[1]
VALLE J W, LAMARCA A, GOYAL L, et al. New horizons for precision medicine in biliary tract cancers[J]. Cancer Discov, 2017, 7(9): 943-962.
[本文引用: 2]
[2]
RIZVI S, KHAN S A, HALLEMEIER C L, et al. Cholangiocarcinoma: evolving concepts and therapeutic strategies[J]. Nat Rev Clin Oncol, 2018, 15(2): 95-111.
[本文引用: 1]
[3]
BENAVIDES M, ANTÓN A, GALLEGO J, et al. Biliary tract cancers: seom clinical guidelines[J]. Clin Transl Oncol, 2015, 17(12): 982-987.
[本文引用: 1]
[4]
EVERHART J E, RUHL C E. Burden of digestive diseases in the United States Part Ⅲ: liver, biliary tract, and pancreas[J]. Gastroenterology, 2009, 136(4): 1134-1144.
[本文引用: 1]
[5]
WAN J C M, MASSIE C, GARCIA-CORBACHO J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA[J]. Nat Rev Cancer, 2017, 17(4): 223-238.
[本文引用: 1]
[6]
VALLE J W, KELLEY R K, NERVI B, et al. Biliary tract cancer[J]. Lancet, 2021, 397(10272): 428-444.
[本文引用: 1]
[7]
BANALES J M, MARIN J J G, LAMARCA A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management[J]. Nat Rev Gastroenterol Hepatol, 2020, 17(9): 557-588.
[本文引用: 1]
[8]
ROA J C, GARCÍA P, KAPOOR V K, et al. Gallbladder cancer[J]. Nat Rev Dis Primers, 2022, 8(1): 69.
[本文引用: 1]
[9]
梁后杰, 秦叔逵, 沈锋, 等. CSCO胆道系统肿瘤诊断治疗专家共识(2019年版)[J]. 临床肿瘤学杂志, 2019, 24(9): 828-838.
[本文引用: 2]
LIANG H J, QIN S K, SHEN F et al. Expert consensus on diagnosis and treatment of CSCO biliary systerm tumors (2019 edition)[J]. Chinese Clinical Oncology, 2019, 24(9): 828-838.
[本文引用: 2]
[10]
VALLE J W. Advances in the treatment of metastatic or unresectable biliary tract cancer[J]. Ann Oncol, 2010, 21(Suppl 7): vii345-vii348.
[本文引用: 1]
[11]
BLECHACZ B, KOMUTA M, ROSKAMS T, et al. Clinical diagnosis and staging of cholangiocarcinoma[J]. Nat Rev Gastroenterol Hepatol, 2011, 8(9): 512-522.
[本文引用: 1]
[12]
蔡晨, 龚伟. 胆囊癌辅助治疗的研究进展[J]. 外科理论与实践, 2021, 26(2): 167-170.
[本文引用: 1]
CAI C, GONG W. Study on gallbladder cancer adjuvant therapy[J]. Surgery Theory and Practice, 2021, 26(2): 167-170.
[本文引用: 1]
[13]
LONE S N, NISAR S, MASOODI T, et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments[J]. Mol Cancer, 2022, 21(1): 79.
[本文引用: 2]
[14]
CROWLEY E, DI NICOLANTONIO F, LOUPAKIS F, et al. Liquid biopsy: monitoring cancer-genetics in the blood[J]. Nat Rev Clin Oncol, 2013, 10(8): 472-484.
[15]
ALIX-PANABIÈRES C, PANTEL K. Liquid biopsy: from discovery to clinical application[J]. Cancer Discov, 2021, 11(4): 858-873.
[本文引用: 1]
[16]
LEON S A, SHAPIRO B, SKLAROFF D M, et al. Free DNA in the serum of cancer patients and the effect of therapy[J]. Cancer Res, 1977, 37(3): 646-650.
[本文引用: 3]
[17]
VESSIES D C L, GREUTER M J E, VAN ROOIJEN K L, et al. Performance of four platforms for KRAS mutation detection in plasma cell-free DNA: ddpcr, Idylla, COBAS z480 and BEAMing[J]. Sci Rep, 2020, 10(1): 8122.
[本文引用: 1]
[18]
HEITZER E, ULZ P, GEIGL J B. Circulating tumor DNA as a liquid biopsy for cancer[J]. Clin Chem, 2015, 61(1): 112-123.
[本文引用: 2]
[19]
OLMEDILLAS-LÓPEZ S, GARCÍA-ARRANZ M, GARCÍA-OLMO D. Current and emerging applications of droplet digital PCR in oncology[J]. Mol Diagn Ther, 2017, 21(5): 493-510.
[本文引用: 1]
[20]
IGNATIADIS M, SLEDGE G W, JEFFREY S S. Liquid biopsy enters the clinic: implementation issues and future challenges[J]. Nat Rev Clin Oncol, 2021, 18(5): 297-312.
[本文引用: 1]
[21]
SIRAVEGNA G, MUSSOLIN B, VENESIO T, et al. How liquid biopsies can change clinical practice in oncology[J]. Ann Oncol, 2019, 30(10): 1580-1590.
[本文引用: 1]
[22]
SHEN N J, ZHANG D D, YIN L, et al. Bile cell‑free DNA as a novel and powerful liquid biopsy for detecting somatic variants in biliary tract cancer[J]. Oncol Rep, 2019, 42(2): 549-560.
[23]
央茂, 李永盛, 吴文广, 等. 液态活检技术在胆道肿瘤诊治中的应用进展[J]. 中华肝胆外科杂志, 2021, 27(6): 472-476.
[本文引用: 1]
YANG M, LI Y S, WU W G et al. Application progress of liquid biopsy in the diagnosis and treatment of biliary tract cancer[J]. Chinese Journal of Hepatobiliary Surgery, 2021, 27(6): 472-476.
[本文引用: 1]
[24]
LIBERATI A, ALTMAN D G, TETZLAFF J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration[J]. BMJ, 2009, 339: b2700.
[本文引用: 2]
[25]
HAN J Y, AHN K S, KIM T S, et al. Liquid biopsy from bile-circulating tumor DNA in patients with biliary tract cancer[J]. Cancers, 2021, 13(18): 4581.
[本文引用: 3]
[26]
HE S, ZENG F X, YIN H H, et al. Molecular diagnosis of pancreatobiliary tract cancer by detecting mutations and methylation changes in bile samples[J]. EClinicalMedicine, 2023, 55: 101736.
[本文引用: 5]
[27]
HUA Y, SUN F Y, HU F, et al. Diagnostic value of quantification of circulating free DNA for gall bladder cancer using a chemiluminescence DNA biosensor system based on DNA G-quadruplex/hemin enzyme[J]. Transl Oncol, 2021, 14(1): 100928.
[本文引用: 2]
[28]
KINUGASA H, NOUSO K, AKO S, et al. Liquid biopsy of bile for the molecular diagnosis of gallbladder cancer[J]. Cancer Biol Ther, 2018, 19(10): 934-938.
[本文引用: 5]
[29]
KUMARI S, HUSAIN N, AGARWAL A, et al. Diagnostic value of circulating free DNA integrity and global methylation status in gall bladder carcinoma[J]. Pathol Oncol Res, 2019, 25(3): 925-936.
[本文引用: 2]
[30]
KUMARI S, MISHRA S, HUSAIN N, et al. Comparison of circulating DNA in malignant neoplasia from diverse locations: investigating a diagnostic role[J]. Indian J Pathol Microbiol, 2022, 65(1): 93-99.
[本文引用: 2]
[31]
KUMARI S, TEWARI S, HUSAIN N, et al. Quantification of circulating free DNA as a diagnostic marker in gall bladder cancer[J]. Pathol Oncol Res, 2017, 23(1): 91-97.
[本文引用: 2]
[32]
WANG X, FU X H, QIAN Z L, et al. Non-invasive detection of biliary tract cancer by low-coverage whole genome sequencing from plasma cell-free DNA: a prospective cohort study[J]. Transl Oncol, 2021, 14(1): 100908.
[本文引用: 2]
[33]
WASENANG W, CHAIYARIT P, PROUNGVITAYA S, et al. Serum cell-free DNA methylation of OPCML and HOXD9 as a biomarker that may aid in differential diagnosis between cholangiocarcinoma and other biliary diseases[J]. Clin Epigenetics, 2019, 11(1): 39.
[本文引用: 2]
[34]
WINTACHAI P, LIM J Q, TECHASEN A, et al. Diagnostic and prognostic value of circulating cell-free DNA for cholangiocarcinoma[J]. Diagnostics, 2021, 11(6): 999.
[本文引用: 2]
[35]
莫迪, 李梦雨, 李华洋, 等. CA199、CEA及cfDNA的联合检测对胆管癌的辅助诊断价值[J]. 牡丹江医学院学报, 2020, 41(3): 18-21.
[本文引用: 2]
MO D, LI M Y, LI H Y, et al. Diagnostic value of combined detection of CA, CEA and plasma cell-free DNA for cholangiocarcinoma[J]. Journal of Mudanjiang Medical University, 2020, 41(3): 18-21.
[本文引用: 2]
[36]
张俊, 徐志伟, 李克. 诊断性试验meta分析的效应指标评价[J]. 中国循证医学杂志, 2013, 13(7): 890-895.
[本文引用: 1]
ZHANG J, XU Z W, LI K. Evaluation on the effect index of diagnostic test[J]. Chinese Journal of Evidence-Based Medicine, 2013, 13(7): 890-895.
[本文引用: 1]
[37]
KHAN S A, TAVOLARI S, BRANDI G. Cholangiocarcinoma: epidemiology and risk factors[J]. Liver Int, 2019, 39(S1): 19-31.
[本文引用: 1]
[38]
ARRICHIELLO G, NACCA V, PARAGLIOLA F, et al. Liquid biopsy in biliary tract cancer from blood and bile samples: current knowledge and future perspectives[J]. Explor Target Antitumor Ther, 2022, 3(3): 362-374.
[本文引用: 1]
[39]
SINGH A, DWIVEDI A. Circulating miRNA and cell-free DNA as a potential diagnostic tool in early detection of biliary tract cancer: a meta-analysis[J]. Biomarkers, 2022, 27(5): 399-406.
[本文引用: 1]
[40]
龚伟, 吴向嵩, 杨自逸. 胆囊癌转化治疗模式探索与思考[J]. 中国实用外科杂志, 2022, 42(2): 163-166.
[本文引用: 1]
GONG W, WU X S, YANG Z Y. Exploring novel therapeutic approach for advanced gallbladder cancer[J]. Chinese Journal of Practical Surgery, 2022, 42(2): 163-166.
