凋亡.CASILLAS等[30 ] 的研究发现,经长期冷水应激的大鼠,其体内卵母细胞活力较低,异常卵母细胞的百分比较高.GAO等[31 ] 的研究显示,随着不可预测应激时间的延长,小鼠卵母细胞氧化应激和窦状卵泡凋亡逐渐增多.由此可见,长期暴露于应激源会降低卵母细胞质量,且随着暴露时间的延长和程度的增加,卵母细胞受到的损害随之加剧. ...
1
... 卵母细胞的质量会直接影响生育结局.在动物实验中,本课题组发现慢性束缚应激会降低细胞周期蛋白B1的表达,增加卵母细胞异常双极纺锤体百分比,从而使生发囊泡破裂的百分比降低并延长其破裂时间[25 ] .本课题组还发现,慢性束缚应激会增加卵母细胞中活性氧水平,使卵母细胞中线粒体脱氧核糖核酸急剧减少,并导致其线粒体功能发生障碍、线粒体膜电位显著降低,致使卵母细胞中三磷酸腺苷含量降低,从而导致染色质错位[26 ] .ZHAO等[27 ] 的研究发现,长期经历慢性不可预测应激可干扰小鼠卵母细胞中的线粒体分布.在哺乳动物卵母细胞中,减数分裂停滞受细胞内高水平的环腺苷酸(cyclic adenylic acid,cAMP)调节[28 ] .JIANG等[21 ] 研究发现,慢性束缚应激能够显著下调小鼠卵母细胞中的G蛋白偶联受体3、壁颗粒细胞中的利钠肽C和卵丘细胞中的利钠肽受体2水平,从而干扰cAMP信号通路;此外,该团队还发现慢性束缚应激小鼠的减数分裂停滞失败(meiotic arrest failure,MAF)显著增加,卵母细胞的中期染色体发生浓缩、纺锤体组装或第一极体被释放,且MAF卵泡中大多数间隙连接被破坏[21 ] .由于卵泡中的间隙连接参与了cAMP的合成和扩散过程[28 ] ,继而推测慢性束缚应激通过干扰卵母细胞中cAMP的产生过程导致MAF.ZHAO等[29 ] 发现,强烈的慢性不可预测应激还会降低小鼠卵母细胞的发育潜力及受精率,并诱发囊胚出现显著的细胞凋亡.CASILLAS等[30 ] 的研究发现,经长期冷水应激的大鼠,其体内卵母细胞活力较低,异常卵母细胞的百分比较高.GAO等[31 ] 的研究显示,随着不可预测应激时间的延长,小鼠卵母细胞氧化应激和窦状卵泡凋亡逐渐增多.由此可见,长期暴露于应激源会降低卵母细胞质量,且随着暴露时间的延长和程度的增加,卵母细胞受到的损害随之加剧. ...
1
... 卵母细胞的质量会直接影响生育结局.在动物实验中,本课题组发现慢性束缚应激会降低细胞周期蛋白B1的表达,增加卵母细胞异常双极纺锤体百分比,从而使生发囊泡破裂的百分比降低并延长其破裂时间[25 ] .本课题组还发现,慢性束缚应激会增加卵母细胞中活性氧水平,使卵母细胞中线粒体脱氧核糖核酸急剧减少,并导致其线粒体功能发生障碍、线粒体膜电位显著降低,致使卵母细胞中三磷酸腺苷含量降低,从而导致染色质错位[26 ] .ZHAO等[27 ] 的研究发现,长期经历慢性不可预测应激可干扰小鼠卵母细胞中的线粒体分布.在哺乳动物卵母细胞中,减数分裂停滞受细胞内高水平的环腺苷酸(cyclic adenylic acid,cAMP)调节[28 ] .JIANG等[21 ] 研究发现,慢性束缚应激能够显著下调小鼠卵母细胞中的G蛋白偶联受体3、壁颗粒细胞中的利钠肽C和卵丘细胞中的利钠肽受体2水平,从而干扰cAMP信号通路;此外,该团队还发现慢性束缚应激小鼠的减数分裂停滞失败(meiotic arrest failure,MAF)显著增加,卵母细胞的中期染色体发生浓缩、纺锤体组装或第一极体被释放,且MAF卵泡中大多数间隙连接被破坏[21 ] .由于卵泡中的间隙连接参与了cAMP的合成和扩散过程[28 ] ,继而推测慢性束缚应激通过干扰卵母细胞中cAMP的产生过程导致MAF.ZHAO等[29 ] 发现,强烈的慢性不可预测应激还会降低小鼠卵母细胞的发育潜力及受精率,并诱发囊胚出现显著的细胞凋亡.CASILLAS等[30 ] 的研究发现,经长期冷水应激的大鼠,其体内卵母细胞活力较低,异常卵母细胞的百分比较高.GAO等[31 ] 的研究显示,随着不可预测应激时间的延长,小鼠卵母细胞氧化应激和窦状卵泡凋亡逐渐增多.由此可见,长期暴露于应激源会降低卵母细胞质量,且随着暴露时间的延长和程度的增加,卵母细胞受到的损害随之加剧. ...
