研究显示,IBD与微环境中过量堆积的活性氧(reactive oxygen species,ROS)密切相关。一方面,来自自由基诱导的学说提示在IBD的病理情况下,上皮细胞代谢产生的氧化物引起氧化应激失衡,产生过多的ROS以破坏上皮细胞结构蛋白及其紧密连接,并导致肠黏膜上皮屏障受损。损伤的肠道上皮细胞通过模式识别受体与肠腔细菌等微生物结合激活核因子-κB(nuclear factor-κB,NF-κB),促进白细胞介素-1(interleukin-1,IL-1)、IL-6、TNF-α和γ干扰素(interferon-γ,IFN-γ)等基因的表达和生成,参与炎症反应[5]。另一方面,固有免疫失调也是IBD肠黏膜上皮无法完全愈合的重要因素之一。作为固有免疫中重要的细胞之一,巨噬细胞在IBD炎症微环境中表现为促炎表型,通过NF-κB等信号通路的激活生成并释放大量IL-1β、IL-6、TNF-α、IFN-γ等细胞因子参与炎症反应,并产生过量ROS促进炎症进展。同时微环境中过量的ROS是维持巨噬细胞促炎表型的重要因素,IL-1β、IL-6、TNF-α、IFN-γ和ROS等促炎介质的持续释放导致肠道炎症微环境的进一步恶化,肠上皮黏膜无法完全愈合[6]。因此在本研究中,我们尝试通过消除ROS的策略改善肠道炎症微环境,从而缓解IBD炎症。
研究[7]显示,直径小于5 nm的氧化纳米铈颗粒(ceria nanoparticle,CeNP)表面同时存在Ce3+与Ce4+,表现出超氧化物歧化酶和过氧化氢酶拟酶活性,可以有效清除超氧阴离子(O2•-)、羟基自由基(•OH)和过氧化氢(H2O2)。除此之外,CeNP可以进行自我催化,使Ce3+和Ce4+能相互转化,具有循环利用、持久有效的抗氧化性质。我们先前的研究[8]证实了CeNP-聚乙二醇(CeNP-polyethylene glycol,CeNP-PEG)是一种无毒性、具有良好生物相容性以及良好抗氧化效应的纳米颗粒。同时,已有研究[7,9-11]证实CeNP-PEG优良的抗氧化性在肝缺血再灌注损伤、脑卒中、帕金森病和肾脏纤维化等多种疾病动物模型中具有良好的应用价值。本研究中,我们合成CeNP-PEG,评估其水合粒径和zeta电位,并通过体外实验及小鼠模型,检测其对IBD的治疗效果。
1 材料与方法
1.1 材料
1.1.1 实验动物及细胞
SPF级4~6周龄雄性C57BL/6小鼠购自浙江维通利华公司,实验动物生产许可证号为SCXK(浙)2019-0001。所有实验用小鼠均饲养在上海交通大学医学院附属第九人民医院SPF级动物房中,实验动物使用许可证号为SYXK(沪)2020-0025,12 h间隔明暗交替,温度(25±2)℃,允许小鼠自由饮食。小鼠单核巨噬细胞系Raw264.7购自美国模式培养物保藏所(American type culture collection,ATCC)细胞库。
1.1.2 主要实验试剂及仪器
重组小鼠IFN-γ(苏州新民科技股份有限公司),脂多糖(LPS,美国MedChemExpress),高糖DMEM培养基(大连美仑生物技术有限公司),胎牛血清(美国Gibco),RIPA裂解缓冲液、BCA蛋白定量检测试剂盒、细胞核蛋白与细胞浆蛋白抽提试剂盒、辣根过氧化物酶(HRP)标记羊抗兔抗体(A0208,1∶1 000)、HRP标记羊抗鼠抗体(A0216,1∶1 