
上海交通大学学报(医学版) ›› 2024, Vol. 44 ›› Issue (8): 1023-1029.doi: 10.3969/j.issn.1674-8115.2024.08.011
吴望舒( ), 王旻洲, 宋阿会, 赵冰茹, 鲁嘉越, 洪文凯, 顾乐怡, 谢可炜, 陆任华(
), 王旻洲, 宋阿会, 赵冰茹, 鲁嘉越, 洪文凯, 顾乐怡, 谢可炜, 陆任华( )
)
收稿日期:2024-04-10
接受日期:2024-05-13
出版日期:2024-08-28
发布日期:2024-08-27
通讯作者:
陆任华,电子信箱:lurenhua1977@hotmail.com。作者简介:吴望舒(1999—),女,博士生;电子信箱:wangshu0825@qq.com。
基金资助:
WU Wangshu( ), WANG Minzhou, SONG Ahui, ZHAO Bingru, LU Jiayue, HONG Wenkai, GU Leyi, XIE Kewei, LU Renhua(
), WANG Minzhou, SONG Ahui, ZHAO Bingru, LU Jiayue, HONG Wenkai, GU Leyi, XIE Kewei, LU Renhua( )
)
Received:2024-04-10
Accepted:2024-05-13
Online:2024-08-28
Published:2024-08-27
Contact:
LU Renhua, E-mail: lurenhua1977@hotmail.com.Supported by:摘要:
目的·探索复方氨基酸胶囊治疗维持性血液透析患者营养不良及钙磷代谢障碍的有效性和安全性。方法·采用前瞻性、随机、对照、单中心的研究设计,40例上海交通大学医学院附属仁济医院的维持性血液透析患者随机分入治疗组(n=21)和对照组(n=19),治疗组在维持性血液透析常规治疗的基础上予口服复方氨基酸胶囊,对照组无特殊营养干预,每3月检测1次2组患者的血清白蛋白、前白蛋白、血红蛋白、铁蛋白,血钙、血磷、1, 25-二羟维生素D3[1, 25-(OH)2-D3]和全段甲状旁腺激素水平,并记录死亡、心脑血管意外、血管通路失功等不良事件的发生情况,共随访9个月。结果·治疗组血清白蛋白和前白蛋白水平在第6月和第9月时较基线升高(白蛋白t=3.574、5.599,均P<0.05;前白蛋白t/Z=-2.485、2.921,均P<0.05),对照组白蛋白在第9月开始升高,但增幅显著小于治疗组(t=3.877,P=0.001),前白蛋白水平则无明显变化。治疗组血红蛋白和铁蛋白水平在第3月即出现升高(血红蛋白t=2.192,铁蛋白t=2.994,均P<0.05)。治疗组血磷在第3月和第9月时较基线明显降低(t/Z=-2.743、-2.103,均P<0.05),而对照组血磷在第3月和第6月时无明显变化,第9月时较基线升高(Z=-2.178,P=0.029)。治疗组血钙和1, 25-(OH)2-D3水平在第3月和第6月均较基线升高(血钙t=4.581、4.922,均P=0.000;1, 25-(OH)2-D3t/Z=4.504、-2.374,均P<0.05),对照组血钙增幅显著小于同时期治疗组,1, 25-(OH)2-D3水平则无明显变化。2组患者的血全段甲状旁腺素水平、不良事件发生率及其他实验室指标在随访期内无统计学差异。结论·复方氨基酸胶囊在改善维持性血液透析患者营养状况、调节钙磷代谢方面有一定的疗效,且安全性高。
中图分类号:
吴望舒, 王旻洲, 宋阿会, 赵冰茹, 鲁嘉越, 洪文凯, 顾乐怡, 谢可炜, 陆任华. 复方氨基酸胶囊治疗维持性血液透析患者营养不良及钙磷代谢障碍的有效性和安全性[J]. 上海交通大学学报(医学版), 2024, 44(8): 1023-1029.