2
... 卵母细胞的质量会直接影响生育结局.在动物实验中,本课题组发现慢性束缚应激会降低细胞周期蛋白B1的表达,增加卵母细胞异常双极纺锤体百分比,从而使生发囊泡破裂的百分比降低并延长其破裂时间[25 ] .本课题组还发现,慢性束缚应激会增加卵母细胞中活性氧水平,使卵母细胞中线粒体脱氧核糖核酸急剧减少,并导致其线粒体功能发生障碍、线粒体膜电位显著降低,致使卵母细胞中三磷酸腺苷含量降低,从而导致染色质错位[26 ] .ZHAO等[27 ] 的研究发现,长期经历慢性不可预测应激可干扰小鼠卵母细胞中的线粒体分布.在哺乳动物卵母细胞中,减数分裂停滞受细胞内高水平的环腺苷酸(cyclic adenylic acid,cAMP)调节[28 ] .JIANG等[21 ] 研究发现,慢性束缚应激能够显著下调小鼠卵母细胞中的G蛋白偶联受体3、壁颗粒细胞中的利钠肽C和卵丘细胞中的利钠肽受体2水平,从而干扰cAMP信号通路;此外,该团队还发现慢性束缚应激小鼠的减数分裂停滞失败(meiotic arrest failure,MAF)显著增加,卵母细胞的中期染色体发生浓缩、纺锤体组装或第一极体被释放,且MAF卵泡中大多数间隙连接被破坏[21 ] .由于卵泡中的间隙连接参与了cAMP的合成和扩散过程[28 ] ,继而推测慢性束缚应激通过干扰卵母细胞中cAMP的产生过程导致MAF.ZHAO等[29 ] 发现,强烈的慢性不可预测应激还会降低小鼠卵母细胞的发育潜力及受精率,并诱发囊胚出现显著的细胞凋亡.CASILLAS等[30 ] 的研究发现,经长期冷水应激的大鼠,其体内卵母细胞活力较低,异常卵母细胞的百分比较高.GAO等[31 ] 的研究显示,随着不可预测应激时间的延长,小鼠卵母细胞氧化应激和窦状卵泡凋亡逐渐增多.由此可见,长期暴露于应激源会降低卵母细胞质量,且随着暴露时间的延长和程度的增加,卵母细胞受到的损害随之加剧. ...
... [28 ],继而推测慢性束缚应激通过干扰卵母细胞中cAMP的产生过程导致MAF.ZHAO等[29 ] 发现,强烈的慢性不可预测应激还会降低小鼠卵母细胞的发育潜力及受精率,并诱发囊胚出现显著的细胞凋亡.CASILLAS等[30 ] 的研究发现,经长期冷水应激的大鼠,其体内卵母细胞活力较低,异常卵母细胞的百分比较高.GAO等[31 ] 的研究显示,随着不可预测应激时间的延长,小鼠卵母细胞氧化应激和窦状卵泡凋亡逐渐增多.由此可见,长期暴露于应激源会降低卵母细胞质量,且随着暴露时间的延长和程度的增加,卵母细胞受到的损害随之加剧. ...