000)(上海碧云天生物技术有限公司),超氧化物指示剂二氢乙啶(dihydroethidium,DHE)、实时荧光定量PCR(RT-qPCR)试剂盒、反转录试剂盒、DAPI细胞核染色剂(美国Thermo Fisher Scientific),兔抗磷酸化P65(p-P65)多克隆抗体(ab86299,1∶5 000)、兔抗核因子-κB抑制蛋白α(NF-κB inhibitor-α,IκB-α)单克隆抗体(ab32518,1∶5 000)(英国Abcam),兔抗甘油醛-3-磷酸脱氢酶(GAPDH)单克隆抗体(60004-1-1g,1∶2 000)(美国Proteintech),葡聚糖硫酸钠(DSS,美国MP Biomedicals),粪便隐血试剂盒(上海源叶生物科技有限公司)。
倒置荧光显微镜(DMi8,德国Leica),化学成像显影机(Fusion FX.EDGE,德国VILBER),透射电子显微镜(TEM,JEM-2100,日本JEOL),纳米粒度及zeta电位仪(Zetasizer Nano-ZS,英国Malvern)。
1.2 实验方法
1.2.1 CeNP-PEG的合成、纯化与表征检测
取0.43 g醋酸铈的水合物、15 mL油胺与15 mL二甲苯相互混合,放置于超声仪,经超声处理至溶液澄清透明,然后在110 ℃、氮气环境中加入1 mL双蒸水搅拌3 h得到CeNP。冷却至室温后,加入甲醇,在室温下11 600×g离心10 min得到纯化的CeNP。称量50 mg聚乙二醇-二硬脂酰磷脂酰乙醇胺(mPEG-DPSE,0.0185 mmol)与10 mg CeNP,在2.0 mL氯仿中混合溶解后室温搅拌2 h。在60 ℃的真空条件下用旋转蒸发器蒸发30 min,加入10 mL双蒸水,超声震荡5 min后用袋式过滤器(截留相对分子质量为10 000)过滤掉多余的mPEG-DPSE,得到纯化的CeNP-PEG。
TEM检测粒径:TEM加速电压设置为200 kV,选择电子衍射区域观察。动态光散射(DLS)检测:在25 ℃下,使用纳米粒度及zeta电位仪测量CeNP-PEG的流体力学直径和zeta电位。
1.2.2 细胞培养
将细胞分为空白对照组(Control组,无任何处理)、阳性刺激组(Positive组,100 ng/mL LPS+20 ng/mL IFN-γ刺激)、0.5Ce组(100 ng/mL LPS+20 ng/mL IFN-γ刺激+0.5 µg/mL CeNP-PEG干预)和1.0Ce组(100 ng/mL LPS+20 ng/mL IFN-γ刺激+1.0 µg/mL CeNP-PEG干预)。复苏后的Raw264.7细胞系使用含10%FBS的高糖DMEM培养液置于10 cm培养皿培养,至细胞密度约为70%时,0.5Ce组和1.0Ce组培养液分别更换成含0.5 µg/mL和1.0 µg/mL CeNP-PEG的新鲜培养液,培养2 h,然后将Positive组、0.5Ce组和1.0Ce组培养液换成含100 ng/mL LPS+20 ng/mL IFN-γ的新鲜培养液,Control组仍使用含10%FBS的高糖DMEM培养液培养,24 h后获取细胞样本。
1.2.3 Western blotting检测
使用RIPA裂解液提取Raw264.7细胞的蛋白,另取部分细胞用细胞核蛋白与细胞浆蛋白抽提试剂盒分别提取细胞核与细胞质蛋白,并使用BCA蛋白定量检测试剂盒检测总蛋白浓度。每孔给予等量的蛋白质进行电泳后,转移到PVDF膜上,5%脱脂奶粉封闭2 h,TBST清洗3遍;然后与p-P65、IκB-α、GAPDH一抗孵育,4 ℃过夜;翌日,吸去多余的一抗,TBST清洗3遍;与相应的二抗孵育1 h后,使用显影剂上机显影。