[本文引用: 1]
2
... 胆道癌(biliary tract cancer,BTC)是指起源于胆道系统的恶性肿瘤,通常被分为肝内胆管癌、肝门部胆管癌、远端胆管癌和胆囊癌[1 -2 ] ,约占所有消化系肿瘤的3%[3 ] .近年来,胆道癌尤其是肝内胆管癌的发病率和死亡率正在逐年上升[4 -6 ] ,中国等亚洲国家胆管癌(cholangiocarcinoma,CCA)的发病率居全球前列[7 ] .胆道癌被认为是预后较差的疾病,对放射治疗和化学治疗不敏感,手术切除是唯一具有治愈性意义的治疗方法[1 ,8 -9 ] .但因其在病程早期通常无特异性症状且侵袭性极强,超过65%的患者在诊断时就已失去根治性切除的机会,5年存活率<5%[9 -10 ] .因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
... [1 ,8 -9 ].但因其在病程早期通常无特异性症状且侵袭性极强,超过65%的患者在诊断时就已失去根治性切除的机会,5年存活率<5%[9 -10 ] .因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
1
... 胆道癌(biliary tract cancer,BTC)是指起源于胆道系统的恶性肿瘤,通常被分为肝内胆管癌、肝门部胆管癌、远端胆管癌和胆囊癌[1 -2 ] ,约占所有消化系肿瘤的3%[3 ] .近年来,胆道癌尤其是肝内胆管癌的发病率和死亡率正在逐年上升[4 -6 ] ,中国等亚洲国家胆管癌(cholangiocarcinoma,CCA)的发病率居全球前列[7 ] .胆道癌被认为是预后较差的疾病,对放射治疗和化学治疗不敏感,手术切除是唯一具有治愈性意义的治疗方法[1 ,8 -9 ] .但因其在病程早期通常无特异性症状且侵袭性极强,超过65%的患者在诊断时就已失去根治性切除的机会,5年存活率<5%[9 -10 ] .因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
1
... 胆道癌(biliary tract cancer,BTC)是指起源于胆道系统的恶性肿瘤,通常被分为肝内胆管癌、肝门部胆管癌、远端胆管癌和胆囊癌[1 -2 ] ,约占所有消化系肿瘤的3%[3 ] .近年来,胆道癌尤其是肝内胆管癌的发病率和死亡率正在逐年上升[4 -6 ] ,中国等亚洲国家胆管癌(cholangiocarcinoma,CCA)的发病率居全球前列[7 ] .胆道癌被认为是预后较差的疾病,对放射治疗和化学治疗不敏感,手术切除是唯一具有治愈性意义的治疗方法[1 ,8 -9 ] .但因其在病程早期通常无特异性症状且侵袭性极强,超过65%的患者在诊断时就已失去根治性切除的机会,5年存活率<5%[9 -10 ] .因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
1
... 胆道癌(biliary tract cancer,BTC)是指起源于胆道系统的恶性肿瘤,通常被分为肝内胆管癌、肝门部胆管癌、远端胆管癌和胆囊癌[1 -2 ] ,约占所有消化系肿瘤的3%[3 ] .近年来,胆道癌尤其是肝内胆管癌的发病率和死亡率正在逐年上升[4 -6 ] ,中国等亚洲国家胆管癌(cholangiocarcinoma,CCA)的发病率居全球前列[7 ] .胆道癌被认为是预后较差的疾病,对放射治疗和化学治疗不敏感,手术切除是唯一具有治愈性意义的治疗方法[1 ,8 -9 ] .但因其在病程早期通常无特异性症状且侵袭性极强,超过65%的患者在诊断时就已失去根治性切除的机会,5年存活率<5%[9 -10 ] .因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
1
... cfDNA以微创方式提供肿瘤细胞的基因组信息[5 ,21 -23 ] ,但目前在胆道癌的诊断上的应用价值尚无定论,且具体选择何种检测方法和样本意见仍不统一,因此在临床开展不多.本研究拟检索目前国内外各项相关研究,全面评价cfDNA对胆道癌的诊断准确性,探讨样本来源、样本来源、检测方法以及截断值选择等对研究的影响,以期为更好开展临床应用提供依据. ...
1
... 胆道癌(biliary tract cancer,BTC)是指起源于胆道系统的恶性肿瘤,通常被分为肝内胆管癌、肝门部胆管癌、远端胆管癌和胆囊癌[1 -2 ] ,约占所有消化系肿瘤的3%[3 ] .近年来,胆道癌尤其是肝内胆管癌的发病率和死亡率正在逐年上升[4 -6 ] ,中国等亚洲国家胆管癌(cholangiocarcinoma,CCA)的发病率居全球前列[7 ] .胆道癌被认为是预后较差的疾病,对放射治疗和化学治疗不敏感,手术切除是唯一具有治愈性意义的治疗方法[1 ,8 -9 ] .但因其在病程早期通常无特异性症状且侵袭性极强,超过65%的患者在诊断时就已失去根治性切除的机会,5年存活率<5%[9 -10 ] .因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
1
... 胆道癌(biliary tract cancer,BTC)是指起源于胆道系统的恶性肿瘤,通常被分为肝内胆管癌、肝门部胆管癌、远端胆管癌和胆囊癌[1 -2 ] ,约占所有消化系肿瘤的3%[3 ] .近年来,胆道癌尤其是肝内胆管癌的发病率和死亡率正在逐年上升[4 -6 ] ,中国等亚洲国家胆管癌(cholangiocarcinoma,CCA)的发病率居全球前列[7 ] .胆道癌被认为是预后较差的疾病,对放射治疗和化学治疗不敏感,手术切除是唯一具有治愈性意义的治疗方法[1 ,8 -9 ] .但因其在病程早期通常无特异性症状且侵袭性极强,超过65%的患者在诊断时就已失去根治性切除的机会,5年存活率<5%[9 -10 ] .因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
1
... 胆道癌(biliary tract cancer,BTC)是指起源于胆道系统的恶性肿瘤,通常被分为肝内胆管癌、肝门部胆管癌、远端胆管癌和胆囊癌[1 -2 ] ,约占所有消化系肿瘤的3%[3 ] .近年来,胆道癌尤其是肝内胆管癌的发病率和死亡率正在逐年上升[4 -6 ] ,中国等亚洲国家胆管癌(cholangiocarcinoma,CCA)的发病率居全球前列[7 ] .胆道癌被认为是预后较差的疾病,对放射治疗和化学治疗不敏感,手术切除是唯一具有治愈性意义的治疗方法[1 ,8 -9 ] .但因其在病程早期通常无特异性症状且侵袭性极强,超过65%的患者在诊断时就已失去根治性切除的机会,5年存活率<5%[9 -10 ] .因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
2
... 胆道癌(biliary tract cancer,BTC)是指起源于胆道系统的恶性肿瘤,通常被分为肝内胆管癌、肝门部胆管癌、远端胆管癌和胆囊癌[1 -2 ] ,约占所有消化系肿瘤的3%[3 ] .近年来,胆道癌尤其是肝内胆管癌的发病率和死亡率正在逐年上升[4 -6 ] ,中国等亚洲国家胆管癌(cholangiocarcinoma,CCA)的发病率居全球前列[7 ] .胆道癌被认为是预后较差的疾病,对放射治疗和化学治疗不敏感,手术切除是唯一具有治愈性意义的治疗方法[1 ,8 -9 ] .但因其在病程早期通常无特异性症状且侵袭性极强,超过65%的患者在诊断时就已失去根治性切除的机会,5年存活率<5%[9 -10 ] .因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
... [9 -10 ].因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
2
... 胆道癌(biliary tract cancer,BTC)是指起源于胆道系统的恶性肿瘤,通常被分为肝内胆管癌、肝门部胆管癌、远端胆管癌和胆囊癌[1 -2 ] ,约占所有消化系肿瘤的3%[3 ] .近年来,胆道癌尤其是肝内胆管癌的发病率和死亡率正在逐年上升[4 -6 ] ,中国等亚洲国家胆管癌(cholangiocarcinoma,CCA)的发病率居全球前列[7 ] .胆道癌被认为是预后较差的疾病,对放射治疗和化学治疗不敏感,手术切除是唯一具有治愈性意义的治疗方法[1 ,8 -9 ] .但因其在病程早期通常无特异性症状且侵袭性极强,超过65%的患者在诊断时就已失去根治性切除的机会,5年存活率<5%[9 -10 ] .因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
... [9 -10 ].因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
1
... 胆道癌(biliary tract cancer,BTC)是指起源于胆道系统的恶性肿瘤,通常被分为肝内胆管癌、肝门部胆管癌、远端胆管癌和胆囊癌[1 -2 ] ,约占所有消化系肿瘤的3%[3 ] .近年来,胆道癌尤其是肝内胆管癌的发病率和死亡率正在逐年上升[4 -6 ] ,中国等亚洲国家胆管癌(cholangiocarcinoma,CCA)的发病率居全球前列[7 ] .胆道癌被认为是预后较差的疾病,对放射治疗和化学治疗不敏感,手术切除是唯一具有治愈性意义的治疗方法[1 ,8 -9 ] .但因其在病程早期通常无特异性症状且侵袭性极强,超过65%的患者在诊断时就已失去根治性切除的机会,5年存活率<5%[9 -10 ] .因此,尽早识别胆道癌并与胆道良性疾病进行区分对于改善患者预后将有很大帮助. ...
1
... 目前,胆道癌的初步诊断首选方法为超声检查,CT等其他影像学检查和CA19-9、CA125等肿瘤标志物等也有一定提示作用,确诊以组织活检或细胞学检查为唯一依据[11 -12 ] .标本主要来源包括外科手术、内窥镜逆行胰胆管造影(endoscopic retrograde cholangiopancreatography,ERCP)术和超声引导下穿刺活检术等.此类传统活检的局限在于有创、时间长、对患者整体情况反映差及标本质量和数量不可控.近十年来,液态活检(liquid biopsy,LB)技术在肿瘤学领域掀起了一场巨大变革,它可提供DNA突变、基因拷贝数改变、转录组/蛋白质组分析、表观遗传改变、代谢物分析等信息,将癌症诊断和监测引向无创时代[13 -15 ] . ...
1
... 目前,胆道癌的初步诊断首选方法为超声检查,CT等其他影像学检查和CA19-9、CA125等肿瘤标志物等也有一定提示作用,确诊以组织活检或细胞学检查为唯一依据[11 -12 ] .标本主要来源包括外科手术、内窥镜逆行胰胆管造影(endoscopic retrograde cholangiopancreatography,ERCP)术和超声引导下穿刺活检术等.此类传统活检的局限在于有创、时间长、对患者整体情况反映差及标本质量和数量不可控.近十年来,液态活检(liquid biopsy,LB)技术在肿瘤学领域掀起了一场巨大变革,它可提供DNA突变、基因拷贝数改变、转录组/蛋白质组分析、表观遗传改变、代谢物分析等信息,将癌症诊断和监测引向无创时代[13 -15 ] . ...