1
... 卵母细胞的质量会直接影响生育结局.在动物实验中,本课题组发现慢性束缚应激会降低细胞周期蛋白B1的表达,增加卵母细胞异常双极纺锤体百分比,从而使生发囊泡破裂的百分比降低并延长其破裂时间[25 ] .本课题组还发现,慢性束缚应激会增加卵母细胞中活性氧水平,使卵母细胞中线粒体脱氧核糖核酸急剧减少,并导致其线粒体功能发生障碍、线粒体膜电位显著降低,致使卵母细胞中三磷酸腺苷含量降低,从而导致染色质错位[26 ] .ZHAO等[27 ] 的研究发现,长期经历慢性不可预测应激可干扰小鼠卵母细胞中的线粒体分布.在哺乳动物卵母细胞中,减数分裂停滞受细胞内高水平的环腺苷酸(cyclic adenylic acid,cAMP)调节[28 ] .JIANG等[21 ] 研究发现,慢性束缚应激能够显著下调小鼠卵母细胞中的G蛋白偶联受体3、壁颗粒细胞中的利钠肽C和卵丘细胞中的利钠肽受体2水平,从而干扰cAMP信号通路;此外,该团队还发现慢性束缚应激小鼠的减数分裂停滞失败(meiotic arrest failure,MAF)显著增加,卵母细胞的中期染色体发生浓缩、纺锤体组装或第一极体被释放,且MAF卵泡中大多数间隙连接被破坏[21 ] .由于卵泡中的间隙连接参与了cAMP的合成和扩散过程[28 ] ,继而推测慢性束缚应激通过干扰卵母细胞中cAMP的产生过程导致MAF.ZHAO等[29 ] 发现,强烈的慢性不可预测应激还会降低小鼠卵母细胞的发育潜力及受精率,并诱发囊胚出现显著的细胞凋亡.CASILLAS等[30 ] 的研究发现,经长期冷水应激的大鼠,其体内卵母细胞活力较低,异常卵母细胞的百分比较高.GAO等[31 ] 的研究显示,随着不可预测应激时间的延长,小鼠卵母细胞氧化应激和窦状卵泡凋亡逐渐增多.由此可见,长期暴露于应激源会降低卵母细胞质量,且随着暴露时间的延长和程度的增加,卵母细胞受到的损害随之加剧. ...
1
... 卵母细胞的质量会直接影响生育结局.在动物实验中,本课题组发现慢性束缚应激会降低细胞周期蛋白B1的表达,增加卵母细胞异常双极纺锤体百分比,从而使生发囊泡破裂的百分比降低并延长其破裂时间[25 ] .本课题组还发现,慢性束缚应激会增加卵母细胞中活性氧水平,使卵母细胞中线粒体脱氧核糖核酸急剧减少,并导致其线粒体功能发生障碍、线粒体膜电位显著降低,致使卵母细胞中三磷酸腺苷含量降低,从而导致染色质错位[26 ] .ZHAO等[27 ] 的研究发现,长期经历慢性不可预测应激可干扰小鼠卵母细胞中的线粒体分布.在哺乳动物卵母细胞中,减数分裂停滞受细胞内高水平的环腺苷酸(cyclic adenylic acid,cAMP)调节[28 ] .JIANG等[21 ] 研究发现,慢性束缚应激能够显著下调小鼠卵母细胞中的G蛋白偶联受体3、壁颗粒细胞中的利钠肽C和卵丘细胞中的利钠肽受体2水平,从而干扰cAMP信号通路;此外,该团队还发现慢性束缚应激小鼠的减数分裂停滞失败(meiotic arrest failure,MAF)显著增加,卵母细胞的中期染色体发生浓缩、纺锤体组装或第一极体被释放,且MAF卵泡中大多数间隙连接被破坏[21 ] .由于卵泡中的间隙连接参与了cAMP的合成和扩散过程[28 ] ,继而推测慢性束缚应激通过干扰卵母细胞中cAMP的产生过程导致MAF.ZHAO等[29 ] 发现,强烈的慢性不可预测应激还会降低小鼠卵母细胞的发育潜力及受精率,并诱发囊胚出现显著的细胞凋亡.CASILLAS等[30 ] 的研究发现,经长期冷水应激的大鼠,其体内卵母细胞活力较低,异常卵母细胞的百分比较高.GAO等[31 ] 的研究显示,随着不可预测应激时间的延长,小鼠卵母细胞氧化应激和窦状卵泡凋亡逐渐增多.由此可见,长期暴露于应激源会降低卵母细胞质量,且随着暴露时间的延长和程度的增加,卵母细胞受到的损害随之加剧. ...
