胰腺切除术(pancreatectomy)属于复杂且高风险的普外科手术。胰瘘是胰腺切除术后常见且严重的并发症,推测其总体发生率在中国内地医院可达近30%,显著影响胰腺肿瘤患者的预后[1-2]。根据2016年国际胰瘘研究小组(International Study Group of Pancreatic Fistula,ISGPF)提出的胰瘘诊断与分级标准,术后体液淀粉酶水平大于正常血清阈值3倍以上,同时伴有相关感染症状或体征时,即可诊断为术后临床胰瘘(clinically relevant post-operative pancreatic fistula,CR-POPF),包括B/C级胰瘘[3]。术前通过高敏感度、无创的影像学方法预测患者胰腺切除术后并发CR-POPF的风险,有助于高危患者的围手术期临床管理,对于指导术后并发症的处理及改善胰腺肿瘤患者预后有重要的临床价值[4-5]。
既往研究[6-7]回顾性地对胰十二指肠切除术后并发CR-POPF的潜在危险因素进行了探索,并提出基于围手术期临床参数的CR-POPF预测模型。目前临床上较为广泛应用的模型包括2013年由CALLERY等[6]提出的胰瘘风险评分(fistula risk score,FRS),其纳入的危险因素包括胰腺质地较软、主胰管内径较窄、术中失血量较多及特定的胰腺肿瘤病理类型(如胰腺腺癌),均与CR-POPF的发生显著相关。2019年,MUNGROOP等[7]提出了改良胰瘘风险评分(alternative fistula risk score,a-FRS)模型,保留了胰腺质地较软与主胰管内径较窄2项高危因素,同时增加了患者体质量指数(body mass index,BMI)较高作为危险因素;通过大样本量回顾,进一步验证了其在预测CR-POPF中的诊断效力。胰腺质地较软作为独立的危险因素,与CR-POPF的发生和严重程度显著相关;既往外科医师多通过术中触诊的方式,定性评估胰腺质地的软硬度,但触诊结果无法提供足够的客观性与可重复性[8],同时也无法实现术前诊断,限制了其在临床实践中的进一步应用。
本研究的目的是通过结合SWE定量参数与临床关键危险因素,构建改良的CR-POPF预测模型,实现术前无创、定量、客观地评估胰腺切除术后并发CR-POPF的风险。
1 对象与方法
1.1 研究对象
本研究为前瞻性研究,纳入2021年9月至2022年3月复旦大学附属中山医院就诊的胰腺肿瘤患者。纳入标准:①患者年龄≥18岁,充分知情本研究内容并自愿参与。②经影像学诊断为胰腺肿瘤,且评估为手术可切除的病灶,拟于复旦大学附属中山医院接受胰腺切除术(胰十二指肠切除术/胰体尾切除术)者。③手术前未接受放射治疗、化学治疗等其他治疗方式。④胰腺病灶及周边正常腺体实质在二维灰阶超声(B mode ultrasound,BMUS)图像上可清晰显示,病灶与体表深度介于2~10 cm,内部以实质回声为主。排除标准:①患者一般情况较差,存在周围组织或远处转移,或具有其他手术禁忌证者。②患者计划接受姑息性手术、剖腹探查、穿刺活检或内科治疗者。③接受再手术者。④无法配合完成术前超声评估或其他必要检查者。⑤要求退出研究者。
1.2 研究方法
1.2.1 收集患者术前临床资料
收集患者术前的临床特征与指标,包括性别、年龄、BMI和实验室检查结果[血清γ-谷氨酰转移酶(γ-glutamyltransferase,γ-GT)、三酰甘油(triacylglycerol,TAG)和胆固醇水平]。
1.2.2 术前BMUS检查与SWE定量评估胰腺质地
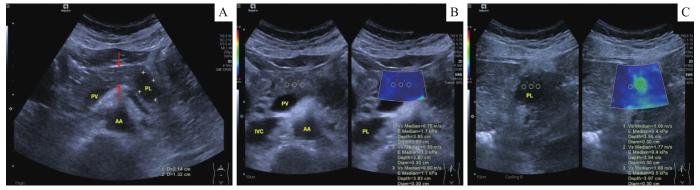
术前超声检查使用Siemens ACUSON Sequoia超声诊断仪及腹部探头5C-1(1~6 MHz)进行,配备声触诊组织弹性成像与量化技术(virtual touch tissue imaging and quantification technology,VTIQ)完成胰腺术前SWE的定量评估(图1)。首先在BMUS清晰显示病灶的情况下,观察并记录病灶大小、边界、形态、内部回声特征、有无囊变/钙化/坏死区及主胰管内径大小,并通过彩色血流成像检测病灶内部血流情况。然后切换至VTIQ模式,在病灶内设置3个相同深度的感兴趣区域(region of interest,ROI)测量组织弹性定量剪切波速度(shear wave velocity,SWV)值(m/s)。重复此步骤3次,计算平均SWV值以代表病灶组织软硬度。最后,在距离病灶边缘至少1 cm的门静脉前方胰体部正常腺体实质内重复上述的测量步骤,以获得正常胰腺组织的SWV参考值。
图1
图1