1
... 目前,胆道癌的初步诊断首选方法为超声检查,CT等其他影像学检查和CA19-9、CA125等肿瘤标志物等也有一定提示作用,确诊以组织活检或细胞学检查为唯一依据[11 -12 ] .标本主要来源包括外科手术、内窥镜逆行胰胆管造影(endoscopic retrograde cholangiopancreatography,ERCP)术和超声引导下穿刺活检术等.此类传统活检的局限在于有创、时间长、对患者整体情况反映差及标本质量和数量不可控.近十年来,液态活检(liquid biopsy,LB)技术在肿瘤学领域掀起了一场巨大变革,它可提供DNA突变、基因拷贝数改变、转录组/蛋白质组分析、表观遗传改变、代谢物分析等信息,将癌症诊断和监测引向无创时代[13 -15 ] . ...
2
... 目前,胆道癌的初步诊断首选方法为超声检查,CT等其他影像学检查和CA19-9、CA125等肿瘤标志物等也有一定提示作用,确诊以组织活检或细胞学检查为唯一依据[11 -12 ] .标本主要来源包括外科手术、内窥镜逆行胰胆管造影(endoscopic retrograde cholangiopancreatography,ERCP)术和超声引导下穿刺活检术等.此类传统活检的局限在于有创、时间长、对患者整体情况反映差及标本质量和数量不可控.近十年来,液态活检(liquid biopsy,LB)技术在肿瘤学领域掀起了一场巨大变革,它可提供DNA突变、基因拷贝数改变、转录组/蛋白质组分析、表观遗传改变、代谢物分析等信息,将癌症诊断和监测引向无创时代[13 -15 ] . ...
... 细胞游离DNA(cell-free DNA,cfDNA)是存在于细胞外的游离DNA分子,可由正常细胞或肿瘤细胞释放.然而,有研究[16 -18 ] 发现癌症患者cfDNA水平明显高于健康人群.cfDNA的检测技术尚未完全标准化,并且缺乏完整的分析共识,目前主要包括聚合酶链式反应(polymerase chain reaction,PCR)、实时荧光定量PCR(quantitative real-time PCR,qPCR)、数字PCR(digital PCR,dPCR)、甲基化特异度PCR(methylation-specific PCR,MSP)和下一代测序(next-generation sequencing,NGS)等[13 ,17 ,19 -20 ] . ...
1
... 目前,胆道癌的初步诊断首选方法为超声检查,CT等其他影像学检查和CA19-9、CA125等肿瘤标志物等也有一定提示作用,确诊以组织活检或细胞学检查为唯一依据[11 -12 ] .标本主要来源包括外科手术、内窥镜逆行胰胆管造影(endoscopic retrograde cholangiopancreatography,ERCP)术和超声引导下穿刺活检术等.此类传统活检的局限在于有创、时间长、对患者整体情况反映差及标本质量和数量不可控.近十年来,液态活检(liquid biopsy,LB)技术在肿瘤学领域掀起了一场巨大变革,它可提供DNA突变、基因拷贝数改变、转录组/蛋白质组分析、表观遗传改变、代谢物分析等信息,将癌症诊断和监测引向无创时代[13 -15 ] . ...
3
... 细胞游离DNA(cell-free DNA,cfDNA)是存在于细胞外的游离DNA分子,可由正常细胞或肿瘤细胞释放.然而,有研究[16 -18 ] 发现癌症患者cfDNA水平明显高于健康人群.cfDNA的检测技术尚未完全标准化,并且缺乏完整的分析共识,目前主要包括聚合酶链式反应(polymerase chain reaction,PCR)、实时荧光定量PCR(quantitative real-time PCR,qPCR)、数字PCR(digital PCR,dPCR)、甲基化特异度PCR(methylation-specific PCR,MSP)和下一代测序(next-generation sequencing,NGS)等[13 ,17 ,19 -20 ] . ...
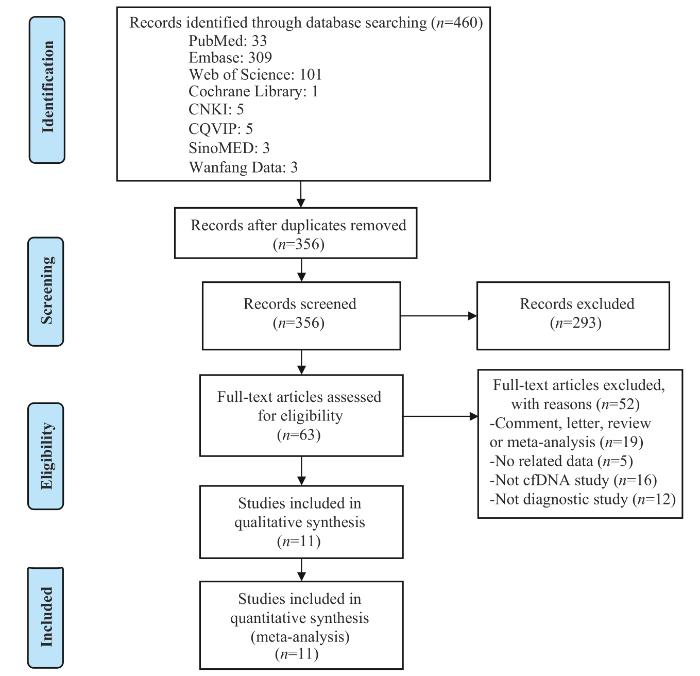
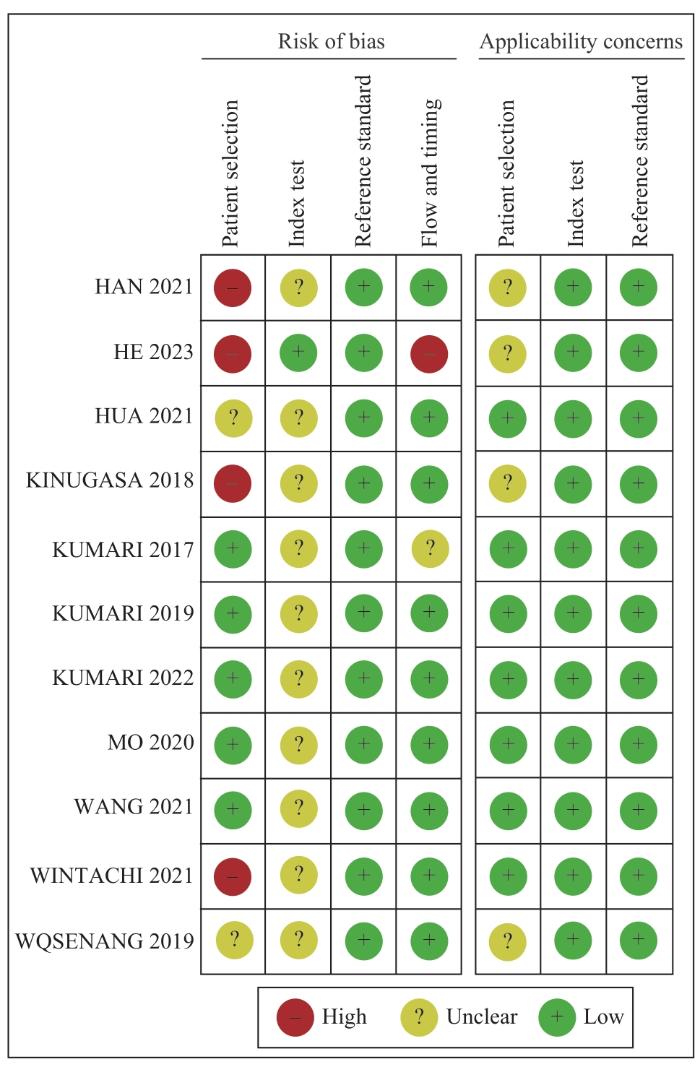
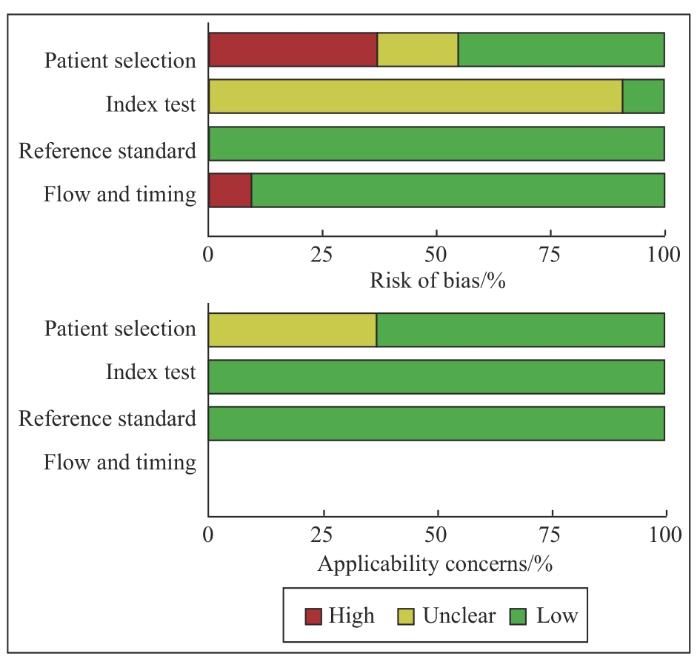
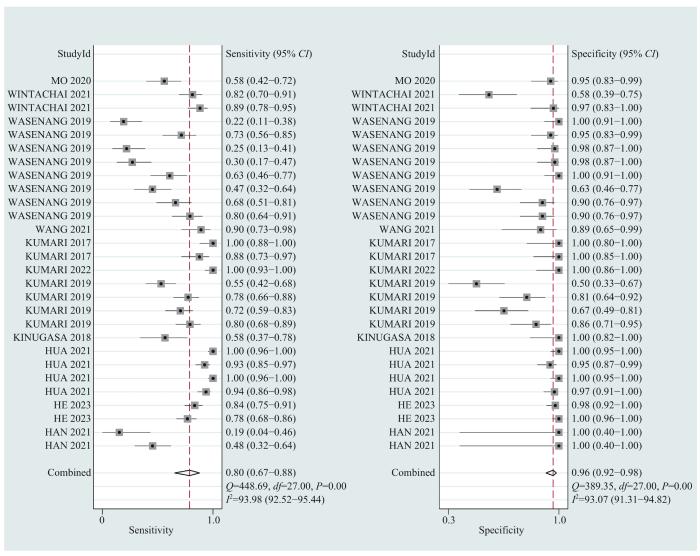
... 胆道癌起病隐匿,早期症状不明显,大部分患者在疾病晚期才被诊断,严重影响患者预后.近年来,随着精准医学的不断发展,液态活检技术在肿瘤学的应用越来越广泛,cfDNA检测已被证明对多种恶性肿瘤有较好的诊断价值[16 -18 ] .cfDNA在胆道癌的应用集中在分析与疾病预后的相关性以及复发监测上,针对BTC诊断应用的研究数量有限,且检测方式、检测指标和截断值选择等尚无统一标准,亟需规范的行业标准以加速临床转化.因此,为进一步评估循环游离DNA与胆道癌诊断之间的联系,本文对此领域现有的前瞻性和回顾性诊断试验展开meta分析.本文共纳入28项诊断性试验,入选实验组1 476例,对照组1 152例.采用QUADAS-2评价体系进行质量评价,提示纳入的文献均为中高等质量. ...