1
... 卵母细胞的质量会直接影响生育结局.在动物实验中,本课题组发现慢性束缚应激会降低细胞周期蛋白B1的表达,增加卵母细胞异常双极纺锤体百分比,从而使生发囊泡破裂的百分比降低并延长其破裂时间[25 ] .本课题组还发现,慢性束缚应激会增加卵母细胞中活性氧水平,使卵母细胞中线粒体脱氧核糖核酸急剧减少,并导致其线粒体功能发生障碍、线粒体膜电位显著降低,致使卵母细胞中三磷酸腺苷含量降低,从而导致染色质错位[26 ] .ZHAO等[27 ] 的研究发现,长期经历慢性不可预测应激可干扰小鼠卵母细胞中的线粒体分布.在哺乳动物卵母细胞中,减数分裂停滞受细胞内高水平的环腺苷酸(cyclic adenylic acid,cAMP)调节[28 ] .JIANG等[21 ] 研究发现,慢性束缚应激能够显著下调小鼠卵母细胞中的G蛋白偶联受体3、壁颗粒细胞中的利钠肽C和卵丘细胞中的利钠肽受体2水平,从而干扰cAMP信号通路;此外,该团队还发现慢性束缚应激小鼠的减数分裂停滞失败(meiotic arrest failure,MAF)显著增加,卵母细胞的中期染色体发生浓缩、纺锤体组装或第一极体被释放,且MAF卵泡中大多数间隙连接被破坏[21 ] .由于卵泡中的间隙连接参与了cAMP的合成和扩散过程[28 ] ,继而推测慢性束缚应激通过干扰卵母细胞中cAMP的产生过程导致MAF.ZHAO等[29 ] 发现,强烈的慢性不可预测应激还会降低小鼠卵母细胞的发育潜力及受精率,并诱发囊胚出现显著的细胞凋亡.CASILLAS等[30 ] 的研究发现,经长期冷水应激的大鼠,其体内卵母细胞活力较低,异常卵母细胞的百分比较高.GAO等[31 ] 的研究显示,随着不可预测应激时间的延长,小鼠卵母细胞氧化应激和窦状卵泡凋亡逐渐增多.由此可见,长期暴露于应激源会降低卵母细胞质量,且随着暴露时间的延长和程度的增加,卵母细胞受到的损害随之加剧. ...
1
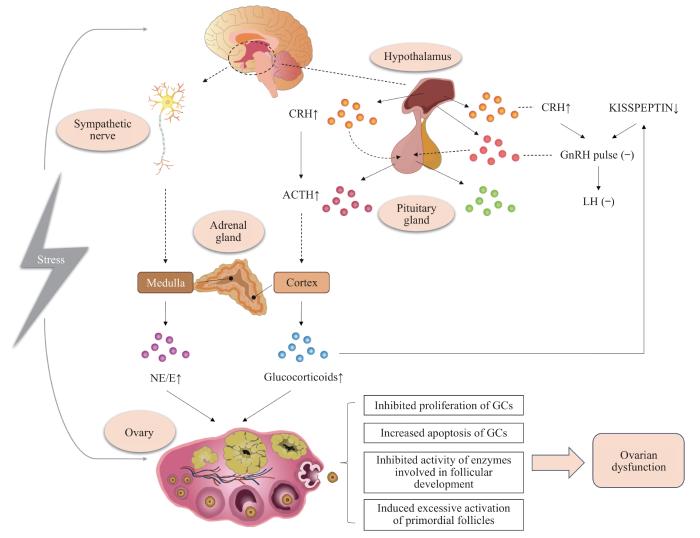
... 研究[32 ] 显示,应激系统的激活可导致机体的行为和外周神经系统发生变化,从而提高其调节稳态的能力并增加生存机会.然而,任何一种应激源超过阈值时均会使机体发生广泛应激反应,给机体带来损害.如机体长期暴露于超过阈值的应激源中,下丘脑-垂体-肾上腺(hypothalamic-pituitary-adrenal,HPA)轴及交感-肾上腺-髓质(sympathetic-adrenal-medullary,SAM)轴将被激活,从而引起下丘脑、垂体和肾上腺释放各类激素,包括促肾上腺皮质激素释放激素(corticotropin releasing hormone,CRH)、皮质醇、儿茶酚胺[如肾上腺素(epinephrine,E)和去甲肾上腺素(norepinephrine,NE)]等[33 ] ,从而加速卵巢功能损伤.目前的研究认为,经长期应激源刺激后HPA轴和SAM轴产生的激素可通过直接或间接的方式与HPO轴相互作用,从而引发卵巢功能紊乱甚至卵巢功能减退(图1 ). ...
