PE动物模型治疗性研究的评价大多需要终止妊娠来观察妊娠结局,因此针对干预后整个妊娠过程中胎盘血流灌注的改善情况,缺乏有效的监测手段。既往研究[11-13]多通过核磁共振、超声造影的方式进行检测,但这些方法复杂且昂贵,因此选择简单、方便、可信度较高的检测手段来反映PE治疗性效果非常必要。彩色超声多普勒检查在临床上既可检测胎儿的系统生长发育,也可对子宫胎盘的血流动力学进行检测,是一种重要的、无创辅助检查手段。本研究通过检测PE大鼠动物模型的脐动脉、子宫动脉以及胎鼠大脑中动脉超声多普勒流参数,并与胎盘微血管密度、妊娠结局包括胎盘、胎鼠质量进行相关性分析,以期对PE动物模型的胎盘血管灌注情况的评估提供参考。
1 对象与方法
1.1 实验对象
10周龄左右的SPF级别Sprague-Dawley(SD)大鼠,体质量为250~280 g,雌性20只、雄性10只,购自北京维通利华实验动物技术有限公司,饲养于华中科技大学同济医学院实验动物中心。
1.2 实验仪器及试剂
超声多普勒成像系统(西门子公司,美国),无创血压计(北京软隆公司),显微镜(Nikon公司,美国),酶标仪(Thermo公司,美国)。L-NAME(Abcam公司,美国),10%水合氯醛(武汉协和医院药剂科),戊巴比妥钠(国药集团化学试剂有限公司),0.9%氯化钠溶液(武汉协和医院药剂科),CBB试剂盒(南京建成科技有限公司),CD31抗体(Abcam公司,美国),过氧化物酶染色试剂盒(北京索莱宝科技有限公司),苏木素染色液(珠海贝索生物技术有限公司)。
1.3 研究方法
1.3.1 PE孕鼠模型的建立及分组
每日19点按照雌雄鼠2∶1的比例进行合笼,次日使用阴道涂片法,若显微镜下观察见精子,则为妊娠(gestational day,GD)第1日(GD1)。将成功妊娠的大鼠纳入研究,最终按照动物体质量匹配随机分为2组,2组均为8只孕鼠。在GD10、11、12时,随机对其中一组进行腹腔注射剂量为50 mg/kg的L-NAME,以建立PE动物模型,即为PE组。另一组则腹腔注射相同剂量的无菌性0.9%氯化钠溶液,即为NP组。
1.3.2 血压测量和尿蛋白浓度检测
在GD15、19时,使用无创血压仪测量大鼠尾动脉收缩压(systolic blood pressure,SBP)并记录,共测量6次,除去前2次测量结果,最后取平均值。
测量血压后利用代谢笼收集24 h尿液,利用CCB试剂盒测尿蛋白浓度并记录。
1.3.3 超声多普勒成像系统检测胎盘血流灌注的情况
GD19时,使用10 %水合氯醛对每只孕鼠进行麻醉,剂量为0.3 mL/100g。超声仪器设置条件:频率15 MHz,功率100%,增益20 dB。超声探头选择浅表超声探头。
(1)超声检测
由具备丰富超声经验的超声科医师操作,麻醉后大鼠在37 ℃的加热平台上与温度/生理监护仪相连。检测区域为大鼠腹部,对该区域继续备皮处理。由于大鼠多胎的特性及肠管、前部胎鼠的遮挡等情况,远离腹壁的胎鼠无法准确成像,因此选择距离腹壁最近的胎鼠进行检测,以找到最准确清晰的图像。涂抹预热的耦合剂后,轻柔使用探头对腹部进行多切面扫描成像,寻找最合适切面评估,选择脐动脉、子宫动脉以及胎鼠大脑中动脉作为评估血管。分别记录收缩期峰值流速(peak systolic velocity,PS)、舒张末期流速(end diastolic flow velocity,ED)和时间平均血流速度(time average velocity,TAV)。计算脐动脉及子宫动脉以及胎鼠大脑中动脉的阻力指数(resistance index,RI)、S/D和搏动指数(pulsation index,PI),PI=(PS-ED)/TAV,RI=(PS-ED)/PS,S/D=PS/ED。