1.2.4 DSS诱导的结肠炎小鼠模型构建与CeNP-PEG干预
小鼠分为3组,分别为正常对照组(Normal组)、模型组(DSS组)、CeNP-PEG治疗组。结肠炎造模周期为9 d,第1~7日将DSS组小鼠饮用水更换为3%~3.5%的DSS水溶液,第8~9日改为双蒸水;CeNP-PEG治疗组小鼠给水策略与DSS组一致,但在造模第3、5、7日尾静脉注射1.0 mg/mL的CeNP-PEG,Normal组和DSS组分别注射PBS;Normal组小鼠造模全程给予双蒸水。造模全程记录小鼠的体质量变化与粪便性状,并使用粪便隐血试剂盒检测小鼠的大便隐血情况。
1.2.5 肠道组织的ROS水平检测
于造模终止日取小鼠结肠组织,PBS清洗肠腔至无任何内容物并用吸水纸吸干结肠组织多余水分,使用OCT包埋剂包埋。包埋好的肠道组织使用冰冻切片机制成厚度4 µm的冰冻切片。冰冻切片置于PBS中清洗多余杂质,使用组化笔在组织周围划圈,向圈内滴加100 µL DHE,置于暗盒室温反应30 min后,使用PBS清洗,滴加DAPI染料封片。使用倒置荧光显微镜在发射波长580 nm、激发波长480 nm处获取荧光图像。
1.2.6 RT-qPCR
于造模终止日取小鼠结肠组织,TRIzol提取总RNA并使用反转录试剂盒进行反转录。使用RT-qPCR试剂盒进行目的基因的扩增,以actin作为内参,引物由上海铂尚生物技术有限公司设计并合成,序列如表1。
表1 RT-qPCR引物序列
Tab 1
| Gene | Sequence (5′→3′) |
|---|---|
| Tnf-α | Forward: CCTGTAGCCCACGTCGTAGC Reverse: AGCAATGACTCCAAAGTAGACC |
| Il-6 | Forward: ATCCAGTTGCCTTCTTGGGACTGA Reverse: TGGCTAAGGACCAAGACCATCCAA |
| Il-1β | Forward: CTTCAGGCAGGCAGTATCACTC Reverse: TGCAGTTGTCTAATGGGAACGT |
| Ifn-γ | Forward: AGCAACAGCAAGGCGAAA Reverse: CTGGACCTGTGGGTTGTTGA |
| actin | Forward: CAGCCTTCCTTCTTGGGTATG Reverse: GGCATAGAGGTCTTTACGGATG |
1.2.7 小鼠疾病活动指数监测
小鼠疾病活动指数(disease activity index,DAI)从体质量变化、大便情况进行评分:体质量减少≤3%或体质量增加记为0分,体质量减少4%~10%记为1分,体质量减少11%~20%记为2分,体质量减少>20%记为3分。大便情况从有无血便及有无稀便2个方面进行评估,无血便、无稀便记为0分,有血便且有稀便记为2分,有血便无稀便或无血便有稀便记为1分。各评分相加得到DAI得分。
1.3 统计学分析
用GraphPad Prism 9.0软件进行统计学分析。定量资料用x±
2 结果
2.1 CeNP-PEG的粒径和zeta电位检测
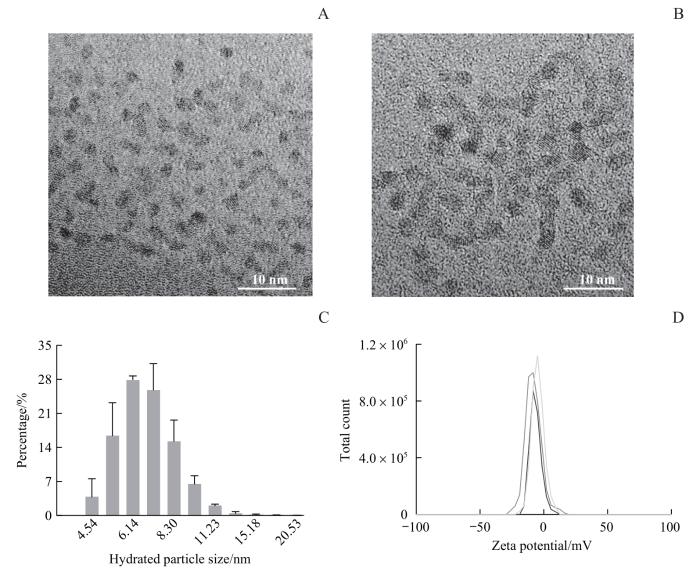