2
... 研究[32 ] 显示,应激系统的激活可导致机体的行为和外周神经系统发生变化,从而提高其调节稳态的能力并增加生存机会.然而,任何一种应激源超过阈值时均会使机体发生广泛应激反应,给机体带来损害.如机体长期暴露于超过阈值的应激源中,下丘脑-垂体-肾上腺(hypothalamic-pituitary-adrenal,HPA)轴及交感-肾上腺-髓质(sympathetic-adrenal-medullary,SAM)轴将被激活,从而引起下丘脑、垂体和肾上腺释放各类激素,包括促肾上腺皮质激素释放激素(corticotropin releasing hormone,CRH)、皮质醇、儿茶酚胺[如肾上腺素(epinephrine,E)和去甲肾上腺素(norepinephrine,NE)]等[33 ] ,从而加速卵巢功能损伤.目前的研究认为,经长期应激源刺激后HPA轴和SAM轴产生的激素可通过直接或间接的方式与HPO轴相互作用,从而引发卵巢功能紊乱甚至卵巢功能减退(图1 ). ...
... NE是一种儿茶酚胺类激素,来源于肾上腺髓质和交感神经.研究[33 ] 显示当处于慢性应激环境中,个体大脑感知的压力会刺激SAM轴,从而促使肾上腺髓质产生E和NE.其中,NE与卵巢的功能密切相关.在卵巢组织中肾上腺素能受体较为丰富,有助于调节类固醇分泌、卵泡的发育和排卵[37 ] .有研究[38 ] 表明,冷应激可增加大鼠的NE水平,从而使其卵巢功能出现急剧下降.LI等[39 ] 采用NE处理GC 24 h后发现,NE可促进GC凋亡且凋亡率呈浓度依赖性.以上结果初步表明,慢性应激促使的NE水平增加可能是导致卵巢功能异常的原因之一. ...
1
... 在慢性应激诱导的卵巢功能减退过程中,CRH起到了重要作用.CRH是由下丘脑室旁核小细胞神经元分泌的一种41个氨基酸组成的多肽.在中枢神经系统中,CRH是应激系统的主要调节因子,可间接调节免疫系统和免疫反应[34 ] .本课题组的前期研究[22 ] 发现慢性束缚应激小鼠的血清CRH水平较高,而采用100 nmol/L CRH体外培养3日龄正常小鼠的卵巢后可显著提升卵巢中活化原始卵泡的数目,提示CRH是促进原始卵泡激活的重要内分泌因子.此外,束缚应激还能够增强小鼠卵巢中CRH及其受体的表达,激活GC和卵母细胞中的凋亡相关因子/凋亡相关因子配体系统,以诱导细胞凋亡的发生[35 ] .DI NATALE等[36 ] 研究发现,应激可引起CRH的释放和γ-氨基丁酸能信号的激活,并进一步抑制GnRH脉冲,从而影响排卵.上述结果表明,慢性应激可通过释放CRH来影响卵泡发育并抑制HPO轴,从而影响卵巢功能. ...
1
... 在慢性应激诱导的卵巢功能减退过程中,CRH起到了重要作用.CRH是由下丘脑室旁核小细胞神经元分泌的一种41个氨基酸组成的多肽.在中枢神经系统中,CRH是应激系统的主要调节因子,可间接调节免疫系统和免疫反应[34 ] .本课题组的前期研究[22 ] 发现慢性束缚应激小鼠的血清CRH水平较高,而采用100 nmol/L CRH体外培养3日龄正常小鼠的卵巢后可显著提升卵巢中活化原始卵泡的数目,提示CRH是促进原始卵泡激活的重要内分泌因子.此外,束缚应激还能够增强小鼠卵巢中CRH及其受体的表达,激活GC和卵母细胞中的凋亡相关因子/凋亡相关因子配体系统,以诱导细胞凋亡的发生[35 ] .DI NATALE等[36 ] 研究发现,应激可引起CRH的释放和γ-氨基丁酸能信号的激活,并进一步抑制GnRH脉冲,从而影响排卵.上述结果表明,慢性应激可通过释放CRH来影响卵泡发育并抑制HPO轴,从而影响卵巢功能. ...