(2)脐动脉血流检测方法
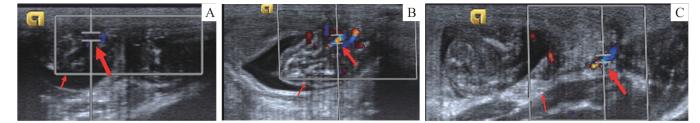
均选择近胎鼠端相对固定的部位进行脐带血流的检测。通过彩超在反复滑动操作中寻找胎鼠脐带,找到后,将取样容积放置于需要测定段,适当调整取样容积大小并调整多普勒取样角度。当观察到一个较清晰、均一的多普勒频谱时,进行图像冻结,每只胎鼠至少测量3个形态相似的多普勒频谱图,分别对此3个频谱数据进行测量(图1A)。
图1
图1
SD大鼠脐动脉、大脑中动脉、子宫动脉的超声成像
Note: A. Umbilical artery. Small arrow—fetal rat, large arrow—umbilical cord. B. Middle cerebral artery. Small arrow—fetal rat head, large arrow—middle cerebral artery. C. Uterine artery. Small arrow—lateral edge of the rat uterus, large arrow—uterine artery.
Fig 1
Ultrasound imaging of the umbilical artery, middle cerebral artery and uterine artery in SD rats
(3)大脑中动脉血流检测方法
适当调整移动探头角度确定切面,寻找胎鼠的头围切面,找到大脑脚,使用彩色超声模式显示基底动脉环,此环左右两侧即为大脑中动脉。同上述脐动脉检测手段,将取样容积置于动脉中部即可以获取到多普勒血流频谱图,同样每只胎鼠至少取3张图片(图1B)。
(4)子宫动脉血流检测方法
SD大鼠的子宫形态同人类稍有不同,呈“V”型,孕后呈现似串珠样。在寻找子宫动脉时,需要找到大鼠子宫纵切面的图形,向左右两侧沿着子宫肌壁外侧缘缓慢且仔细寻找子宫动脉。由于大鼠子宫动脉来源于卵巢动脉,一般在近卵巢处可以找到该动脉。同上述方法获取多普勒血流频谱图(图1C)。
1.3.4 孕鼠的胎盘、胎鼠组织
在GD20时,使用3%戊巴比妥钠(30 mg/kg)对孕鼠进行麻醉,并在操作台上进行剖宫产,取出胎盘和胎鼠,计数并称量质量。将胎盘组织保存在4%多聚甲醛溶液中,进行下一步的免疫组化染色。
1.3.5 胎盘免疫组化染色
进行胎盘CD31免疫组化染色,明确胎盘微血管变化。多聚甲醛固定胎盘组织后,石蜡包埋及切片。石蜡切片脱蜡至水、抗原修复:将切片放入二甲苯Ⅰ中10 min,再次放入二甲苯Ⅱ中10 min,再利用无水乙醇Ⅰ固定10 min,无水乙醇Ⅱ浸泡10 min,再利用85 %乙醇和75 %乙醇分别浸泡5 min后用双蒸水清洗。然后将组织切片置于盛满EDTA抗原修复缓冲液的修复盒中。利用微波炉进行抗原修复冷却后用PBS清洗3次,并用3%H2O2进行内消,用双蒸水清洗5 min。孵育抗体:用5%BSA封闭液放于湿盒内室温封闭1 h。甩干封闭液后在切片上滴加CD31抗体,稀释比例1∶100。4 ℃过夜,第2日将切片洗涤甩干后加入二抗,稀释比例为1∶500。染核封片镜检:片子滴加DAB溶液,镜下观察显色后即可终止染色,并用苏木精复染细胞核。脱水封片后镜下观察。