WU Wangshu, WANG Minzhou, SONG Ahui, ZHAO Bingru, LU Jiayue, HONG Wenkai, GU Leyi, XIE Kewei, LU Renhua. Efficacy and safety of compound amino acid capsules in the treatment of malnutrition and calcium and phosphorus metabolism disorders in maintenance hemodialysis patients[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2024, 44(8): 1023-1029.
| Item | Treatment group (n=21) | Control group (n=19) | t/χ2/U value | P value |
|---|---|---|---|---|
| Age/year | 64.67±10.75 | 59.37±12.78 | 1.424 | 0.163 |
| Male/n(%) | 14 (66.7) | 16 (84.2%) | 0.835① | 0.361 |
| Dialysis duration/month | 29.1 (64.4) | 34.9 (58.1) | 181.000② | 0.616 |
| Baseline body mass index/(kg·m-2) | 20.34±2.59 | 22.91±4.40 | -1.251 | 0.219 |
| Baseline Kt /V | 1.60±0.40 | 1.57±0.26 | 0.211 | 0.834 |
| Baseline ultrafiltration volume/mL | 2 400.00±989.95 | 2 742.11±697.07 | -1.273 | 0.211 |
| Primary renal disease/n(%) | 10.608① | 0.035 | ||
| Chronic glomerulonephritis | 6 (28.6) | 6 (31.6) | ||
| Hypertensive nephrosclerosis | 4 (20.0) | 4 (21.1) | ||
| Diabetic nephropathy | 0 (0) | 1 (5.3) | ||
| Polycystic kidney | 3 (7.5) | 0 (0) | ||
| Other | 5 (12.5) | 0 (0) | ||
| Unknown causes | 3 (27.5) | 8 (42.1) |
表1 2组一般资料比较
Tab 1 Comparison of general characteristics between the two groups
| Item | Treatment group (n=21) | Control group (n=19) | t/χ2/U value | P value |
|---|---|---|---|---|
| Age/year | 64.67±10.75 | 59.37±12.78 | 1.424 | 0.163 |
| Male/n(%) | 14 (66.7) | 16 (84.2%) | 0.835① | 0.361 |
| Dialysis duration/month | 29.1 (64.4) | 34.9 (58.1) | 181.000② | 0.616 |
| Baseline body mass index/(kg·m-2) | 20.34±2.59 | 22.91±4.40 | -1.251 | 0.219 |
| Baseline Kt /V | 1.60±0.40 | 1.57±0.26 | 0.211 | 0.834 |
| Baseline ultrafiltration volume/mL | 2 400.00±989.95 | 2 742.11±697.07 | -1.273 | 0.211 |
| Primary renal disease/n(%) | 10.608① | 0.035 | ||
| Chronic glomerulonephritis | 6 (28.6) | 6 (31.6) | ||
| Hypertensive nephrosclerosis | 4 (20.0) | 4 (21.1) | ||
| Diabetic nephropathy | 0 (0) | 1 (5.3) | ||
| Polycystic kidney | 3 (7.5) | 0 (0) | ||
| Other | 5 (12.5) | 0 (0) | ||
| Unknown causes | 3 (27.5) | 8 (42.1) |
| Item | Treatment group (n=21) | Control group (n=19) | t/U value | P value |
|---|---|---|---|---|
| Albumin/(g·L-1) | 35.20±3.05 | 37.47±2.19 | 102.000① | 0.008 |
| Prealbumin/(mg·L-1) | 284.51±77.54 | 312.38±61.80 | 1.248 | 0.220 |