1
... 在慢性应激诱导的卵巢功能减退过程中,CRH起到了重要作用.CRH是由下丘脑室旁核小细胞神经元分泌的一种41个氨基酸组成的多肽.在中枢神经系统中,CRH是应激系统的主要调节因子,可间接调节免疫系统和免疫反应[34 ] .本课题组的前期研究[22 ] 发现慢性束缚应激小鼠的血清CRH水平较高,而采用100 nmol/L CRH体外培养3日龄正常小鼠的卵巢后可显著提升卵巢中活化原始卵泡的数目,提示CRH是促进原始卵泡激活的重要内分泌因子.此外,束缚应激还能够增强小鼠卵巢中CRH及其受体的表达,激活GC和卵母细胞中的凋亡相关因子/凋亡相关因子配体系统,以诱导细胞凋亡的发生[35 ] .DI NATALE等[36 ] 研究发现,应激可引起CRH的释放和γ-氨基丁酸能信号的激活,并进一步抑制GnRH脉冲,从而影响排卵.上述结果表明,慢性应激可通过释放CRH来影响卵泡发育并抑制HPO轴,从而影响卵巢功能. ...
1
... NE是一种儿茶酚胺类激素,来源于肾上腺髓质和交感神经.研究[33 ] 显示当处于慢性应激环境中,个体大脑感知的压力会刺激SAM轴,从而促使肾上腺髓质产生E和NE.其中,NE与卵巢的功能密切相关.在卵巢组织中肾上腺素能受体较为丰富,有助于调节类固醇分泌、卵泡的发育和排卵[37 ] .有研究[38 ] 表明,冷应激可增加大鼠的NE水平,从而使其卵巢功能出现急剧下降.LI等[39 ] 采用NE处理GC 24 h后发现,NE可促进GC凋亡且凋亡率呈浓度依赖性.以上结果初步表明,慢性应激促使的NE水平增加可能是导致卵巢功能异常的原因之一. ...
1
... NE是一种儿茶酚胺类激素,来源于肾上腺髓质和交感神经.研究[33 ] 显示当处于慢性应激环境中,个体大脑感知的压力会刺激SAM轴,从而促使肾上腺髓质产生E和NE.其中,NE与卵巢的功能密切相关.在卵巢组织中肾上腺素能受体较为丰富,有助于调节类固醇分泌、卵泡的发育和排卵[37 ] .有研究[38 ] 表明,冷应激可增加大鼠的NE水平,从而使其卵巢功能出现急剧下降.LI等[39 ] 采用NE处理GC 24 h后发现,NE可促进GC凋亡且凋亡率呈浓度依赖性.以上结果初步表明,慢性应激促使的NE水平增加可能是导致卵巢功能异常的原因之一. ...
1
... NE是一种儿茶酚胺类激素,来源于肾上腺髓质和交感神经.研究[33 ] 显示当处于慢性应激环境中,个体大脑感知的压力会刺激SAM轴,从而促使肾上腺髓质产生E和NE.其中,NE与卵巢的功能密切相关.在卵巢组织中肾上腺素能受体较为丰富,有助于调节类固醇分泌、卵泡的发育和排卵[37 ] .有研究[38 ] 表明,冷应激可增加大鼠的NE水平,从而使其卵巢功能出现急剧下降.LI等[39 ] 采用NE处理GC 24 h后发现,NE可促进GC凋亡且凋亡率呈浓度依赖性.以上结果初步表明,慢性应激促使的NE水平增加可能是导致卵巢功能异常的原因之一. ...