1例胰腺导管腺癌患者的术前BMUS影像及SWE定量评估
Note: A. The body part of pancreatic parenchyma (red arrows) in the superficial layer of portal vein was clearly displayed on BMUS with a lesion located in the body-tail part of pancreas (yellow cursors). B. VTIQ assessment makes the visualization of relative elasticity of target tissues. The body part of pancreatic parenchyma appeared in an evenly blue color, which indicated a relatively low elasticity. C. VTIQ revealed an unevenly green color in the pancreatic lesion, which indicated a relatively high elasticity. The circles in B and C indicate the ROIs in the pancreatic parenchyma and the lesions, respectively, and the SWV values were detected. AA—abdominal aorta; IVC—inferior vena cava; PL—pancreatic lesion; PV—portal vein.
Fig 1
Preoperative BMUS imaging and quantitative evaluation of SWE in a patient with pancreatic ductal adenocarcinoma
1.2.3 收集患者术中临床信息
术中的临床信息包括手术类型(胰十二指肠切除术/胰体尾切除术)、手术持续时间、术中失血量和通过外科医师直接触诊定性评估的胰腺质地(软、中等-硬)。术中触诊由一位经验丰富的高年资外科医师进行,且外科医师术前未被告知SWE结果。术后经组织病理学检查,确定胰腺肿瘤的最终诊断。
1.2.4 CR-POPF的诊断与分级
术后胰瘘的诊断与分级是根据ISGPF于2016年制定的标准[3]。在本研究中,只有当术后引流液淀粉酶水平达到正常血清值上限的3倍以上,同时出现感染相关症状/体征时,诊断为B级胰瘘;若出现严重术后出血、多器官衰竭或患者需要再手术,甚至死亡时,则诊断为C级胰瘘。B/C级胰瘘均属于CR-POPF。
1.3 统计学分析
应用SPSS 26.0软件进行统计分析。定量资料用x±s表示,2组之间比较采用t检验;定性资料用频数(百分比)表示,2组之间比较采用χ2检验。通过单因素和多因素Logistic回归分析建立CR-POPF预测模型,通过受试者工作特征曲线(receiver-operating characteristic curve,ROC曲线)和决策曲线分析(decision curve analysis,DCA)评估模型的诊断效力及临床效益。所有检验均为双侧检验,当P<0.05时表示有统计学意义。
2 结果
2.1 患者一般信息
2021年9月至2022年3月,按纳排标准本研究共纳入100名胰腺肿瘤患者,包括57名男性和43名女性,年龄为(59.3±13.2)岁,BMI为(23.2±3.2)kg/m2;其中33例(33.0%)患者接受了胰十二指肠切除术,67例(67.0%)患者接受了胰体尾切除术,术后病理结果示于表1。通过术后3周的临床随访,58例(58.0%)患者并发POPF,包括23例(39.7%,23/58)无症状A级胰瘘(生化瘘)、35例(60.3%,35/58)例B级胰瘘,无患者发生C级胰瘘。对于CR-POPF的处理包括引流、抗生素和胰酶抑制剂的应用、营养支持治疗,所有患者预后良好。
表1 CR-POPF阳性和阴性的胰腺肿瘤患者临床特征的比较
Tab 1
| Index | CR-POPF positive (n=35) | CR-POPF negative (n=65) | t/χ2 value | P value |
|---|---|---|---|---|
| Gender/n(%) | 0.396 | 0.529 | ||
| Male | 16 (45.7) | 34 (52.3) | ||