1.4 统计学方法
采用SPSS 15.0软件进行统计分析。所有数据均采用Prism软件记录并进行组间差异分析,定量资料采用x±s表示,定性资料采用n(%)表示。符合正态分布的组间分析采用t检验,不符合正态分布的数据采用秩和检验。选择Pearson相关性分析进行检验。P<0.05表示差异有统计学意义。
2 结果
2.1 血压及尿蛋白定量
GD15时,PE组的孕鼠尾动脉SBP显著高于NP组[(143.90±2.04) mmHg vs (112.60±2.89) mmHg,P=0.001],尿蛋白定量显著高于NP组[(4.00±0.52) μg/μL vs (1.62±0.21) μg/μL,P=0.001],提示PE大鼠动物模型建模成功。GD19时,尾动脉SBP和尿蛋白呈现相同的趋势。(表1)
表1 2组尾动脉血压及尿蛋白比较
Tab 1
| Item | |||
|---|---|---|---|
2.2 胎鼠及胎盘的血流动力学评价
超声多普勒评价胎盘血流灌注情况(表2~4,图2)。与NP组相比,PE组胎鼠脐动脉PS显著增高且差异具有统计学意义(P=0.008,表2),脐动脉ED、TAV、S/D差异无统计学意义(表2),脐动脉PI及RI显著升高且差异具有统计学意义(P=0.000)。关于子宫动脉的血流动力学情况的比较,其PS、ED、TAV在2组间差异无统计学意义(表3),但PE组的S/D、RI、PI显著升高且差异具有统计学意义(均P=0.000)。大脑中动脉的血流动力学情况比较,其PS、TAV在2组间差异无统计学意义(表4),ED在PE组显著升高且差异有统计学意义(表4),其S/D、PI、RI在PE组显著降低且差异有统计学意义(均P=0.000,表4)。
表2 超声评价2组胎鼠脐动脉血流动力学的比较
Tab 2
| Item | NP | PE | P value |
|---|---|---|---|
| 7.05±1.51 | 10.51±2.74 | 0.008 | |
| 2.08±0.78 | 1.59±0.71 | 0.203 | |
| 4.26±0.94 | 5.25±1.16 | 0.083 | |
| 3.61±0.97 | 13.95±18.00 | 0.127 | |
| 1.18±0.16 | 1.72±0.13 | 0.000 | |
| 0.71±0.07 | 0.88±0.06 | 0.000 |
表3 超声评价2组子宫动脉血流动力学的比较
Tab 3
| Item | NP group | PE group | P value |
|---|---|---|---|
| 16.27±3.93 | 0.203 | ||
| 7.98±2.50 | 0.067 | ||
| 11.05±3.26 | 0.751 | ||
| 2.05±0.16 | 0.000 | ||
| 0.77±0.10 | 0.000 | ||
| 0.51±0.44 | 0.000 |
表4 超声评价2组胎鼠大脑中动脉血流动力学的比较
Tab 4
| Item | NP | PE | P value |