1
... 慢性应激可作用于下丘脑和垂体,导致其激素分泌出现异常,从而影响卵巢功能.在HPO轴中,由下丘脑分泌的KISSPEPTIN、GnRH及由垂体分泌的FSH、促黄体素(luteinizing hormone,LH)均发挥着重要作用.研究[40 -41 ] 发现,长期经冷水或束缚应激刺激可导致个体的下丘脑KISSPEPTIN分泌减少,从而抑制GnRH神经元(即调控生殖的关键神经元).KISSPEPTIN是一种由KISSPEPTIN神经元分泌的神经肽,常通过作用于下丘脑的GnRH神经元参与卵巢功能的调控[42 ] .HUANG等[41 ] 发现,慢性束缚应激后的雌性小鼠下丘脑中的Kisspeptin 下调可能是受KISSPEPTIN神经元上的糖皮质激素受体调控.同时,对不同的应激模型开展研究[10 ,24 ,43 ] 也发现,由垂体分泌的FSH、LH水平会出现不同程度的波动,进而影响小鼠的卵泡发育和排卵等功能. ...
2
... 慢性应激可作用于下丘脑和垂体,导致其激素分泌出现异常,从而影响卵巢功能.在HPO轴中,由下丘脑分泌的KISSPEPTIN、GnRH及由垂体分泌的FSH、促黄体素(luteinizing hormone,LH)均发挥着重要作用.研究[40 -41 ] 发现,长期经冷水或束缚应激刺激可导致个体的下丘脑KISSPEPTIN分泌减少,从而抑制GnRH神经元(即调控生殖的关键神经元).KISSPEPTIN是一种由KISSPEPTIN神经元分泌的神经肽,常通过作用于下丘脑的GnRH神经元参与卵巢功能的调控[42 ] .HUANG等[41 ] 发现,慢性束缚应激后的雌性小鼠下丘脑中的Kisspeptin 下调可能是受KISSPEPTIN神经元上的糖皮质激素受体调控.同时,对不同的应激模型开展研究[10 ,24 ,43 ] 也发现,由垂体分泌的FSH、LH水平会出现不同程度的波动,进而影响小鼠的卵泡发育和排卵等功能. ...
... [41 ]发现,慢性束缚应激后的雌性小鼠下丘脑中的Kisspeptin 下调可能是受KISSPEPTIN神经元上的糖皮质激素受体调控.同时,对不同的应激模型开展研究[10 ,24 ,43 ] 也发现,由垂体分泌的FSH、LH水平会出现不同程度的波动,进而影响小鼠的卵泡发育和排卵等功能. ...
1
... 慢性应激可作用于下丘脑和垂体,导致其激素分泌出现异常,从而影响卵巢功能.在HPO轴中,由下丘脑分泌的KISSPEPTIN、GnRH及由垂体分泌的FSH、促黄体素(luteinizing hormone,LH)均发挥着重要作用.研究[40 -41 ] 发现,长期经冷水或束缚应激刺激可导致个体的下丘脑KISSPEPTIN分泌减少,从而抑制GnRH神经元(即调控生殖的关键神经元).KISSPEPTIN是一种由KISSPEPTIN神经元分泌的神经肽,常通过作用于下丘脑的GnRH神经元参与卵巢功能的调控[42 ] .HUANG等[41 ] 发现,慢性束缚应激后的雌性小鼠下丘脑中的Kisspeptin 下调可能是受KISSPEPTIN神经元上的糖皮质激素受体调控.同时,对不同的应激模型开展研究[10 ,24 ,43 ] 也发现,由垂体分泌的FSH、LH水平会出现不同程度的波动,进而影响小鼠的卵泡发育和排卵等功能. ...
1
... 慢性应激可作用于下丘脑和垂体,导致其激素分泌出现异常,从而影响卵巢功能.在HPO轴中,由下丘脑分泌的KISSPEPTIN、GnRH及由垂体分泌的FSH、促黄体素(luteinizing hormone,LH)均发挥着重要作用.研究[40 -41 ] 发现,长期经冷水或束缚应激刺激可导致个体的下丘脑KISSPEPTIN分泌减少,从而抑制GnRH神经元(即调控生殖的关键神经元).KISSPEPTIN是一种由KISSPEPTIN神经元分泌的神经肽,常通过作用于下丘脑的GnRH神经元参与卵巢功能的调控[42 ] .HUANG等[41 ] 发现,慢性束缚应激后的雌性小鼠下丘脑中的Kisspeptin 下调可能是受KISSPEPTIN神经元上的糖皮质激素受体调控.同时,对不同的应激模型开展研究[10 ,24 ,43 ] 也发现,由垂体分泌的FSH、LH水平会出现不同程度的波动,进而影响小鼠的卵泡发育和排卵等功能. ...