| Hemoglobin/(g·L-1) | 102.14±14.64 | 112.05±15.52 | 2.078 | 0.045 |
| Ferritin/(μg·L-1) | 454.50 (390.90) | 247.70 (362.00) | 127.000① | 0.050 |
| Serum phosphorus/(mmol·L-1) | 2.09±0.35 | 1.87±0.38 | 125.500① | 0.045 |
| Serum calcium/(mmol·L-1) | 1.77±0.51 | 2.21±0.25 | 68.500① | 0.000 |
| 1,25-(OH)2-D3/(ng·mL-1) | 14.59±5.03 | 13.87±6.45 | 0.382 | 0.705 |
| iPTH/(pg·mL-1) | 221.00 (248.45) | 147.05 (194.33) | 155.000① | 0.338 |
| hsCRP/(mg·L-1) | 2.55 (8.81) | 1.53 (2.19) | 106.000① | 0.018 |
表2 2组基线期实验室指标比较
Tab 2 Comparison of baseline parameters between the two groups
| Item | Treatment group (n=21) | Control group (n=19) | t/U value | P value |
|---|---|---|---|---|
| Albumin/(g·L-1) | 35.20±3.05 | 37.47±2.19 | 102.000① | 0.008 |
| Prealbumin/(mg·L-1) | 284.51±77.54 | 312.38±61.80 | 1.248 | 0.220 |
| Hemoglobin/(g·L-1) | 102.14±14.64 | 112.05±15.52 | 2.078 | 0.045 |
| Ferritin/(μg·L-1) | 454.50 (390.90) | 247.70 (362.00) | 127.000① | 0.050 |
| Serum phosphorus/(mmol·L-1) | 2.09±0.35 | 1.87±0.38 | 125.500① | 0.045 |
| Serum calcium/(mmol·L-1) | 1.77±0.51 | 2.21±0.25 | 68.500① | 0.000 |
| 1,25-(OH)2-D3/(ng·mL-1) | 14.59±5.03 | 13.87±6.45 | 0.382 | 0.705 |
| iPTH/(pg·mL-1) | 221.00 (248.45) | 147.05 (194.33) | 155.000① | 0.338 |
| hsCRP/(mg·L-1) | 2.55 (8.81) | 1.53 (2.19) | 106.000① | 0.018 |
| Time | Treatment group (n=21) | Control group (n=19) | ||||
|---|---|---|---|---|---|---|
| Serum albumin/(g·L-1) | t value① | P value① | Serum albumin/(g·L-1) | t value① | P value① | |
| Baseline | 35.20±3.05 | ‒ | ‒ | 37.47±2.19 | ‒ | ‒ |
| 3-month | 36.41±3.74 | 1.700 | 0.105 | 38.57±2.92 | 2.057 | 0.054 |
| 6-month | 37.53±2.75 | 3.574 | 0.002 | 38.01±3.22 | 1.173 | 0.256 |
| 9-month | 39.42±2.73 | 5.599 | 0.000 | 38.92±1.73 | 3.670 | 0.002 |
表3 2组血清白蛋白水平比较
Tab 3 Comparison of serum albumin between the two groups
| Time | Treatment group (n=21) | Control group (n=19) | ||||
|---|---|---|---|---|---|---|
| Serum albumin/(g·L-1) | t value① | P value① | Serum albumin/(g·L-1) | t value① | P value① | |
| Baseline | 35.20±3.05 | ‒ | ‒ | 37.47±2.19 | ‒ | ‒ |
| 3-month | 36.41±3.74 | 1.700 | 0.105 | 38.57±2.92 | 2.057 | 0.054 |
| 6-month | 37.53±2.75 | 3.574 | 0.002 | 38.01±3.22 | 1.173 | 0.256 |
| 9-month | 39.42±2.73 | 5.599 | 0.000 | 38.92±1.73 | 3.670 | 0.002 |
| Comparison | Serum albumin increase of treatment group/[g·L-1 (95% CI)] | Serum albumin increase of control group/[g·L-1 (95% CI)] | t value | P value |