通过高分辨率TEM获取的图像可见,合成的CeNP-PEG粒径为(1.816±0.298)nm,符合我们的实验要求;DLS测定结果显示,CeNP-PEG在双蒸水中的流体力学直径(水合粒径)主要分布在(6.96±0.27)nm,平均zeta电位为(-6.02±1.31)mV(图1)。
图1
图1
CeNP和CeNP-PEG的表征
Note: A/B. Representative images of CeNP (A) and CeNP-PEG (B) (
Fig 1
Representation of CeNP and CeNP-PEG
2.2 CeNP-PEG对巨噬细胞NF-κB促炎信号通路的影响
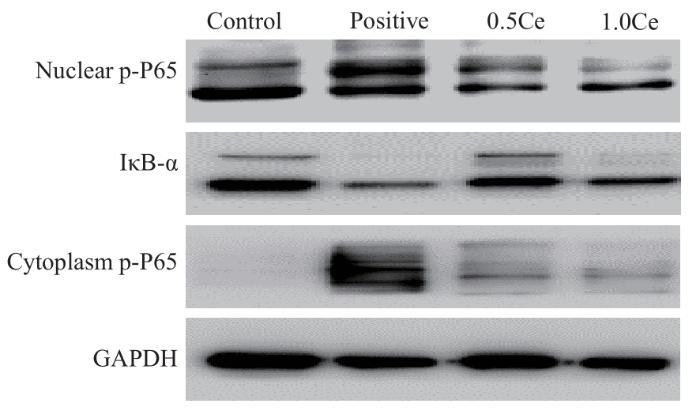
Western blotting结果显示,Positive组即巨噬细胞被诱导为促炎表型后NF-κB通路的相关蛋白p-P65表达增多,IκB-α表达减少,提示M1促炎表型的巨噬细胞中NF-κB通路的活化程度明显增加;在使用0.5 μg/mL或1.0 μg/mL CeNP-PEG干预的0.5Ce组和1.0Ce组中,p-P65表达较Positive组明显减少,而IκB-α表达明显增多,提示0.5 μg/mL 或1.0 μg/mL CeNP-PEG可以抑制体外巨噬细胞NF-κB 通路的活化(图2)。
图2
图2
CeNP-PEG对小鼠巨噬细胞NF-κB促炎信号通路相关蛋白表达水平的影响
Fig 2
Effect of CeNP-PEG on the expression levels of NF-κB pro-inflammatory signaling pathway-associated proteins in murine macrophages
2.3 CeNP-PEG对结肠炎小鼠DAI的改善效果
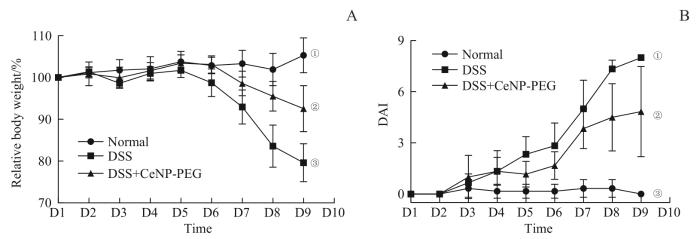
记录造模期间Normal组、DSS组与CeNP-PEG治疗组小鼠的体质量、粪便性状与便血情况,评估造模小鼠的体质量改变与DAI。结果显示,随着造模的进行,DSS组和CeNP-PEG治疗组小鼠的体质量都出现下降,DAI则呈现上升的趋势。至造模终止日(第9日),DSS组小鼠体质量下降至初始值的80%,平均DAI为8.03±1.65;CeNP-PEG治疗组小鼠体质量下降至初始值的92.5%,平均DAI为4.97±1.11,即CeNP-PEG治疗组小鼠的肠道炎症疾病活动度较DSS组明显减轻,提示CeNP-PEG治疗可以显著改善结肠炎小鼠的疾病活动度(图3)。
图3
图3
CeNP-PEG缓解结肠炎小鼠疾病活动度的疗效评价
Note: A. Relative body weight of the mice compared with the body weight on D1. ①P=0.001, compared with the DSS+CeNP-PEG group; ②P=0.001, compared with the DSS group; ③P=0.000, compared with the Normal group. B. DAI of the mice. ①P=0.000, compared with the Normal group; ②P=0.000, compared with the DSS group; ③P=0.000, compared with the DSS+CeNP-PEG group.