1
... 皮质酮是啮齿类动物肾上腺皮质中产生的一种类固醇激素,也是HPA轴调节的重要激素[44 ] ,可调节体内能量代谢、应激反应、免疫和认知[45 ] ,在人体内对应的产物为皮质醇.既往多数研究[19 -20 ] 发现,慢性应激可导致动物体内皮质酮含量增加.LUO等[46 ] 发现皮质酮可强烈抑制雌性小鼠前腹侧脑室周围核和邻近四周核的KISSPEPTIN神经元活性,也可抑制垂体中GnRH受体和Lh 的表达.这些研究表明,慢性应激可能通过分泌的皮质酮/皮质醇影响HPO轴,进而影响卵巢功能;而有关皮质酮/皮质醇浓度的变化对卵巢功能的具体调控,尚有待进一步研究. ...
1
... 皮质酮是啮齿类动物肾上腺皮质中产生的一种类固醇激素,也是HPA轴调节的重要激素[44 ] ,可调节体内能量代谢、应激反应、免疫和认知[45 ] ,在人体内对应的产物为皮质醇.既往多数研究[19 -20 ] 发现,慢性应激可导致动物体内皮质酮含量增加.LUO等[46 ] 发现皮质酮可强烈抑制雌性小鼠前腹侧脑室周围核和邻近四周核的KISSPEPTIN神经元活性,也可抑制垂体中GnRH受体和Lh 的表达.这些研究表明,慢性应激可能通过分泌的皮质酮/皮质醇影响HPO轴,进而影响卵巢功能;而有关皮质酮/皮质醇浓度的变化对卵巢功能的具体调控,尚有待进一步研究. ...
1
... 皮质酮是啮齿类动物肾上腺皮质中产生的一种类固醇激素,也是HPA轴调节的重要激素[44 ] ,可调节体内能量代谢、应激反应、免疫和认知[45 ] ,在人体内对应的产物为皮质醇.既往多数研究[19 -20 ] 发现,慢性应激可导致动物体内皮质酮含量增加.LUO等[46 ] 发现皮质酮可强烈抑制雌性小鼠前腹侧脑室周围核和邻近四周核的KISSPEPTIN神经元活性,也可抑制垂体中GnRH受体和Lh 的表达.这些研究表明,慢性应激可能通过分泌的皮质酮/皮质醇影响HPO轴,进而影响卵巢功能;而有关皮质酮/皮质醇浓度的变化对卵巢功能的具体调控,尚有待进一步研究. ...
1
... 慢性应激还可通过降低神经生长因子(nerve growth factor,NGF)水平导致卵巢功能减退.研究[47 ] 显示NGF是一种多肽生长因子,可与高亲和力的NGF受体——酪氨酸激酶受体A(tyrosine kinase receptor A,TrkA)结合,影响卵泡的发育;慢性不可预测应激可下调大鼠NGF和TrkA的表达水平,进而促进POI的发生[23 ] .此外,小鼠在经历了母亲分离应激后,其窦前卵泡的抗氧化相关酶活性下降,导致其窦前卵泡的总抗氧化能力降低,从而抑制了卵泡发育[48 ] . ...
1
... 慢性应激还可通过降低神经生长因子(nerve growth factor,NGF)水平导致卵巢功能减退.研究[47 ] 显示NGF是一种多肽生长因子,可与高亲和力的NGF受体——酪氨酸激酶受体A(tyrosine kinase receptor A,TrkA)结合,影响卵泡的发育;慢性不可预测应激可下调大鼠NGF和TrkA的表达水平,进而促进POI的发生[23 ] .此外,小鼠在经历了母亲分离应激后,其窦前卵泡的抗氧化相关酶活性下降,导致其窦前卵泡的总抗氧化能力降低,从而抑制了卵泡发育[48 ] . ...