| Female | 19 (54.3) | 31 (47.7) | ||
| Age/year | 57.7±14.4 | 60.1±12.5 | 0.887 | 0.378 |
| BMI/(kg·m-2) | 23.5±2.9 | 23.1±3.3 | -0.702 | 0.484 |
| γ-GT>150 U·L-1/n(%) | 4 (11.4) | 16 (24.6) | 2.473 | 0.116 |
| TAG>1.7 mmol·L-1/n(%) | 10 (28.6) | 18 (27.7) | 0.009 | 0.926 |
| Cholesterol>5.2 mmol·L-1/n(%) | 6 (17.1) | 12 (18.5) | 0.027 | 0.870 |
| Diabetes mellitus/n(%) | 4 (11.4) | 16 (24.6) | 2.473 | 0.116 |
| Lesion size/mm | 29.6±14.8 | 29.8±14.1 | 0.081 | 0.935 |
| MPD diameter≤3 mm/n(%) | 29 (82.9) | 36 (55.4) | 7.547 | 0.006 |
| SWV value/(m·s-1) | ||||
| Pancreatic parenchyma | 0.86±0.18 | 1.24±0.45 | 5.941 | 0.000 |
| Lesion | 1.42±0.60 | 1.41±0.64 | -0.078 | 0.938 |
| Lesion-parenchyma ratio | 1.69±0.72 | 1.24±0.68 | -3.044 | 0.003 |
| Surgery type/n(%) | 1.293 | 0.256 | ||
| Pancreaticoduodenectomy | 9 (25.7) | 24 (36.9) | ||
| Distal pancreatectomy | 26 (74.3) | 41 (63.1) | ||
| Surgery duration/min | 178.3±66.7 | 195.7±80.6 | 1.091 | 0.278 |
| Blood loss/mL | 138.3±131.0 | 119.2±139.8 | -0.664 | 0.508 |
| Palpation of pancreas/n(%) | 1.876 | 0.171 | ||
| Soft | 19 (54.3) | 26 (40.0) | ||
| Medium-hard | 16 (45.7) | 39 (60.0) | ||
| Pathological diagnosis of PDAC/PASC/n(%) | 13 (37.1) | 37 (56.9) | 3.560 | 0.059 |
| FRS/point | 4.9±1.3 | 3.8±1.8 | -2.891 | 0.005 |
| a-FRS/% | 60.6±27.2 | 21.3±23.2 | -7.593 | 0.000 |
CR-POPF阳性组和阴性组的性别、年龄、BMI,以及实验室检查结果和术后病理诊断为胰腺腺癌[包括胰腺导管腺癌(pancreatic ductal adenocarcinoma,PDAC)和胰腺腺鳞癌(pancreatic adeno-squamous cancer,PASC)]的患者比例差异均无统计学意义(均P>0.05,表1)。
2.2 术前超声影像学特征与剪切波弹性成像
术前BMUS检查显示,CR-POPF阳性组和阴性组之间病灶大小差异无统计学意义(P=0.935)。CR-POPF阳性组中,主胰管内径≤3 mm的患者比例显著高于CR-POPF阴性组(P=0.006)。VTIQ测量结果显示:CR-POPF阳性组中门静脉前方胰体部正常腺体实质测得的SWV值显著低于CR-POPF阴性组(P=0.000);2组间病灶内SWV值差异无统计学意义(P=0.938);CR-POPF阳性组病灶与周围胰腺实质的SWV比值显著高于CR-POPF阴性组(P=0.003)。见表1。
2.3 患者术中相关临床信息
在CR-POPF阳性组和阴性组之间,接受胰十二指肠切除术或胰体尾切除术的患者比例差异无统计学意义(P=0.256)。通过外科医师术中对胰腺的直接触诊,术者定性评估为质地软和质地中等-硬的比例在CR-POPF阳性组与阴性组间差异无统计学意义(P=0.171)。2组的平均手术时间和术中失血量之间差异也无统计学意义(均P>0.05)。见表1。
2.4 改良CR-POPF模型的建立