|---|---|---|---|---|
| 3-month vs baseline | 1.21 (-0.27‒2.69) | 1.10 (-0.02‒2.22) | 0.121 | 0.904 |
| 6-month vs baseline | 2.33 (0.97‒3.70) | 0.53 (-0.42‒1.48) | 2.222 | 0.032 |
| 9-month vs baseline | 4.22 (2.65‒5.80) | 1.08 (0.46‒1.71) | 3.877 | 0.001 |
表4 2组血清白蛋白增幅比较
Tab 4 Comparison of serum albumin increase between the two groups
| Comparison | Serum albumin increase of treatment group/[g·L-1 (95% CI)] | Serum albumin increase of control group/[g·L-1 (95% CI)] | t value | P value |
|---|---|---|---|---|
| 3-month vs baseline | 1.21 (-0.27‒2.69) | 1.10 (-0.02‒2.22) | 0.121 | 0.904 |
| 6-month vs baseline | 2.33 (0.97‒3.70) | 0.53 (-0.42‒1.48) | 2.222 | 0.032 |
| 9-month vs baseline | 4.22 (2.65‒5.80) | 1.08 (0.46‒1.71) | 3.877 | 0.001 |
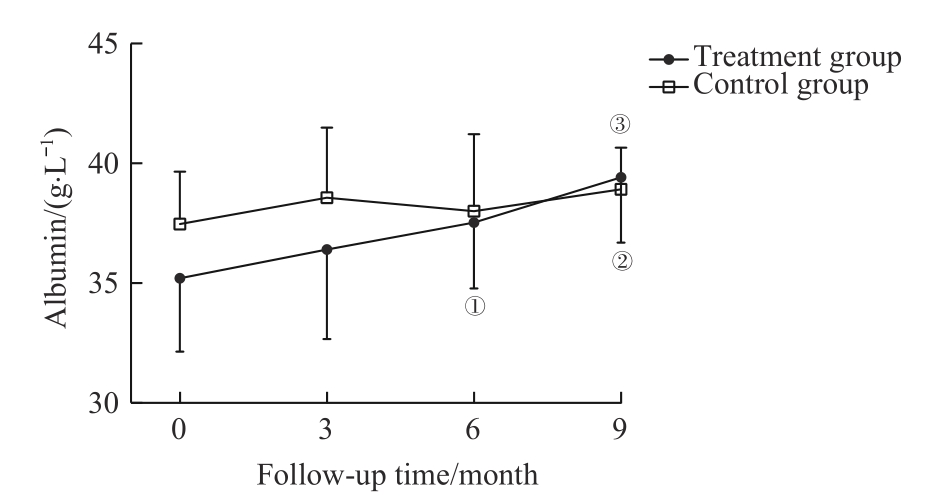
图1 2组血清白蛋白水平比较Note: ①P=0.002, ②P=0.000, compared with the baseline serum albumin of the treatment group; ③P=0.002, compared with the baseline serum albumin of the control group.
Fig 1 Comparison of serum albumin between the two groups
| Group | Serum prealbumin/(mg·L-1) | t/Z value① | P value① | Hemoglobin/ (g·L-1) | t value① | P value① | Serum ferritin/(μg·L-1) | t/Z value① | P value① |
|---|---|---|---|---|---|---|---|---|---|
| Treatment group | |||||||||
| Baseline | 284.51±77.54 | ‒ | ‒ | 102.14±14.64 | ‒ | ‒ | 454.50 (390.90) | ‒ | ‒ |
| 3-month | 267.21±78.38 | -1.382 | 0.183 | 108.24±15.57 | 2.192 | 0.040 | 580.40 (639.70) | 2.994 | 0.007 |
| 6-month | 316.58±80.11 | -2.485② | 0.013 | 106.76±16.32 | 1.199 | 0.245 | 502.20 (450.15) | -1.894② | 0.058 |
| 9-month | 320.77±57.98 | 2.921 | 0.008 | 115.29±13.63 | 3.739 | 0.001 | 488.50 (451.25) | 1.889 | 0.074 |
| Control group | |||||||||
| Baseline | 312.38±61.80 | ‒ | ‒ | 112.05±15.52 | ‒ | ‒ | 247.70 (362.00) | ‒ | ‒ |
| 3-month | 296.45±55.78 | -1.961 | 0.066 | 113.89±20.37 | 0.377 | 0.711 | 350.50 (345.90) | 1.632 | 0.120 |
| 6-month | 313.83±62.77 | 0.155 | 0.879 | 111.63±13.10 | -0.184 | 0.856 | 214.30 (373.10) | -0.080② | 0.936 |
| 9-month | 337.47±61.95 | 1.775 | 0.094 | 119.94±15.93 | 1.704 | 0.107 | 250.25 (384.95) | 0.113 | 0.911 |
表5 2组血清前白蛋白、血红蛋白及血清铁蛋白水平比较
Tab 5 Comparison of serum prealbumin, hemoglobin and ferritin between the two groups
| Group | Serum prealbumin/(mg·L-1) | t/Z value① | P value① | Hemoglobin/ (g·L-1) | t value① | P value① | Serum ferritin/(μg·L-1) | t/Z value① | P value① |