... 敏感度和特异度2个统计量的异质性分析提示各项研究存在较大异质性,可能由包括阈值效应和非阈值效应的其他因素导致.阈值效应约占总体一致性的15%,这也与现在cfDNA检测指标和阈值不固定、检测试剂盒和仪器使用并未统一的检测现状相吻合[16 ] .根据样本量进行分层,I 2 仍>50%,提示异质性研究的病例数关系不大.综合各篇文献的研究设计,笔者认为纳入的28项诊断性试验存在临床异质性,可能与样本选择过程中存在选择倾向性、各项研究的疾病分级分期不明确且差异较大等因素相关. ...
1
... 细胞游离DNA(cell-free DNA,cfDNA)是存在于细胞外的游离DNA分子,可由正常细胞或肿瘤细胞释放.然而,有研究[16 -18 ] 发现癌症患者cfDNA水平明显高于健康人群.cfDNA的检测技术尚未完全标准化,并且缺乏完整的分析共识,目前主要包括聚合酶链式反应(polymerase chain reaction,PCR)、实时荧光定量PCR(quantitative real-time PCR,qPCR)、数字PCR(digital PCR,dPCR)、甲基化特异度PCR(methylation-specific PCR,MSP)和下一代测序(next-generation sequencing,NGS)等[13 ,17 ,19 -20 ] . ...
2
... 细胞游离DNA(cell-free DNA,cfDNA)是存在于细胞外的游离DNA分子,可由正常细胞或肿瘤细胞释放.然而,有研究[16 -18 ] 发现癌症患者cfDNA水平明显高于健康人群.cfDNA的检测技术尚未完全标准化,并且缺乏完整的分析共识,目前主要包括聚合酶链式反应(polymerase chain reaction,PCR)、实时荧光定量PCR(quantitative real-time PCR,qPCR)、数字PCR(digital PCR,dPCR)、甲基化特异度PCR(methylation-specific PCR,MSP)和下一代测序(next-generation sequencing,NGS)等[13 ,17 ,19 -20 ] . ...
... 胆道癌起病隐匿,早期症状不明显,大部分患者在疾病晚期才被诊断,严重影响患者预后.近年来,随着精准医学的不断发展,液态活检技术在肿瘤学的应用越来越广泛,cfDNA检测已被证明对多种恶性肿瘤有较好的诊断价值[16 -18 ] .cfDNA在胆道癌的应用集中在分析与疾病预后的相关性以及复发监测上,针对BTC诊断应用的研究数量有限,且检测方式、检测指标和截断值选择等尚无统一标准,亟需规范的行业标准以加速临床转化.因此,为进一步评估循环游离DNA与胆道癌诊断之间的联系,本文对此领域现有的前瞻性和回顾性诊断试验展开meta分析.本文共纳入28项诊断性试验,入选实验组1 476例,对照组1 152例.采用QUADAS-2评价体系进行质量评价,提示纳入的文献均为中高等质量. ...
1
... 细胞游离DNA(cell-free DNA,cfDNA)是存在于细胞外的游离DNA分子,可由正常细胞或肿瘤细胞释放.然而,有研究[16 -18 ] 发现癌症患者cfDNA水平明显高于健康人群.cfDNA的检测技术尚未完全标准化,并且缺乏完整的分析共识,目前主要包括聚合酶链式反应(polymerase chain reaction,PCR)、实时荧光定量PCR(quantitative real-time PCR,qPCR)、数字PCR(digital PCR,dPCR)、甲基化特异度PCR(methylation-specific PCR,MSP)和下一代测序(next-generation sequencing,NGS)等[13 ,17 ,19 -20 ] . ...
1
... 细胞游离DNA(cell-free DNA,cfDNA)是存在于细胞外的游离DNA分子,可由正常细胞或肿瘤细胞释放.然而,有研究[16 -18 ] 发现癌症患者cfDNA水平明显高于健康人群.cfDNA的检测技术尚未完全标准化,并且缺乏完整的分析共识,目前主要包括聚合酶链式反应(polymerase chain reaction,PCR)、实时荧光定量PCR(quantitative real-time PCR,qPCR)、数字PCR(digital PCR,dPCR)、甲基化特异度PCR(methylation-specific PCR,MSP)和下一代测序(next-generation sequencing,NGS)等[13 ,17 ,19 -20 ] . ...
1
... cfDNA以微创方式提供肿瘤细胞的基因组信息[5 ,21 -23 ] ,但目前在胆道癌的诊断上的应用价值尚无定论,且具体选择何种检测方法和样本意见仍不统一,因此在临床开展不多.本研究拟检索目前国内外各项相关研究,全面评价cfDNA对胆道癌的诊断准确性,探讨样本来源、样本来源、检测方法以及截断值选择等对研究的影响,以期为更好开展临床应用提供依据. ...
1
... cfDNA以微创方式提供肿瘤细胞的基因组信息[5 ,21 -23 ] ,但目前在胆道癌的诊断上的应用价值尚无定论,且具体选择何种检测方法和样本意见仍不统一,因此在临床开展不多.本研究拟检索目前国内外各项相关研究,全面评价cfDNA对胆道癌的诊断准确性,探讨样本来源、样本来源、检测方法以及截断值选择等对研究的影响,以期为更好开展临床应用提供依据. ...
1
... cfDNA以微创方式提供肿瘤细胞的基因组信息[5 ,21 -23 ] ,但目前在胆道癌的诊断上的应用价值尚无定论,且具体选择何种检测方法和样本意见仍不统一,因此在临床开展不多.本研究拟检索目前国内外各项相关研究,全面评价cfDNA对胆道癌的诊断准确性,探讨样本来源、样本来源、检测方法以及截断值选择等对研究的影响,以期为更好开展临床应用提供依据. ...
2
... 在英文数据库PubMed、Web of Science、Embase、Cochrane Library以及中文数据库中国知网、万方数据知识服务平台、维普网和中国生物医学文献服务系统中系统检索有关cfDNA在胆道癌中的诊断试验研究,检索时间设置为从建库至2023年4月.检索策略依据首选报告的条目(preferred reporting items for systematic reviews and meta-analyses,PRISMA)声明[24 ] 制定.搜索的关键词包括“cfDNA”“ctDNA”“液态活检”“胆道癌”“胆管癌”“胆囊癌”“诊断”“ROC曲线”“敏感度”和“特异度”等,检索式涵盖主题词与自由词并依据各数据库的不同要求进行调整,英文检索式与中文格式大致类似. ...
... 使用Stata 17.0软件进行统计分析.对敏感度与(1-特异度)进行Spearman秩相关分析,以判断阈值效应是否存在[24 ] .运用Cochran Q检验、I 2 检验分析纳入研究间的异质性.拟合双变量混合效应模型,将统计量TP、TN、FP和FN进行合并,计算总体敏感度、特异度、阳性似然比(positive likelihood ratio,PLR)、阴性似然比(negative likelihood ratio,NLR)、诊断优势比(diagnostic odds ratio,DOR)和曲线下面积(area under the curve,AUC),以判断诊断性能. ...
3
... Basic characteristics of the included studies
Tab 1 Study Study type Country Reference standard HAN 2021[25 ] Prospective study South Korea BTC: pathological examination HE 2023[26 ] Retrospective and prospective study China BTC: pathological examination; BBD: pathological examination and clinical follow-up; healthy population: pathological examination/clinical follow-up HUA 2021[27 ] Prospective study China BTC: pathological examination KINUGASA 2018[28 ] Prospective study Japan Pathological examination KUMARI 2019[29 ] Retrospective study India Imaging/pathological examination KUMARI 2022[30 ] Retrospective study India Imaging/pathological examination KUMARI 2017[31 ] Retrospective study India Imaging/pathological examination WANG 2021[32 ] Prospective study China Pathological examination WASENANG 2019[33 ] Prospective study Thailand BTC: pathological examination WINTACHAI 2021[34 ] Retrospective study Thailand Pathological examination MO 2020[35 ] Retrospective study China BTC/BBD: imaging examination/pathological examination
Note:
... Collected data of the included studies
Tab 2 Study Sample Method Sample size/n Cut off TP/n FP/n FN/n TN/n HAN 2021[25 ] Bile ddPCR 46 1 500 copies·mL-1 20 0 22 4 Plasma ddPCR 20 60 copies·mL-1 3 0 13 4 HE 2023[26 ] Bile qPCR 188 NA 74 0 21 93 Bile NGS 188 NA 80 2 15 91 HUA 2021[27 ] Serum qPCR 158 403.65 ng·mL-1 78 2 5 73 Serum qPCR 153 113.82 ng·mL-1 83 0 0 70 Serum qPCR 158 364 ng·mL-1 76 7 7 68 Serum qPCR 153 96 ng·mL-1 83 0 0 70 KINUGASA 2018[28 ] Bile NGS 43 NA 14 0 10 19 KUMARI 2019[29 ] Serum qPCR 96 406.582 5 ng·mL-1 48 5 12 31 Serum qPCR 96 1 128.429 ng·mL-1 43 12 17 24 Serum qPCR 96 cfDNA integrity index: 0.356 47 7 13 29 Serum Methylated DNA Quantification Kit 96 Global DNA methylation: 0.713 5 33 18 27 18 KUMARI 2022[30 ] Serum qPCR 75 251.2 ng·mL-1 50 0 0 25 KUMARI 2017[31 ] Serum qPCR 56 372.92 ng·mL-1 30 0 4 22 Serum qPCR 51 218.55 ng·mL-1 34 0 0 17 WANG 2021[32 ] Plasma Low-coverage WGS 47 |Z|-score in UCAD test 2.32 26 2 3 16 WASENANG 2019[33 ] Serum MSP 80 OPCML 3.24%‒50% methylation 32 4 8 36 Serum MSP 80 HOXD9 1.56%‒50% methylation 27 4 13 36 Serum MSP 80 HOXA9 1.56%‒50% methylation 19 15 21 25 Serum MSP 80 OPCML, HOXD9 ...