Fig 3
Evaluation of the effect of CeNP-PEG on reducing disease activity in the mice with colitis
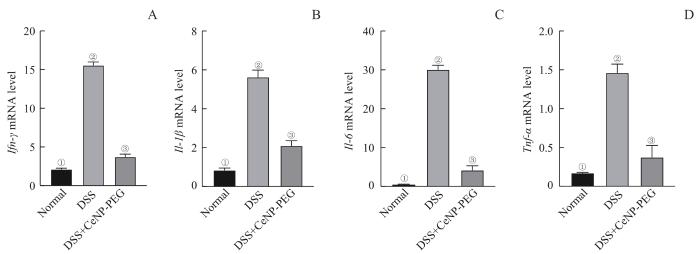
2.4 CeNP-PEG对肠道组织促炎细胞因子表达的影响
提取造模终止日Normal组、DSS组和CeNP-PEG治疗组的小鼠肠道组织RNA,通过RT-qPCR检测肠道促炎细胞因子的mRNA表达水平。结果显示,DSS组小鼠肠道组织Ifn-γ、Il-1β、Il-6和Tnf-α细胞因
子的mRNA水平较Normal组显著上调,而给予CeNP-PEG治疗的小鼠促炎细胞因子mRNA水平较DSS组显著降低(均P=0.000),但仍显著高于Normal组(均P=0.000)(图4)。上述结果提示结肠炎小鼠肠道组织中Ifn-γ、Il-1β、Il-6和Tnf-α等促炎细胞因子的表达显著上调,应用CeNP-PEG治疗可明显抑制肠道促炎细胞因子表达。
图4
图4
CeNP-PEG对结肠炎小鼠肠道促炎细胞因子表达水平的影响
Note: A. Ifn-γ mRNA level. ①P=0.000, compared with the DSS group; ②P=0.000, compared with the DSS+CeNP-PEG group; ③P=0.000, compared with the Normal group. B. Il-1β mRNA level. ①P=0.000, compared with the DSS group; ②P=0.000, compared with the DSS+CeNP-PEG group; ③P=0.000, compared with the Normal group. C. Il-6 mRNA level. ①P=0.000, compared with the DSS group; ②P=0.000, compared with the DSS+CeNP-PEG group; ③P=0.000, compared with the Normal group. D. Tnf-α mRNA level. ①P=0.000, compared with the DSS group; ②P=0.000, compared with the DSS+CeNP-PEG group; ③P=0.000, compared with the Normal group.
Fig 4
Effect of CeNP-PEG on the expression of proinflammatory cytokines in the colonic tissues of colitis mice
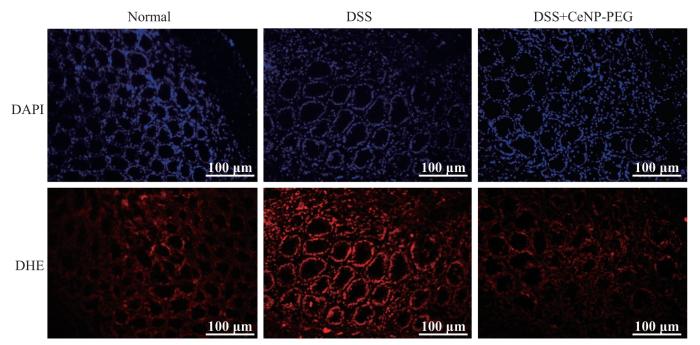
2.5 CeNP-PEG对结肠炎小鼠肠道ROS水平的影响
提取造模终止日Normal组、DSS组和CeNP-PEG治疗组小鼠的结肠组织,制备冰冻切片并进行DHE染色。DHE染色后荧光强度代表ROS的含量,结果显示,DSS组小鼠结肠组织的荧光强度较PBS组明显增加,而CeNP-PEG治疗组结肠组织荧光强度较DSS组明显减弱(图5)。提示DSS组小鼠肠道组织ROS水平明显增加,CeNP-PEG可以有效清除结肠炎小鼠肠道组织中过量的ROS。
图5
图5
CeNP-PEG对结肠炎小鼠结肠组织ROS水平的影响
Fig 5
Effect of CeNP-PEG on ROS levels in the colonic tissues of colitis mice
3 讨论