|---|---|---|---|---|---|---|---|---|---|
| Treatment group | |||||||||
| Baseline | 284.51±77.54 | ‒ | ‒ | 102.14±14.64 | ‒ | ‒ | 454.50 (390.90) | ‒ | ‒ |
| 3-month | 267.21±78.38 | -1.382 | 0.183 | 108.24±15.57 | 2.192 | 0.040 | 580.40 (639.70) | 2.994 | 0.007 |
| 6-month | 316.58±80.11 | -2.485② | 0.013 | 106.76±16.32 | 1.199 | 0.245 | 502.20 (450.15) | -1.894② | 0.058 |
| 9-month | 320.77±57.98 | 2.921 | 0.008 | 115.29±13.63 | 3.739 | 0.001 | 488.50 (451.25) | 1.889 | 0.074 |
| Control group | |||||||||
| Baseline | 312.38±61.80 | ‒ | ‒ | 112.05±15.52 | ‒ | ‒ | 247.70 (362.00) | ‒ | ‒ |
| 3-month | 296.45±55.78 | -1.961 | 0.066 | 113.89±20.37 | 0.377 | 0.711 | 350.50 (345.90) | 1.632 | 0.120 |
| 6-month | 313.83±62.77 | 0.155 | 0.879 | 111.63±13.10 | -0.184 | 0.856 | 214.30 (373.10) | -0.080② | 0.936 |
| 9-month | 337.47±61.95 | 1.775 | 0.094 | 119.94±15.93 | 1.704 | 0.107 | 250.25 (384.95) | 0.113 | 0.911 |
| Group | Serum phosphorus/(mmol·L-1) | t/Z value① | P value① | Serum calcium/(mmol·L-1) | t/Z value① | P value① | 1,25-(OH)2-D3/(ng·mL-1) | t/Z value① | P value① | iPTH/ (pg·mL-1) | t/Z value① | P value① |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | ||||||||||||
| Baseline | 2.09±0.35 | ‒ | ‒ | 1.77±0.51 | ‒ | ‒ | 14.59±5.03 | ‒ | ‒ | 221.00 (248.45) | ‒ | ‒ |
| 3-month | 1.67±0.45 | -2.743 | 0.013 | 2.35±0.21 | 4.581 | 0.000 | 18.03±5.02 | 4.504 | 0.000 | 156.60 (276.15) | -0.362 | 0.722 |
| 6-month | 1.87±0.63 | -1.568② | 0.117 | 2.32±0.19 | 4.922 | 0.000 | 18.31±8.74 | -2.374② | 0.018 | 218.00 (379.25) | 1.045 | 0.308 |
| 9-month | 1.81±0.38 | -2.103② | 0.035 | 2.42±0.25 | 5.008 | 0.000 | 15.50±8.07 | -0.282② | 0.778 | 211.20 (307.15) | -0.330② | 0.741 |
| Control group | ||||||||||||
| Baseline | 1.87±0.38 | ‒ | ‒ | 2.21±0.25 | ‒ | ‒ | 13.87±6.45 | ‒ | ‒ | 147.05 (194.33) | ‒ | ‒ |
| 3-month | 1.79±0.53 | -1.132② | 0.257 | 2.39±0.30 | -2.571② | 0.010 | 16.14±9.48 | -1.087② | 0.277 | 176.50 (270.35) | 0.000② | >0.999 |
| 6-month | 1.84±0.46 | -0.202 | 0.842 | 2.32±0.19 | 2.06 | 0.054 | 15.90±11.08 | -0.563② | 0.573 | 148.60 (189.88) | -0.675 | 0.509 |
| 9-month | 2.23±0.57 | -2.178② | 0.029 | 2.34±0.22 | -2.202② | 0.028 | 12.99±8.14 | -0.679 | 0.506 | 178.50 (209.18) | -0.081 | 0.936 |
表6 2组血磷、血钙、1,25-(OH) 2-D3 及iPTH水平比较
Tab 6 Comparison of serum phosphorus, serum calcium, 1,25-(OH)2-D3 and iPTH between the two groups