先对术前VTIQ测得的SWE定量值与围手术期临床上可能相关的因素进行单因素Logistic回归分析,然后将P<0.05的变量纳入多因素Logistic回归分析;结果显示,门静脉前方胰体部实质的低SWV值[lgOR=-2.934(95%CI -4.387~-1.479),P=0.000]和较窄的主胰管内径(≤3 mm)[lgOR=0.805(95%CI 0.274~1.335),P=0.003]是CR-POPF的独立危险因素(表2)。
表2 单因素和多因素Logistic回归分析CR-POPF发生的相关因素
Tab 2
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| lgOR (95%CI) | P value | B value | lgOR (95%CI) | P value | ||
| Gender (female) | 0.115 (-0.243‒0.473) | 0.530 | ‒ | ‒ | ‒ | |
| Age | -0.006 (-0.020‒0.007) | 0.375 | ‒ | ‒ | ‒ | |
| BMI | 0.020 (-0.036‒0.077) | 0.480 | ‒ | ‒ | ‒ | |
| Diabetes mellitus | 0.043 (-0.111‒0.918) | 0.124 | ‒ | ‒ | ‒ | |
| γ-GT level (>150 U/L) | 0.403 (-0.111‒0.918) | 0.124 | ‒ | ‒ | ‒ | |
| Lesion size | -0.001 (-0.013‒0.012) | 0.935 | ‒ | ‒ | ‒ | |
| MPD diameter (≤3 mm) | -0.590 (-1.027‒ -0.153) | 0.008 | 1.852 | 0.805 (0.274‒1.335) | 0.003 | |
| SWV of pancreatic parenchyma | -2.441 (-3.635‒ -1.248) | 0.000 | -6.755 | -2.934 (-4.387‒ -1.479) | 0.000 | |
| Palpation of pancreas (soft) | -0.251 (-0.611‒0.110) | 0.173 | ‒ | ‒ | ‒ | |
| Surgery type (PD) | 0.228 (-0.167‒0.623) | 0.258 | ‒ | ‒ | ‒ | |
| Constant | ‒ | ‒ | 4.737 | 2.057 | 0.002 | |
CR-POPF的发生概率可基于以下公式计算获得:
发生概率=
当主胰管内径≤3 mm时,[MPD]=1;当主胰管内径>3 mm时,[MPD]=0;[SWV]=门静脉前方胰体部实质SWV值(m/s)。
2.5 CR-POPF风险评估模型的比较
图2
图2
基于SWE的改良模型和既往临床模型对于预测CR-POPF的ROC曲线和决策曲线
Note: A. ROC curves of 3 models. B. Decision curves of 3 models.
Fig 2
ROC curves and decision curves of the modified model based on SWE and previous clinical models in the prediction of CR-POPF
表3 基于SWE的改良模型和既往临床模型对于预测CR-POPF的诊断效能
Tab 3
| Model | AUROC | Sensitivity/% | Specificity/% | PPV/% | NPV/% | Likelihood ratio |
|---|---|---|---|---|---|---|
| FRS | 0.665 | 42.9 | 80.0 | 68.2 | 58.4 | 2.143 |
| a-FRS | 0.744 | 68.6 | 73.9 | 72.4 | 70.2 | 2.622 |
| Modified model | 0.842 | 85.7 | 64.6 | 70.5 | 81.8 | 2.422 |
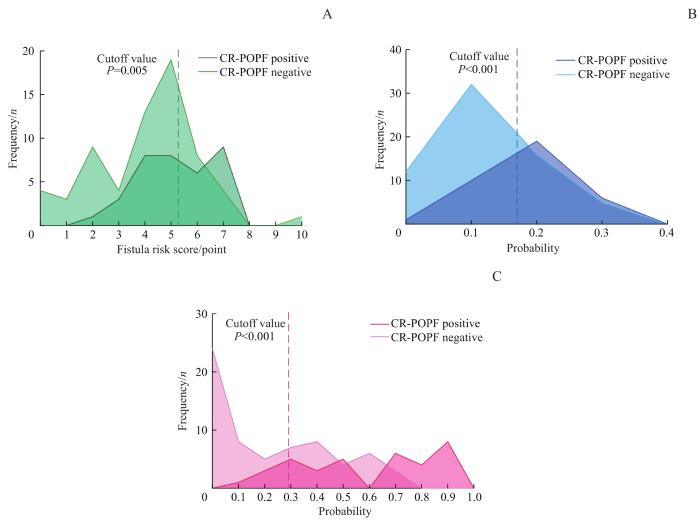
图3
图3
CR-POPF阳性组和阴性组患者采用基于SWE的改良模型和既往临床模型计算后的频数分布
Note: A. FRS. B. a-FRS. C. Modified model.