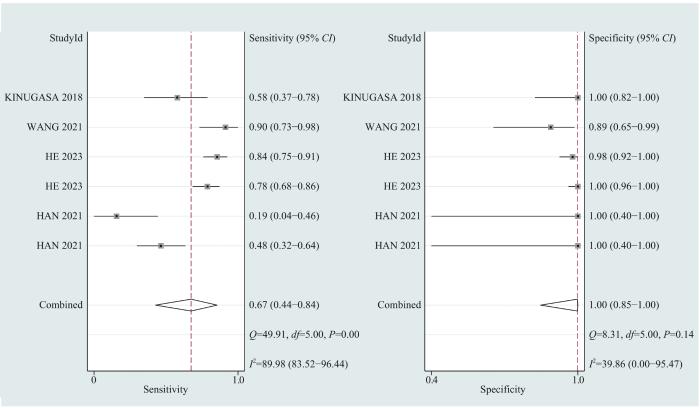
... 本文对样本量大小、检测方式和样本来源进行亚组分析.在检测方式选择方面,利用qPCR技术检测cfDNA浓度的敏感度和特异度均较高,诊断准确性较高,且成本较低,适用于早期筛查.胆道癌的精准分期对治疗方法选择有较大影响[40 ] ,cfDNA的浓度可提示肿瘤的全身负荷情况,便于临床医师精准地了解肿瘤的分级分期,准确评估手术的可行性,选择对患者更有利的治疗方案.使用NGS、WGS和ddPCR等方法进行基因或突变位点分析对BTC进行诊断敏感度不高(0.67),但特异度突出,提示该种方法用于排除诊断效果较好.同时,NGS和WGS等方法可以提供肿瘤的突变的位置信息,为临床医师的个性化用药提供依据.纳入的诊断性试验关注的突变位点主要有KRAS[25 -26 ,28 ] 、TP53[26 ,28 ] 和SMAD4[26 ,28 ] 等.3种检测方法中,甲基化检测效能最低,为避免漏诊和误诊,在诊断过程中需结合其他辅助检查结果. ...
5
... Basic characteristics of the included studies
Tab 1 Study Study type Country Reference standard HAN 2021[25 ] Prospective study South Korea BTC: pathological examination HE 2023[26 ] Retrospective and prospective study China BTC: pathological examination; BBD: pathological examination and clinical follow-up; healthy population: pathological examination/clinical follow-up HUA 2021[27 ] Prospective study China BTC: pathological examination KINUGASA 2018[28 ] Prospective study Japan Pathological examination KUMARI 2019[29 ] Retrospective study India Imaging/pathological examination KUMARI 2022[30 ] Retrospective study India Imaging/pathological examination KUMARI 2017[31 ] Retrospective study India Imaging/pathological examination WANG 2021[32 ] Prospective study China Pathological examination WASENANG 2019[33 ] Prospective study Thailand BTC: pathological examination WINTACHAI 2021[34 ] Retrospective study Thailand Pathological examination MO 2020[35 ] Retrospective study China BTC/BBD: imaging examination/pathological examination
Note:
... Collected data of the included studies
Tab 2 Study Sample Method Sample size/n Cut off TP/n FP/n FN/n TN/n HAN 2021[25 ] Bile ddPCR 46 1 500 copies·mL-1 20 0 22 4 Plasma ddPCR 20 60 copies·mL-1 3 0 13 4 HE 2023[26 ] Bile qPCR 188 NA 74 0 21 93 Bile NGS 188 NA 80 2 15 91 HUA 2021[27 ] Serum qPCR 158 403.65 ng·mL-1 78 2 5 73 Serum qPCR 153 113.82 ng·mL-1 83 0 0 70 Serum qPCR 158 364 ng·mL-1 76 7 7 68 Serum qPCR 153 96 ng·mL-1 83 0 0 70 KINUGASA 2018[28 ] Bile NGS 43 NA 14 0 10 19 KUMARI 2019[29 ] Serum qPCR 96 406.582 5 ng·mL-1 48 5 12 31 Serum qPCR 96 1 128.429 ng·mL-1 43 12 17 24 Serum qPCR 96 cfDNA integrity index: 0.356 47 7 13 29 Serum Methylated DNA Quantification Kit 96 Global DNA methylation: 0.713 5 33 18 27 18 KUMARI 2022[30 ] Serum qPCR 75 251.2 ng·mL-1 50 0 0 25 KUMARI 2017[31 ] Serum qPCR 56 372.92 ng·mL-1 30 0 4 22 Serum qPCR 51 218.55 ng·mL-1 34 0 0 17 WANG 2021[32 ] Plasma Low-coverage WGS 47 |Z|-score in UCAD test 2.32 26 2 3 16 WASENANG 2019[33 ] Serum MSP 80 OPCML 3.24%‒50% methylation 32 4 8 36 Serum MSP 80 HOXD9 1.56%‒50% methylation 27 4 13 36 Serum MSP 80 HOXA9 1.56%‒50% methylation 19 15 21 25 Serum MSP 80 OPCML, HOXD9 ...
... 本文对样本量大小、检测方式和样本来源进行亚组分析.在检测方式选择方面,利用qPCR技术检测cfDNA浓度的敏感度和特异度均较高,诊断准确性较高,且成本较低,适用于早期筛查.胆道癌的精准分期对治疗方法选择有较大影响[40 ] ,cfDNA的浓度可提示肿瘤的全身负荷情况,便于临床医师精准地了解肿瘤的分级分期,准确评估手术的可行性,选择对患者更有利的治疗方案.使用NGS、WGS和ddPCR等方法进行基因或突变位点分析对BTC进行诊断敏感度不高(0.67),但特异度突出,提示该种方法用于排除诊断效果较好.同时,NGS和WGS等方法可以提供肿瘤的突变的位置信息,为临床医师的个性化用药提供依据.纳入的诊断性试验关注的突变位点主要有KRAS[25 -26 ,28 ] 、TP53[26 ,28 ] 和SMAD4[26 ,28 ] 等.3种检测方法中,甲基化检测效能最低,为避免漏诊和误诊,在诊断过程中需结合其他辅助检查结果. ...
... [26 ,28 ]和SMAD4[26 ,28 ] 等.3种检测方法中,甲基化检测效能最低,为避免漏诊和误诊,在诊断过程中需结合其他辅助检查结果. ...
... [26 ,28 ]等.3种检测方法中,甲基化检测效能最低,为避免漏诊和误诊,在诊断过程中需结合其他辅助检查结果. ...
2
... Basic characteristics of the included studies
Tab 1 Study Study type Country Reference standard HAN 2021[25 ] Prospective study South Korea BTC: pathological examination HE 2023[26 ] Retrospective and prospective study China BTC: pathological examination; BBD: pathological examination and clinical follow-up; healthy population: pathological examination/clinical follow-up HUA 2021[27 ] Prospective study China BTC: pathological examination KINUGASA 2018[28 ] Prospective study Japan Pathological examination KUMARI 2019[29 ] Retrospective study India Imaging/pathological examination KUMARI 2022[30 ] Retrospective study India Imaging/pathological examination KUMARI 2017[31 ] Retrospective study India Imaging/pathological examination WANG 2021[32 ] Prospective study China Pathological examination WASENANG 2019[33 ] Prospective study Thailand BTC: pathological examination WINTACHAI 2021[34 ] Retrospective study Thailand Pathological examination MO 2020[35 ] Retrospective study China BTC/BBD: imaging examination/pathological examination
Note:
... Collected data of the included studies
Tab 2 Study Sample Method Sample size/n Cut off TP/n FP/n FN/n TN/n HAN 2021[25 ] Bile ddPCR 46 1 500 copies·mL-1 20 0 22 4 Plasma ddPCR 20 60 copies·mL-1 3 0 13 4 HE 2023[26 ] Bile qPCR 188 NA 74 0 21 93 Bile NGS 188 NA 80 2 15 91 HUA 2021[27 ] Serum qPCR 158 403.65 ng·mL-1 78 2 5 73 Serum qPCR 153 113.82 ng·mL-1 83 0 0 70 Serum qPCR 158 364 ng·mL-1 76 7 7 68 Serum qPCR 153 96 ng·mL-1 83 0 0 70 KINUGASA 2018[28 ] Bile NGS 43 NA 14 0 10 19 KUMARI 2019[29 ] Serum qPCR 96 406.582 5 ng·mL-1 48 5 12 31 Serum qPCR 96 1 128.429 ng·mL-1 43 12 17 24 Serum qPCR 96 cfDNA integrity index: 0.356 47 7 13 29 Serum Methylated DNA Quantification Kit 96 Global DNA methylation: 0.713 5 33 18 27 18 KUMARI 2022[30 ] Serum qPCR 75 251.2 ng·mL-1 50 0 0 25 KUMARI 2017[31 ] Serum qPCR 56 372.92 ng·mL-1 30 0 4 22 Serum qPCR 51 218.55 ng·mL-1 34 0 0 17 WANG 2021[32 ] Plasma Low-coverage WGS 47 |Z|-score in UCAD test 2.32 26 2 3 16 WASENANG 2019[33 ] Serum MSP 80 OPCML 3.24%‒50% methylation 32 4 8 36 Serum MSP 80 HOXD9 1.56%‒50% methylation 27 4 13 36 Serum MSP 80 HOXA9 1.56%‒50% methylation 19 15 21 25 Serum MSP 80 OPCML, HOXD9 ...