| Group | Serum phosphorus/(mmol·L-1) | t/Z value① | P value① | Serum calcium/(mmol·L-1) | t/Z value① | P value① | 1,25-(OH)2-D3/(ng·mL-1) | t/Z value① | P value① | iPTH/ (pg·mL-1) | t/Z value① | P value① |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | ||||||||||||
| Baseline | 2.09±0.35 | ‒ | ‒ | 1.77±0.51 | ‒ | ‒ | 14.59±5.03 | ‒ | ‒ | 221.00 (248.45) | ‒ | ‒ |
| 3-month | 1.67±0.45 | -2.743 | 0.013 | 2.35±0.21 | 4.581 | 0.000 | 18.03±5.02 | 4.504 | 0.000 | 156.60 (276.15) | -0.362 | 0.722 |
| 6-month | 1.87±0.63 | -1.568② | 0.117 | 2.32±0.19 | 4.922 | 0.000 | 18.31±8.74 | -2.374② | 0.018 | 218.00 (379.25) | 1.045 | 0.308 |
| 9-month | 1.81±0.38 | -2.103② | 0.035 | 2.42±0.25 | 5.008 | 0.000 | 15.50±8.07 | -0.282② | 0.778 | 211.20 (307.15) | -0.330② | 0.741 |
| Control group | ||||||||||||
| Baseline | 1.87±0.38 | ‒ | ‒ | 2.21±0.25 | ‒ | ‒ | 13.87±6.45 | ‒ | ‒ | 147.05 (194.33) | ‒ | ‒ |
| 3-month | 1.79±0.53 | -1.132② | 0.257 | 2.39±0.30 | -2.571② | 0.010 | 16.14±9.48 | -1.087② | 0.277 | 176.50 (270.35) | 0.000② | >0.999 |
| 6-month | 1.84±0.46 | -0.202 | 0.842 | 2.32±0.19 | 2.06 | 0.054 | 15.90±11.08 | -0.563② | 0.573 | 148.60 (189.88) | -0.675 | 0.509 |
| 9-month | 2.23±0.57 | -2.178② | 0.029 | 2.34±0.22 | -2.202② | 0.028 | 12.99±8.14 | -0.679 | 0.506 | 178.50 (209.18) | -0.081 | 0.936 |
| Scope | Treatment group (n=21) | Control group (n=19) | χ2 value | P value |
|---|---|---|---|---|
| Total adverse event/n(%) | 1 (4.76) | 4 (21.05) | 1.160 | 0.281 |
| Cardio-cerebrovascular accidents/n(%) | 1 (4.76) | 3 (15.79) | 0.401 | 0.527 |
| Vascular access failure/n(%) | 0 (0) | 1 (4.76) | ‒ | 0.475 |
表7 2组不良事件发生情况比较
Tab 7 Comparison of adverse event incidence between the two groups
| Scope | Treatment group (n=21) | Control group (n=19) | χ2 value | P value |
|---|---|---|---|---|
| Total adverse event/n(%) | 1 (4.76) | 4 (21.05) | 1.160 | 0.281 |
| Cardio-cerebrovascular accidents/n(%) | 1 (4.76) | 3 (15.79) | 0.401 | 0.527 |
| Vascular access failure/n(%) | 0 (0) | 1 (4.76) | ‒ | 0.475 |
| 1 | LIYANAGE T, NINOMIYA T, JHA V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review[J]. Lancet, 2015, 385(9981): 1975-1982. |
| 2 | SABATINO A, REGOLISTI G, KARUPAIAH T, et al. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis[J]. Clin Nutr, 2017, 36(3): 663-671. |