Fig 3
Frequency distributions of CR-POPF-positive group and -negative group calculated by the modified model based on SWE and previous clinical models
3 讨论
术前预测CR-POPF可以确保高危患者在围手术期获得及时有效的临床干预,避免手术相关腹腔内感染、大出血甚至是患者死亡的情况发生;改善胰腺肿瘤患者的预后,一直以来得到外科医师的广泛关注。2013年CALLERY等[6]提出的FRS模型及2019年MUNGROOP等[7]提出的a-FRS模型是目前临床使用较为广泛的CR-POPF预测模型,通过综合评估围手术期CR-POPF的危险因素,为临床提供了预测CR-POPF的可行方法。然而,FRS中的许多危险因素,例如胰腺质地软硬度及术后病理诊断,难以在术前被有效地定量评估,影响了其临床实际应用的价值[10]。另外术中通过外科医师触诊定性评估的胰腺质地,对于操作者的经验依赖性高,触诊结果在不同术者间存在较大差异,导致预测值的偏差及不可重复性[11]。因此,亟需一种术前无创、定量的CR-POPF改良风险模型预测方法,这将有助于提高临床术前预测的客观性与准确性。
既往的研究[13-14]曾应用CT或MRI的方法定量评估胰腺的纤维化程度或脂肪浸润,并探讨其在评估胰十二指肠切除术后并发CR-POPF的可能性。CT根据靶组织的造影剂积聚程度评估组织纤维化程度,从而间接推测组织软硬度,评估的结果可能无法提供足够的准确性与可重复性[13]。此外,胰腺实质的其他弥漫性改变,如扩张主胰管的支持作用,也可直接影响胰腺组织的软硬度[15]。MRI弹性成像虽然可以客观地定量评估靶组织弹性,敏感度与特异度均可达70%以上,但其检查过程十分耗时且费用昂贵[14]。两者均不适合作为常规术前检查项目。与CT或MRI定量方法相比,超声SWE检测具有实时成像、可定量、观察角度灵活多变、无创、无辐射等诸多优势,适合在临床推广应用。
我们基于超声SWE定量测值和术前临床关键危险因素,构建了改良的CR-POPF预测模型,实现了影像学对胰腺切除术前CR-POPF风险定量、客观的评估。ROC曲线分析结果显示其预测CR-POPF的ROC曲线下面积达0.842,敏感度、特异度、阳性预测值、阴性预测值及似然比分别为85.7%、64.6%、70.5%、81.8%及2.422,具有足够的诊断效能。DCA显示,相较于既往的临床预测模型,改良模型可以提供更好的临床效益。最终被纳入改良模型的参数包括主胰管内径(是否≤3 mm)以及门静脉前方胰体部正常实质的超声弹性定量SWV值。两者均可通过术前常规BMUS影像学表现,结合SWE准确定量评估,对于高危患者的围手术期管理可以提供重要的参考信息。
本研究的局限性为样本量较小,以及缺乏外部验证。在未来的研究中,我们计划通过持续增加样本量来进一步验证改良模型的预测效力,并通过多中心队列研究进行外部验证。
综上所述,基于SWE对胰腺组织质地的定量评估,并结合临床关键危险因素的改良胰瘘风险评估模型,可以提高CR-POPF术前评估的客观性及可靠性,为临床决策提供有效的参考。
作者贡献声明
董怡、楼文晖、田晓梵、王文平参与了研究设计;田晓梵负责实施研究、搜集并整理数据;田晓梵、左丹参与了数据分析;田晓梵、张琪、邱艺杰参与了论文的写作和修改。所有作者均阅读并同意了最终稿件的提交。
AUTHOR's CONTRIBUTIONS
The study was designed by DONG Yi, LOU Wenhui, TIAN Xiaofan, and WANG Wenping. TIAN Xiaofan conducted the research,and collected and organized the original data. Data analysis was conducted by TIAN Xiaofan and ZUO Dan. The manuscript was drafted and revised by TIAN Xiaofan, ZHANG Qi, and QIU Yijie. All the authors have read the last version of paper and consented for submission.
利益冲突声明
所有作者声明不存在利益冲突。
COMPETING INTERESTS
All authors disclose no relevant conflict of interests.
参考文献