5
... Basic characteristics of the included studies
Tab 1 Study Study type Country Reference standard HAN 2021[25 ] Prospective study South Korea BTC: pathological examination HE 2023[26 ] Retrospective and prospective study China BTC: pathological examination; BBD: pathological examination and clinical follow-up; healthy population: pathological examination/clinical follow-up HUA 2021[27 ] Prospective study China BTC: pathological examination KINUGASA 2018[28 ] Prospective study Japan Pathological examination KUMARI 2019[29 ] Retrospective study India Imaging/pathological examination KUMARI 2022[30 ] Retrospective study India Imaging/pathological examination KUMARI 2017[31 ] Retrospective study India Imaging/pathological examination WANG 2021[32 ] Prospective study China Pathological examination WASENANG 2019[33 ] Prospective study Thailand BTC: pathological examination WINTACHAI 2021[34 ] Retrospective study Thailand Pathological examination MO 2020[35 ] Retrospective study China BTC/BBD: imaging examination/pathological examination
Note:
... Collected data of the included studies
Tab 2 Study Sample Method Sample size/n Cut off TP/n FP/n FN/n TN/n HAN 2021[25 ] Bile ddPCR 46 1 500 copies·mL-1 20 0 22 4 Plasma ddPCR 20 60 copies·mL-1 3 0 13 4 HE 2023[26 ] Bile qPCR 188 NA 74 0 21 93 Bile NGS 188 NA 80 2 15 91 HUA 2021[27 ] Serum qPCR 158 403.65 ng·mL-1 78 2 5 73 Serum qPCR 153 113.82 ng·mL-1 83 0 0 70 Serum qPCR 158 364 ng·mL-1 76 7 7 68 Serum qPCR 153 96 ng·mL-1 83 0 0 70 KINUGASA 2018[28 ] Bile NGS 43 NA 14 0 10 19 KUMARI 2019[29 ] Serum qPCR 96 406.582 5 ng·mL-1 48 5 12 31 Serum qPCR 96 1 128.429 ng·mL-1 43 12 17 24 Serum qPCR 96 cfDNA integrity index: 0.356 47 7 13 29 Serum Methylated DNA Quantification Kit 96 Global DNA methylation: 0.713 5 33 18 27 18 KUMARI 2022[30 ] Serum qPCR 75 251.2 ng·mL-1 50 0 0 25 KUMARI 2017[31 ] Serum qPCR 56 372.92 ng·mL-1 30 0 4 22 Serum qPCR 51 218.55 ng·mL-1 34 0 0 17 WANG 2021[32 ] Plasma Low-coverage WGS 47 |Z|-score in UCAD test 2.32 26 2 3 16 WASENANG 2019[33 ] Serum MSP 80 OPCML 3.24%‒50% methylation 32 4 8 36 Serum MSP 80 HOXD9 1.56%‒50% methylation 27 4 13 36 Serum MSP 80 HOXA9 1.56%‒50% methylation 19 15 21 25 Serum MSP 80 OPCML, HOXD9 ...
... 本文对样本量大小、检测方式和样本来源进行亚组分析.在检测方式选择方面,利用qPCR技术检测cfDNA浓度的敏感度和特异度均较高,诊断准确性较高,且成本较低,适用于早期筛查.胆道癌的精准分期对治疗方法选择有较大影响[40 ] ,cfDNA的浓度可提示肿瘤的全身负荷情况,便于临床医师精准地了解肿瘤的分级分期,准确评估手术的可行性,选择对患者更有利的治疗方案.使用NGS、WGS和ddPCR等方法进行基因或突变位点分析对BTC进行诊断敏感度不高(0.67),但特异度突出,提示该种方法用于排除诊断效果较好.同时,NGS和WGS等方法可以提供肿瘤的突变的位置信息,为临床医师的个性化用药提供依据.纳入的诊断性试验关注的突变位点主要有KRAS[25 -26 ,28 ] 、TP53[26 ,28 ] 和SMAD4[26 ,28 ] 等.3种检测方法中,甲基化检测效能最低,为避免漏诊和误诊,在诊断过程中需结合其他辅助检查结果. ...
... ,28 ]和SMAD4[26 ,28 ] 等.3种检测方法中,甲基化检测效能最低,为避免漏诊和误诊,在诊断过程中需结合其他辅助检查结果. ...
... ,28 ]等.3种检测方法中,甲基化检测效能最低,为避免漏诊和误诊,在诊断过程中需结合其他辅助检查结果. ...
2
... Basic characteristics of the included studies
Tab 1 Study Study type Country Reference standard HAN 2021[25 ] Prospective study South Korea BTC: pathological examination HE 2023[26 ] Retrospective and prospective study China BTC: pathological examination; BBD: pathological examination and clinical follow-up; healthy population: pathological examination/clinical follow-up HUA 2021[27 ] Prospective study China BTC: pathological examination KINUGASA 2018[28 ] Prospective study Japan Pathological examination KUMARI 2019[29 ] Retrospective study India Imaging/pathological examination KUMARI 2022[30 ] Retrospective study India Imaging/pathological examination KUMARI 2017[31 ] Retrospective study India Imaging/pathological examination WANG 2021[32 ] Prospective study China Pathological examination WASENANG 2019[33 ] Prospective study Thailand BTC: pathological examination WINTACHAI 2021[34 ] Retrospective study Thailand Pathological examination MO 2020[35 ] Retrospective study China BTC/BBD: imaging examination/pathological examination
Note:
... Collected data of the included studies
Tab 2 Study Sample Method Sample size/n Cut off TP/n FP/n FN/n TN/n HAN 2021[25 ] Bile ddPCR 46 1 500 copies·mL-1 20 0 22 4 Plasma ddPCR 20 60 copies·mL-1 3 0 13 4 HE 2023[26 ] Bile qPCR 188 NA 74 0 21 93 Bile NGS 188 NA 80 2 15 91 HUA 2021[27 ] Serum qPCR 158 403.65 ng·mL-1 78 2 5 73 Serum qPCR 153 113.82 ng·mL-1 83 0 0 70 Serum qPCR 158 364 ng·mL-1 76 7 7 68 Serum qPCR 153 96 ng·mL-1 83 0 0 70 KINUGASA 2018[28 ] Bile NGS 43 NA 14 0 10 19 KUMARI 2019[29 ] Serum qPCR 96 406.582 5 ng·mL-1 48 5 12 31 Serum qPCR 96 1 128.429 ng·mL-1 43 12 17 24 Serum qPCR 96 cfDNA integrity index: 0.356 47 7 13 29 Serum Methylated DNA Quantification Kit 96 Global DNA methylation: 0.713 5 33 18 27 18 KUMARI 2022[30 ] Serum qPCR 75 251.2 ng·mL-1 50 0 0 25 KUMARI 2017[31 ] Serum qPCR 56 372.92 ng·mL-1 30 0 4 22 Serum qPCR 51 218.55 ng·mL-1 34 0 0 17 WANG 2021[32 ] Plasma Low-coverage WGS 47 |Z|-score in UCAD test 2.32 26 2 3 16 WASENANG 2019[33 ] Serum MSP 80 OPCML 3.24%‒50% methylation 32 4 8 36 Serum MSP 80 HOXD9 1.56%‒50% methylation 27 4 13 36 Serum MSP 80 HOXA9 1.56%‒50% methylation 19 15 21 25 Serum MSP 80 OPCML, HOXD9 ...
2
... Basic characteristics of the included studies
Tab 1 Study Study type Country Reference standard HAN 2021[25 ] Prospective study South Korea BTC: pathological examination HE 2023[26 ] Retrospective and prospective study China BTC: pathological examination; BBD: pathological examination and clinical follow-up; healthy population: pathological examination/clinical follow-up HUA 2021[27 ] Prospective study China BTC: pathological examination KINUGASA 2018[28 ] Prospective study Japan Pathological examination KUMARI 2019[29 ] Retrospective study India Imaging/pathological examination KUMARI 2022[30 ] Retrospective study India Imaging/pathological examination KUMARI 2017[31 ] Retrospective study India Imaging/pathological examination WANG 2021[32 ] Prospective study China Pathological examination WASENANG 2019[33 ] Prospective study Thailand BTC: pathological examination WINTACHAI 2021[34 ] Retrospective study Thailand Pathological examination MO 2020[35 ] Retrospective study China BTC/BBD: imaging examination/pathological examination
Note:
... Collected data of the included studies
Tab 2 Study Sample Method Sample size/n Cut off TP/n FP/n FN/n TN/n HAN 2021[25 ] Bile ddPCR 46 1 500 copies·mL-1 20 0 22 4 Plasma ddPCR 20 60 copies·mL-1 3 0 13 4 HE 2023[26 ] Bile qPCR 188 NA 74 0 21 93 Bile NGS 188 NA 80 2 15 91 HUA 2021[27 ] Serum qPCR 158 403.65 ng·mL-1 78 2 5 73 Serum qPCR 153 113.82 ng·mL-1 83 0 0 70 Serum qPCR 158 364 ng·mL-1 76 7 7 68 Serum qPCR 153 96 ng·mL-1 83 0 0 70 KINUGASA 2018[28 ] Bile NGS 43 NA 14 0 10 19 KUMARI 2019[29 ] Serum qPCR 96 406.582 5 ng·mL-1 48 5 12 31 Serum qPCR 96 1 128.429 ng·mL-1 43 12 17 24 Serum qPCR 96 cfDNA integrity index: 0.356 47 7 13 29 Serum Methylated DNA Quantification Kit 96 Global DNA methylation: 0.713 5 33 18 27 18 KUMARI 2022[30 ] Serum qPCR 75 251.2 ng·mL-1 50 0 0 25 KUMARI 2017[31 ] Serum qPCR 56 372.92 ng·mL-1 30 0 4 22 Serum qPCR 51 218.55 ng·mL-1 34 0 0 17 WANG 2021[32 ] Plasma Low-coverage WGS 47 |Z|-score in UCAD test 2.32 26 2 3 16 WASENANG 2019[33 ] Serum MSP 80 OPCML 3.24%‒50% methylation 32 4 8 36 Serum MSP 80 HOXD9 1.56%‒50% methylation 27 4 13 36 Serum MSP 80 HOXA9 1.56%‒50% methylation 19 15 21 25 Serum MSP 80 OPCML, HOXD9 ...