IBD传统的治疗药物具有疗效短暂、不良反应大、缓解率低等劣势[12]。虽然新型的生物制剂显著提升了IBD患者的疗效,但长期缓解率仍然不尽如人意。究其原因,IBD的难治愈性跟其持续存在的促炎微环境密切相关,巨噬细胞、T细胞和上皮细胞等在促炎微环境中发生交互作用,维持或扩大促炎微环境,阻碍肠道黏膜上皮的完全修复与愈合。巨噬细胞作为固有免疫最重要的细胞之一,也是肠道微环境中数量最多的免疫细胞。巨噬细胞具有良好的可塑性,表现为在不同的微环境中转化为不同的表型发挥不同的作用[13]。在IBD的炎症微环境中,它表现为促炎型,通过激活NF-κB转录因子,生成释放IFN-γ、IL-1β、IL-6、TNF-α和ROS,促进炎症发展[14]。LIU等人[15]对UC和CD患者炎症部位的结肠黏膜进行单细胞测序(scRNA-sequence,scRNA-seq),分析巨噬细胞的基因型特征,发现具有促炎效应的M1型亚群基因型明显增强,提示促炎型巨噬细胞在IBD炎症发展中的重要作用。本次研究结果显示,CeNP-PEG干预后巨噬细胞相关促炎NF-κB通路活化状态明显减弱。这与本课题组之前的研究结果一致。之前的研究[8]结果提示CeNP-PEG可以消除氧化应激中产生的过量ROS,抑制NF-κB信号通路,使小胶质细胞由M1促炎表型转化为M2抑炎表型。DAI评分是DSS诱导的小鼠结肠炎模型的通用评分标准,通过体质量和大便情况来评估造模小鼠的肠道炎症活动度。本研究的DAI评分和DHE染色结果显示:CeNP-PEG干预后,DSS小鼠肠道炎症减轻,疾病活动度明显降低,肠道组织ROS含量明显减少,显示出CeNP-PEG对肠道炎症组织ROS的清除效果,提示CeNP-PEG可能通过消除ROS改善结肠炎小鼠的疾病活动度。越来越多的研究显示,ROS对于巨噬细胞促炎基因的表达至关重要[16]。本研究结果显示,CeNP-PEG能够显著抑制Il-1β、Il-6、Tnf-α和Ifn-γ的基因表达。ORÓ等人[17]发现CeO2治疗可以下调IL-1β、TNF-α等促炎因子,以及巨噬细胞促炎表型标志物诱导型一氧化氮合酶(INOS)的基因表
达及生成,减轻肝纤维化引起的炎症反应。类似地,在其他炎症相关性疾病的模型中应用纳米铈颗粒治疗后也观察到NF-κB通路的活化减弱。如KHURANA等人[18]使用纳米铈颗粒治疗1型糖尿病模型取得了良好的效果,机制研究显示纳米铈可以明显抑制NF-κB通路的活化并使促炎因子IL-6和TNF-α的水平显著下降。
综上所述,本研究应用CeNP-PEG治疗可明显减轻结肠炎小鼠的炎症活动度并且改善IBD的促炎微环境,是一种极具潜力的IBD治疗方法。本研究结果有望为IBD治疗提供以改善免疫微环境并重塑肠道稳态为导向的治疗新策略。
作者贡献声明
卢雨涵、石亚红和吴颖为参与实验设计,卢雨涵和吴颖为参与论文的写作和修改。卢雨涵、石亚红和龙满美参与实验操作。石亚红负责数据分析。王子负责CeNP-PEG合成、优化和表征测定。所有作者均阅读并同意了最终稿件的提交。
AUTHOR's CONTRIBUTIONS
The study was designed by LU Yuhan, SHI Yahong and WU Yingwei. The manuscript was drafted and revised by LU Yuhan and WU Yingwei. The experiments were performed by LU Yuhan, SHI Yahong and LONG Manmei. The data were analyzed by SHI Yahong. The CeNP-PEG was synthesized, optimized and characterized by WANG Zi. All the authors have read the last version of paper and consented for submission.
利益冲突声明
所有作者声明不存在利益冲突。
COMPETING INTERESTS
All authors disclose no relevant conflict of interests.
参考文献