|---|---|---|---|
| 0.264 | |||
| 0.000 | |||
| 0.073 | |||
| 0.000 | |||
| 0.000 | |||
| 0.000 |
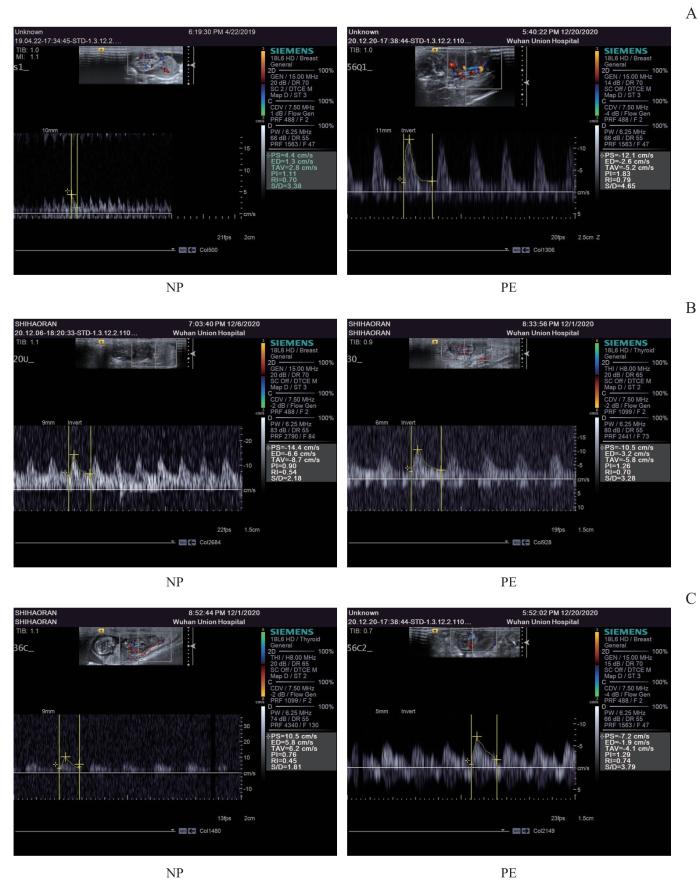
图2
图2
2组孕鼠的超声多普勒频谱图
Note: A. Umbilical artery. B. Uterine artery. C. Middle cerebral artery
Fig 2
Ultrasound Doppler spectrogram between the two groups
2.3 胎盘质量、胎鼠的数量和质量比较
通过大鼠解剖获得胎盘质量、胎鼠的数量和质量,结果见表5。胎鼠的数量在PE组和NP组间差异无统计学意义,PE组的胎盘质量(P=0.006)和胎鼠质量(P=0.000)均小于NP组。
表5 2组胎盘质量和胎鼠数量、质量比较
Tab 5
| Item | NP group | PE group | P value |
|---|---|---|---|
| Placental quality/ | 0.006 | ||
| Fetal rat quality/ | 4.20 | 0.000 | |
| Fetal number/n | 0.526 |
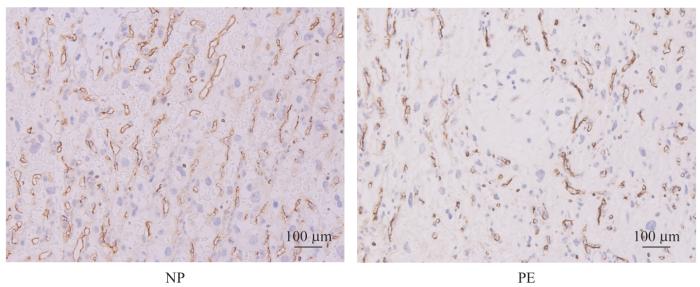
2.4 胎盘血管病理
表6 2组MVD比较
Tab 6
| P value | |||
|---|---|---|---|
图3
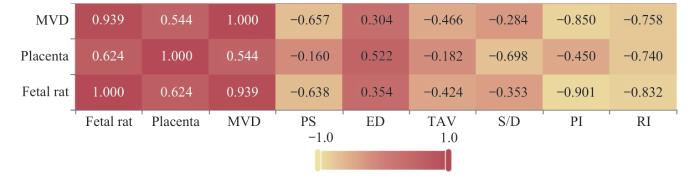
2.5 脐动脉、子宫动脉及大脑中动脉相关指标与胎盘质量、胎鼠质量及胎盘MVD的相关性分析
表7 脐动脉相关参数指标与胎盘质量、胎鼠质量及胎盘MVD的相关性分析[r (P)]
Tab 7
| Item | MVD | Placental quality | Fetal rat quality |
|---|---|---|---|
| PS | -0.657 (0.006) | -0.160 (0.554) | -0.638 (0.008) |
| ED | 0.304 (0.253) | 0.522 (0.038) | 0.354 (0.178) |
| TAV | -0.466 (0.069) | -0.182 (0.500) | -0.424 (0.102) |
| S/D | -0.284 (0.287) | -0.698 (0.003) | -0.353 (0.179) |
| PI | -0.850 (0.000) | -0.450 (0.081) | -0.901 (0.000) |
| RI | -0.758 (0.001) | -0.740 (0.001) | -0.832 (0.000) |
图4
图4
脐动脉相关参数指标与胎盘质量、胎鼠质量及胎盘MVD相关性分析热图
Note: Each number in the cells represents a correlation coefficient, with the depth of color indicating the magnitude of the value.