2
... Basic characteristics of the included studies
Tab 1 Study Study type Country Reference standard HAN 2021[25 ] Prospective study South Korea BTC: pathological examination HE 2023[26 ] Retrospective and prospective study China BTC: pathological examination; BBD: pathological examination and clinical follow-up; healthy population: pathological examination/clinical follow-up HUA 2021[27 ] Prospective study China BTC: pathological examination KINUGASA 2018[28 ] Prospective study Japan Pathological examination KUMARI 2019[29 ] Retrospective study India Imaging/pathological examination KUMARI 2022[30 ] Retrospective study India Imaging/pathological examination KUMARI 2017[31 ] Retrospective study India Imaging/pathological examination WANG 2021[32 ] Prospective study China Pathological examination WASENANG 2019[33 ] Prospective study Thailand BTC: pathological examination WINTACHAI 2021[34 ] Retrospective study Thailand Pathological examination MO 2020[35 ] Retrospective study China BTC/BBD: imaging examination/pathological examination
Note:
... Collected data of the included studies
Tab 2 Study Sample Method Sample size/n Cut off TP/n FP/n FN/n TN/n HAN 2021[25 ] Bile ddPCR 46 1 500 copies·mL-1 20 0 22 4 Plasma ddPCR 20 60 copies·mL-1 3 0 13 4 HE 2023[26 ] Bile qPCR 188 NA 74 0 21 93 Bile NGS 188 NA 80 2 15 91 HUA 2021[27 ] Serum qPCR 158 403.65 ng·mL-1 78 2 5 73 Serum qPCR 153 113.82 ng·mL-1 83 0 0 70 Serum qPCR 158 364 ng·mL-1 76 7 7 68 Serum qPCR 153 96 ng·mL-1 83 0 0 70 KINUGASA 2018[28 ] Bile NGS 43 NA 14 0 10 19 KUMARI 2019[29 ] Serum qPCR 96 406.582 5 ng·mL-1 48 5 12 31 Serum qPCR 96 1 128.429 ng·mL-1 43 12 17 24 Serum qPCR 96 cfDNA integrity index: 0.356 47 7 13 29 Serum Methylated DNA Quantification Kit 96 Global DNA methylation: 0.713 5 33 18 27 18 KUMARI 2022[30 ] Serum qPCR 75 251.2 ng·mL-1 50 0 0 25 KUMARI 2017[31 ] Serum qPCR 56 372.92 ng·mL-1 30 0 4 22 Serum qPCR 51 218.55 ng·mL-1 34 0 0 17 WANG 2021[32 ] Plasma Low-coverage WGS 47 |Z|-score in UCAD test 2.32 26 2 3 16 WASENANG 2019[33 ] Serum MSP 80 OPCML 3.24%‒50% methylation 32 4 8 36 Serum MSP 80 HOXD9 1.56%‒50% methylation 27 4 13 36 Serum MSP 80 HOXA9 1.56%‒50% methylation 19 15 21 25 Serum MSP 80 OPCML, HOXD9 ...
2
... Basic characteristics of the included studies
Tab 1 Study Study type Country Reference standard HAN 2021[25 ] Prospective study South Korea BTC: pathological examination HE 2023[26 ] Retrospective and prospective study China BTC: pathological examination; BBD: pathological examination and clinical follow-up; healthy population: pathological examination/clinical follow-up HUA 2021[27 ] Prospective study China BTC: pathological examination KINUGASA 2018[28 ] Prospective study Japan Pathological examination KUMARI 2019[29 ] Retrospective study India Imaging/pathological examination KUMARI 2022[30 ] Retrospective study India Imaging/pathological examination KUMARI 2017[31 ] Retrospective study India Imaging/pathological examination WANG 2021[32 ] Prospective study China Pathological examination WASENANG 2019[33 ] Prospective study Thailand BTC: pathological examination WINTACHAI 2021[34 ] Retrospective study Thailand Pathological examination MO 2020[35 ] Retrospective study China BTC/BBD: imaging examination/pathological examination
Note:
... Collected data of the included studies
Tab 2 Study Sample Method Sample size/n Cut off TP/n FP/n FN/n TN/n HAN 2021[25 ] Bile ddPCR 46 1 500 copies·mL-1 20 0 22 4 Plasma ddPCR 20 60 copies·mL-1 3 0 13 4 HE 2023[26 ] Bile qPCR 188 NA 74 0 21 93 Bile NGS 188 NA 80 2 15 91 HUA 2021[27 ] Serum qPCR 158 403.65 ng·mL-1 78 2 5 73 Serum qPCR 153 113.82 ng·mL-1 83 0 0 70 Serum qPCR 158 364 ng·mL-1 76 7 7 68 Serum qPCR 153 96 ng·mL-1 83 0 0 70 KINUGASA 2018[28 ] Bile NGS 43 NA 14 0 10 19 KUMARI 2019[29 ] Serum qPCR 96 406.582 5 ng·mL-1 48 5 12 31 Serum qPCR 96 1 128.429 ng·mL-1 43 12 17 24 Serum qPCR 96 cfDNA integrity index: 0.356 47 7 13 29 Serum Methylated DNA Quantification Kit 96 Global DNA methylation: 0.713 5 33 18 27 18 KUMARI 2022[30 ] Serum qPCR 75 251.2 ng·mL-1 50 0 0 25 KUMARI 2017[31 ] Serum qPCR 56 372.92 ng·mL-1 30 0 4 22 Serum qPCR 51 218.55 ng·mL-1 34 0 0 17 WANG 2021[32 ] Plasma Low-coverage WGS 47 |Z|-score in UCAD test 2.32 26 2 3 16 WASENANG 2019[33 ] Serum MSP 80 OPCML 3.24%‒50% methylation 32 4 8 36 Serum MSP 80 HOXD9 1.56%‒50% methylation 27 4 13 36 Serum MSP 80 HOXA9 1.56%‒50% methylation 19 15 21 25 Serum MSP 80 OPCML, HOXD9 ...
2
... Basic characteristics of the included studies
Tab 1 Study Study type Country Reference standard HAN 2021[25 ] Prospective study South Korea BTC: pathological examination HE 2023[26 ] Retrospective and prospective study China BTC: pathological examination; BBD: pathological examination and clinical follow-up; healthy population: pathological examination/clinical follow-up HUA 2021[27 ] Prospective study China BTC: pathological examination KINUGASA 2018[28 ] Prospective study Japan Pathological examination KUMARI 2019[29 ] Retrospective study India Imaging/pathological examination KUMARI 2022[30 ] Retrospective study India Imaging/pathological examination KUMARI 2017[31 ] Retrospective study India Imaging/pathological examination WANG 2021[32 ] Prospective study China Pathological examination WASENANG 2019[33 ] Prospective study Thailand BTC: pathological examination WINTACHAI 2021[34 ] Retrospective study Thailand Pathological examination MO 2020[35 ] Retrospective study China BTC/BBD: imaging examination/pathological examination
Note:
... Collected data of the included studies
Tab 2 Study Sample Method Sample size/n Cut off TP/n FP/n FN/n TN/n HAN 2021[25 ] Bile ddPCR 46 1 500 copies·mL-1 20 0 22 4 Plasma ddPCR 20 60 copies·mL-1 3 0 13 4 HE 2023[26 ] Bile qPCR 188 NA 74 0 21 93 Bile NGS 188 NA 80 2 15 91 HUA 2021[27 ] Serum qPCR 158 403.65 ng·mL-1 78 2 5 73 Serum qPCR 153 113.82 ng·mL-1 83 0 0 70 Serum qPCR 158 364 ng·mL-1 76 7 7 68 Serum qPCR 153 96 ng·mL-1 83 0 0 70 KINUGASA 2018[28 ] Bile NGS 43 NA 14 0 10 19 KUMARI 2019[29 ] Serum qPCR 96 406.582 5 ng·mL-1 48 5 12 31 Serum qPCR 96 1 128.429 ng·mL-1 43 12 17 24 Serum qPCR 96 cfDNA integrity index: 0.356 47 7 13 29 Serum Methylated DNA Quantification Kit 96 Global DNA methylation: 0.713 5 33 18 27 18 KUMARI 2022[30 ] Serum qPCR 75 251.2 ng·mL-1 50 0 0 25 KUMARI 2017[31 ] Serum qPCR 56 372.92 ng·mL-1 30 0 4 22 Serum qPCR 51 218.55 ng·mL-1 34 0 0 17 WANG 2021[32 ] Plasma Low-coverage WGS 47 |Z|-score in UCAD test 2.32 26 2 3 16 WASENANG 2019[33 ] Serum MSP 80 OPCML 3.24%‒50% methylation 32 4 8 36 Serum MSP 80 HOXD9 1.56%‒50% methylation 27 4 13 36 Serum MSP 80 HOXA9 1.56%‒50% methylation 19 15 21 25 Serum MSP 80 OPCML, HOXD9 ...