| 3 | HARA H, NAKAMURA Y, HATANO M, et al. Protein energy wasting and sarcopenia in dialysis patients[J]. Contrib Nephrol, 2018, 196: 243-249. |
| 4 | NAGY E, MAHMOUD M, EL-KANNISHY G, et al. Impact of malnutrition on health-related quality of life in patients on maintenance hemodialysis[J]. Ther Apher Dial, 2021, 25(4): 467-474. |
| 5 | 傅鹏, 袁伟杰. 复方氨基酸胶囊纠正慢性肾功能衰竭血液透析伴低蛋白血症的疗效观察[J]. 中国药房, 2005, 16(10): 764-766. |
| FU P, YUAN W J. Effects of compound amino acids capsules on hypoalbuminemia in patients with chronic renal failure undergoing hemodialysis[J]. China Pharmacy, 2005, 16(10): 764-766. | |
| 6 | CARRERO J J, THOMAS F, NAGY K, et al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism[J]. J Ren Nutr, 2018, 28(6): 380-392. |
| 7 | MACLAUGHLIN H L, FRIEDMAN A N, IKIZLER T A. Nutrition in kidney disease: core curriculum 2022[J]. Am J Kidney Dis, 2022, 79(3): 437-449. |
| 8 | HANNA R M, GHOBRY L, WASSEF O, et al. A practical approach to nutrition, protein-energy wasting, sarcopenia, and Cachexia in patients with chronic kidney disease[J]. Blood Purif, 2020, 49(1/2): 202-211. |
| 9 | SHIRAI N, INOUE T, OGAWA M, et al. Relationship between nutrition-related problems and falls in hemodialysis patients: a narrative review[J]. Nutrients, 2022, 14(15): 3225. |
| 10 | BOLASCO P. Hemodialysis-nutritional flaws in diagnosis and prescriptions. could amino acid losses be the sharpest "sword of Damocles"?[J]. Nutrients, 2020, 12(6): 1773. |
| 11 | GRATEROL TORRES F, MOLINA M, SOLER-MAJORAL J, et al. Evolving concepts on inflammatory biomarkers and malnutrition in chronic kidney disease[J]. Nutrients, 2022, 14(20): 4297. |
| 12 | KLOPPENBURG W D, STEGEMAN C A, HOVINGA T K, et al. Effect of prescribing a high protein diet and increasing the dose of dialysis on nutrition in stable chronic haemodialysis patients: a randomized, controlled trial[J]. Nephrol Dial Transplant, 2004, 19(5): 1212-1223. |
| 13 | SAHATHEVAN S, KHOR B H, NG H M, et al. Understanding development of malnutrition in hemodialysis patients: a narrative review[J]. Nutrients, 2020, 12(10): 3147. |
| 14 | HENDRIKS F K, KOOMAN J P, VAN LOON L J C. Dietary protein interventions to improve nutritional status in end-stage renal disease patients undergoing hemodialysis[J]. Curr Opin Clin Nutr Metab Care, 2021, 24(1): 79-87. |
| 15 | IKIZLER T A, BURROWES J D, BYHAM-GRAY L D, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update[J]. Am J Kidney Dis, 2020, 76(3 Suppl 1): S1-S107. |