Fig 4
Heatmap of the correlation analysis between umbilical artery parameter indices and placental quality, fetal rat quality and placental MVD
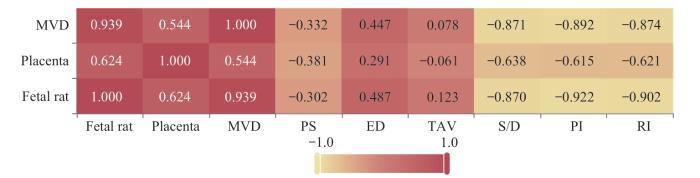
表8 子宫动脉相关参数指标与胎盘质量、胎鼠质量及胎盘MVD的相关性分析[r (P)]
Tab 8
| Item | MVD | Placental quality | Fetal rat quality |
|---|---|---|---|
| PS | -0.332 (0.209) | -0.381 (0.145) | -0.302 (0.256) |
| ED | 0.447 (0.083) | 0.291 (0.274) | 0.487 (0.056) |
| TAV | 0.078 (0.775) | -0.061 (0.824) | 0.123 (0.650) |
| S/D | -0.871 (0.000) | -0.638 (0.008) | -0.870 (0.000) |
| PI | -0.892 (0.000) | -0.615 (0.011) | -0.922 (0.000) |
| RI | -0.874 (0.000) | -0.621 (0.010) | -0.902 (0.000) |
图5
图5
子宫动脉相关参数指标与胎盘质量、胎鼠质量及胎盘MVD相关性分析热图
Note: Each number in the cells represents a correlation coefficient, with the depth of color indicating the magnitude of the value.
Fig 5
Heatmap of the correlation analysis between uterine artery parameter indices and placental quality, fetal rat quality and placental MVD
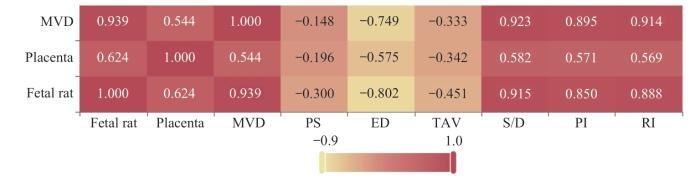
表9 大脑中动脉相关参数指标与胎盘质量、胎鼠质量及胎盘MVD的相关系数
Tab 9
| Item | MVD | Placental quality | Fetal rat quality |
|---|---|---|---|
| PS | -0.148 (0.586) | -0.196 (0.467) | -0.300 (0.260) |
| ED | -0.749 (0.001) | -0.575 (0.020) | -0.802 (0.000) |
| TAV | -0.333 (0.207) | -0.342 (0.195) | -0.451 (0.079) |
| S/D | 0.923 (0.000) | 0.582 (0.018) | 0.915 (0.000) |
| PI | 0.895 (0.000) | 0.571 (0.021) | 0.850 (0.000) |
| RI | 0.914 (0.000) | 0.569 (0.022) | 0.888 (0.000) |
图6
图6
大脑中动脉相关参数指标与胎盘质量、胎鼠质量及胎盘MVD相关性分析热图
Note: Each number in the cells represents a correlation coefficient, with the depth of color indicating the magnitude of the value.