2
... Basic characteristics of the included studies
Tab 1 Study Study type Country Reference standard HAN 2021[25 ] Prospective study South Korea BTC: pathological examination HE 2023[26 ] Retrospective and prospective study China BTC: pathological examination; BBD: pathological examination and clinical follow-up; healthy population: pathological examination/clinical follow-up HUA 2021[27 ] Prospective study China BTC: pathological examination KINUGASA 2018[28 ] Prospective study Japan Pathological examination KUMARI 2019[29 ] Retrospective study India Imaging/pathological examination KUMARI 2022[30 ] Retrospective study India Imaging/pathological examination KUMARI 2017[31 ] Retrospective study India Imaging/pathological examination WANG 2021[32 ] Prospective study China Pathological examination WASENANG 2019[33 ] Prospective study Thailand BTC: pathological examination WINTACHAI 2021[34 ] Retrospective study Thailand Pathological examination MO 2020[35 ] Retrospective study China BTC/BBD: imaging examination/pathological examination
Note:
... all methylated
9 0 31 40 WINTACHAI 2021[34 ] Plasma qPCR 92 0.217 5 ng·µL-1 55 1 7 29 Plasma qPCR 95 0.338 8 ng·µL-1 51 14 11 19 MO 2020[35 ] Plasma qPCR 85 18.06 ng·µL-1 26 2 19 38
Note:
2
... Basic characteristics of the included studies
Tab 1 Study Study type Country Reference standard HAN 2021[25 ] Prospective study South Korea BTC: pathological examination HE 2023[26 ] Retrospective and prospective study China BTC: pathological examination; BBD: pathological examination and clinical follow-up; healthy population: pathological examination/clinical follow-up HUA 2021[27 ] Prospective study China BTC: pathological examination KINUGASA 2018[28 ] Prospective study Japan Pathological examination KUMARI 2019[29 ] Retrospective study India Imaging/pathological examination KUMARI 2022[30 ] Retrospective study India Imaging/pathological examination KUMARI 2017[31 ] Retrospective study India Imaging/pathological examination WANG 2021[32 ] Prospective study China Pathological examination WASENANG 2019[33 ] Prospective study Thailand BTC: pathological examination WINTACHAI 2021[34 ] Retrospective study Thailand Pathological examination MO 2020[35 ] Retrospective study China BTC/BBD: imaging examination/pathological examination
Note:
... all methylated
9 0 31 40 WINTACHAI 2021[34 ] Plasma qPCR 92 0.217 5 ng·µL-1 55 1 7 29 Plasma qPCR 95 0.338 8 ng·µL-1 51 14 11 19 MO 2020[35 ] Plasma qPCR 85 18.06 ng·µL-1 26 2 19 38
Note:
2
... Basic characteristics of the included studies
Tab 1 Study Study type Country Reference standard HAN 2021[25 ] Prospective study South Korea BTC: pathological examination HE 2023[26 ] Retrospective and prospective study China BTC: pathological examination; BBD: pathological examination and clinical follow-up; healthy population: pathological examination/clinical follow-up HUA 2021[27 ] Prospective study China BTC: pathological examination KINUGASA 2018[28 ] Prospective study Japan Pathological examination KUMARI 2019[29 ] Retrospective study India Imaging/pathological examination KUMARI 2022[30 ] Retrospective study India Imaging/pathological examination KUMARI 2017[31 ] Retrospective study India Imaging/pathological examination WANG 2021[32 ] Prospective study China Pathological examination WASENANG 2019[33 ] Prospective study Thailand BTC: pathological examination WINTACHAI 2021[34 ] Retrospective study Thailand Pathological examination MO 2020[35 ] Retrospective study China BTC/BBD: imaging examination/pathological examination
Note:
... all methylated
9 0 31 40 WINTACHAI 2021[34 ] Plasma qPCR 92 0.217 5 ng·µL-1 55 1 7 29 Plasma qPCR 95 0.338 8 ng·µL-1 51 14 11 19 MO 2020[35 ] Plasma qPCR 85 18.06 ng·µL-1 26 2 19 38
Note:
1
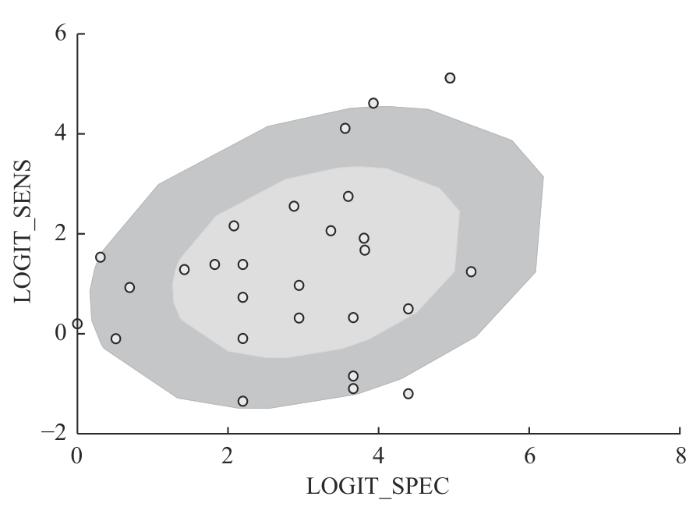
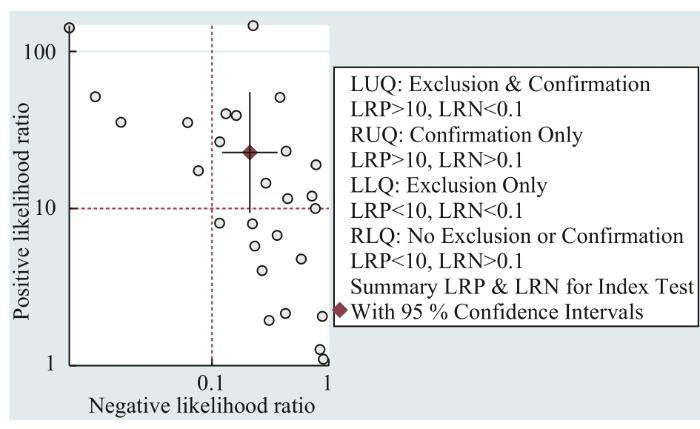
... Spearman相关分析提示敏感度与(1-特异度)呈正相关(r s =-0.082,P =0.677),提示存在阈值效应[36 ] ,并通过双变量混合效应模型进行分析得出15%的异质性可能是由于阈值效应导致的.双变量箱线图(图4 )显示有研究落在箱线图外.敏感度和特异度的Q检验P <0.01,一致性指数I s e n 2 I s e n 2
1
... Spearman相关分析提示敏感度与(1-特异度)呈正相关(r s =-0.082,P =0.677),提示存在阈值效应[36 ] ,并通过双变量混合效应模型进行分析得出15%的异质性可能是由于阈值效应导致的.双变量箱线图(图4 )显示有研究落在箱线图外.敏感度和特异度的Q检验P <0.01,一致性指数I s e n 2 I s e n 2
1
... 在全球范围内,BTC的发病率和死亡率呈现出东方世界明显高于西方世界的特点,主要与肝吸虫感染率等危险因素的分布情况有一定相关性[37 -38 ] .本文纳入的28项研究均来自亚洲国家,也体现了东方国家对于BTC早期诊断更加迫切的需求.cfDNA作为肿瘤领域的新兴技术,相关临床试验首先在这些BTC的高发国家开展. ...
1
... 在全球范围内,BTC的发病率和死亡率呈现出东方世界明显高于西方世界的特点,主要与肝吸虫感染率等危险因素的分布情况有一定相关性[37 -38 ] .本文纳入的28项研究均来自亚洲国家,也体现了东方国家对于BTC早期诊断更加迫切的需求.cfDNA作为肿瘤领域的新兴技术,相关临床试验首先在这些BTC的高发国家开展. ...
1
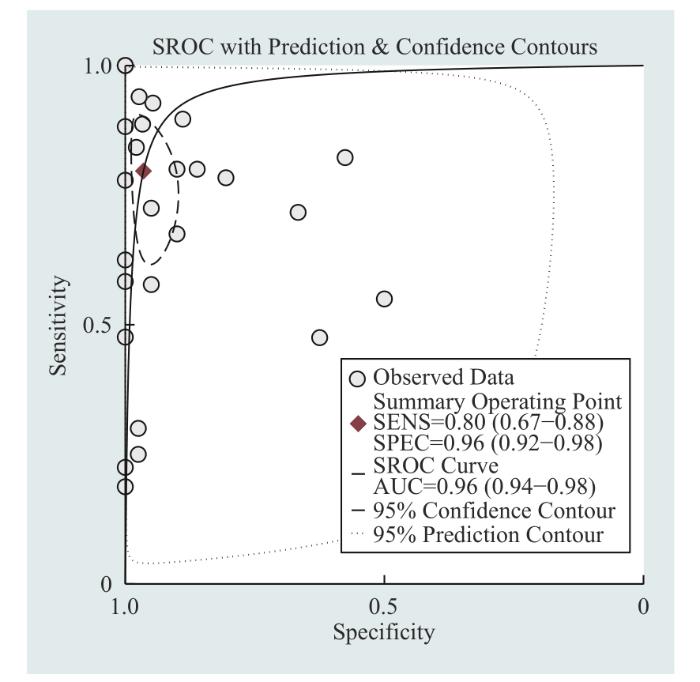
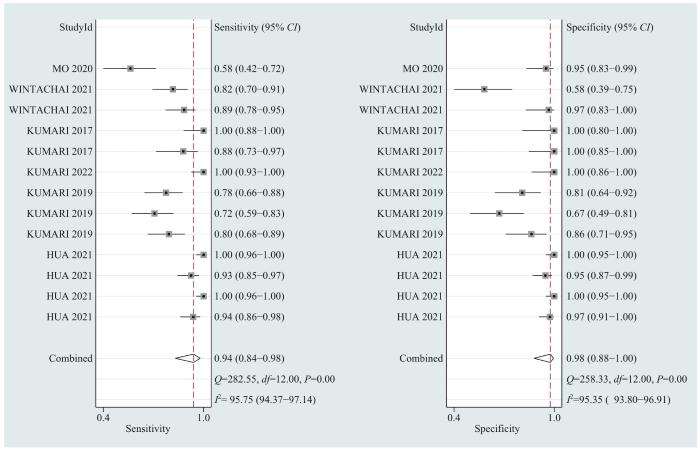
... 目前,临床医师在诊断BTC时,超声、CT等影像学检查以及CA19-9、CA125等肿瘤标志物应用较多,这些方法的提示作用较好,但易出现假阳性结果,特异性稍差,可能还会导致患者焦虑情绪的产生.想要进行鉴别诊断需要进行组织活检,但因其有创且操作复杂,部分患者存有抵抗情绪.本研究中meta分析结果显示,在诊断BTC时cfDNA检测合并后的敏感度为0.80、特异度为0.96、SROC曲线下面积为0.96,提示cfDNA检测是一种诊断效能较高的诊断方法.与SINGH等[39 ] 发表的涵盖miRNA、cfDNA和CTC的meta分析结果相比,本研究只对cfDNA单种方法进行分析,尤其是在利用qPCR法时,表现出更突出的敏感度.因此,本项meta分析提示cfDNA检测因具有高敏感度和高特异度,创伤小,无辐射和二次致癌风险,可用于BTC的早期筛查和鉴别诊断. ...
1
... 本文对样本量大小、检测方式和样本来源进行亚组分析.在检测方式选择方面,利用qPCR技术检测cfDNA浓度的敏感度和特异度均较高,诊断准确性较高,且成本较低,适用于早期筛查.胆道癌的精准分期对治疗方法选择有较大影响[40 ] ,cfDNA的浓度可提示肿瘤的全身负荷情况,便于临床医师精准地了解肿瘤的分级分期,准确评估手术的可行性,选择对患者更有利的治疗方案.使用NGS、WGS和ddPCR等方法进行基因或突变位点分析对BTC进行诊断敏感度不高(0.67),但特异度突出,提示该种方法用于排除诊断效果较好.同时,NGS和WGS等方法可以提供肿瘤的突变的位置信息,为临床医师的个性化用药提供依据.纳入的诊断性试验关注的突变位点主要有KRAS[25 -26 ,28 ] 、TP53[26 ,28 ] 和SMAD4[26 ,28 ] 等.3种检测方法中,甲基化检测效能最低,为避免漏诊和误诊,在诊断过程中需结合其他辅助检查结果. ...
1
... 本文对样本量大小、检测方式和样本来源进行亚组分析.在检测方式选择方面,利用qPCR技术检测cfDNA浓度的敏感度和特异度均较高,诊断准确性较高,且成本较低,适用于早期筛查.胆道癌的精准分期对治疗方法选择有较大影响[40 ] ,cfDNA的浓度可提示肿瘤的全身负荷情况,便于临床医师精准地了解肿瘤的分级分期,准确评估手术的可行性,选择对患者更有利的治疗方案.使用NGS、WGS和ddPCR等方法进行基因或突变位点分析对BTC进行诊断敏感度不高(0.67),但特异度突出,提示该种方法用于排除诊断效果较好.同时,NGS和WGS等方法可以提供肿瘤的突变的位置信息,为临床医师的个性化用药提供依据.纳入的诊断性试验关注的突变位点主要有KRAS[25 -26 ,28 ] 、TP53[26 ,28 ] 和SMAD4[26 ,28 ] 等.3种检测方法中,甲基化检测效能最低,为避免漏诊和误诊,在诊断过程中需结合其他辅助检查结果. ...
〈
〉