Fig 6
Heatmap of the correlation analysis between middle cerebral artery parameter indices and placental quality, fetal rat quality and placental MVD
关(r=-0.575),与胎鼠质量呈现较强负相关(r=-0.802)。
3 讨论
PE是妊娠期特有的一种疾病,属于妊娠期高血压疾病,临床表现为血压异常升高及母体器官功能障碍[14-16]。其在孕产妇中的发病率为2%~5%,是发生孕产妇和新生儿死亡的主要原因之一[16]。PE的发病机制目前仍在研究中,但通常认为子宫螺旋动脉重铸不足在其中发挥了重要的作用。螺旋动脉重铸不足进而导致胎盘血流灌注减少,引发缺血缺氧、细胞因子释放,进一步诱发高血压、多器官损伤等一系列临床表现[17]。本研究PE动物模型的妊娠结果提示:PE组的胎鼠及胎盘质量显著低于NP组,原因可能是由于胎盘质量与胎盘中绒毛数量呈正相关性[18];而胎鼠及胎盘的数量在2组间不具有统计学差异,其原因可能是在妊娠中期使用L-NAME建模较妊娠早期建模对胎鼠数量的影响小;除此之外,不同种系对于妊娠并发症的敏感性可能不同,未导致明显的胎鼠流产,而仅发生胎鼠生长受限。
TRAVIS等[25]使用超声多普勒监测PE大鼠的子宫动脉,证实干扰素γ可以降低PE大鼠的子宫动脉PI。BIBEAU等[26]通过多普勒超声发现宫内生长受限的大鼠模型中存在子宫动脉、脐动脉的血流变化。本研究通过超声多普勒检测PE大鼠模型子宫动脉、脐动脉、大脑中动脉的PS、ED、TAV、S/D、PI、RI指标,并与胎盘MVD、胎盘、胎鼠质量进行相关性分析,结果发现PE大鼠动物模型中PI显著升高,反映了全身血管痉挛、外周阻力升高的病理生理机制,而脐动脉及胎鼠大脑中动脉的超声多普勒血流频谱则反映胎盘胎儿血流循环。随着PE的严重程度的增加,脐动脉PI增加,而大脑中动脉RI降低,且均与围产期的不良预后相关[27-28]。在本研究的PE大鼠模型中,呈现同样的趋势。
选择一种在动物模型妊娠期可重复性使用、创伤小的胎盘血供监测手段十分重要。相较于核磁共振、CT等昂贵设备,超声多普勒检测可在建模、干预治疗后多次使用,具有无创、方便等优势,更具可行性。然而,本研究尚存在样本量较小等不足之处,后续将进一步增加样本量为大鼠胎盘血流动力学变化的情况提供参考区间,进一步细化胎盘病理结果,深入研究胎盘病理变化情况。
作者贡献声明
赵茵负责课题和实验的设计,刘雨、徐桂香和史昊然负责实验操作,杨泽俊和毛艳辅助实验操作,刘雨和马瑞琳负责数据分析及作图,马瑞琳、刘雨、崔俭俭和赵茵参与论文的写作和修改。所有作者均阅读并同意了最终稿件的提交。
AUTHOR's CONTRIBUTIONS
The study was conceived and designed by ZHAO Yin.The study was implemented by LIU Yu, XU Guixiang and SHI Haoran, with YANG Zejun and MAO Yan providing assistance. The statistical analysis and graphing were performed by LIU Yu and MA Ruilin. The manuscript was drafted and revised by MA Ruilin, LIU Yu, CUI Jianjian and ZHAO Yin. All the authors have read the last version of paper and consented for submission.
利益冲突声明
所有作者声明不存在利益冲突。
COMPETING INTERESTS
All authors disclose no relevant conflict of interests.
参考文献