| 16 | MA L J, ZHAO S M. Risk factors for mortality in patients undergoing hemodialysis: a systematic review and meta-analysis[J]. Int J Cardiol, 2017, 238: 151-158. |
| 17 | JIANG N, QIAN J Q, LIN A W, et al. Low-protein diet supplemented with keto acids is associated with suppression of small-solute peritoneal transport rate in peritoneal dialysis patients[J]. Int J Nephrol, 2011, 2011: 542704. |
| 18 | 李福红, 孙彬, 田玉梅. 复方氨基酸胶囊辅助治疗血液透析患者肾性贫血的临床观察[J]. 临床肾脏病杂志, 2017, 17(10): 618-620. |
| LI F H, SUN B, TIAN Y M. Clinical observation of compound amino acid capsules in the supplementary treatment of renal anemia in hemodialysis patients[J]. Journal of Clinical Nephrology, 2017, 17(10): 618-620. | |
| 19 | HU L, NAPOLETANO A, PROVENZANO M, et al. Mineral bone disorders in kidney disease patients: the ever-current topic[J]. Int J Mol Sci, 2022, 23(20): 12223. |
| 20 | ZHOU C, SHI Z Y, OUYANG N, et al. Hyperphosphatemia and cardiovascular disease[J]. Front Cell Dev Biol, 2021, 9: 644363. |
| 21 | LI H M, LONG Q, SHAO C H, et al. Effect of short-term low-protein diet supplemented with keto acids on hyperphosphatemia in maintenance hemodialysis patients[J]. Blood Purif, 2011, 31(1/2/3): 33-40. |
| 22 | CASES A, CIGARRÁN-GULDRÍS S, MAS S, et al. Vegetable-based diets for chronic kidney disease? it is time to reconsider[J]. Nutrients, 2019, 11(6): 1263. |
| 23 | CARRERO J J, GONZÁLEZ-ORTIZ A, AVESANI C M, et al. Plant-based diets to manage the risks and complications of chronic kidney disease[J]. Nat Rev Nephrol, 2020, 16(9): 525-542. |
| 24 | HOSHINO J. Renal rehabilitation: exercise intervention and nutritional support in dialysis patients[J]. Nutrients, 2021, 13(5): 1444. |
| [1] | 魏珊, 纪鸥洋, 陈志豪, 黄泽慧, 李璞, 方均燕, 刘英莉. 维持性透析患者安全用药知信行现状调查及相关因素分析[J]. 上海交通大学学报(医学版), 2023, 43(1): 88-94. |
| [2] | 王亚琨, 许佳瑞, 吴茜茜, 张晓华, 朱迎春, 白寿军. 医养结合综合干预对上海郊区老年慢性肾脏病患者生活质量和精神状态的影响[J]. 上海交通大学学报(医学版), 2022, 42(7): 904-910. |
| [3] | 沈剑箫, 王万鹏, 邵兴华, 吴晶魁, 李舒, 车霞静, 倪兆慧. 顺铂诱导的急性肾损伤中肾脏组织m6A甲基化水平的变化[J]. 上海交通大学学报(医学版), 2021, 41(12): 1603-1611. |
| [4] | 黄泽慧, 胡春, 李璞, 张春丽, 方均燕, 宋阿会, 魏珊, 纪鸥洋, 佟琰, 邓海, 刘英莉. 居家腹膜透析患者自我管理量表的编制及信度、效度分析研究[J]. 上海交通大学学报(医学版), 2021, 41(7): 942-948. |
| [5] | 周悦玲,丁 巍,艾红兰,卢建新,丁 峰,胡 春. 维持性血液透析的终末期肾病患者脑结构性异常及认知功能分析[J]. 上海交通大学学报(医学版), 2020, 40(7): 962-967. |
| [6] | 王丽 1, 2,章倩莹 1,林涛 1,徐天 1,黄晓敏 1,张春燕 1,徐耀文 1,吴珮 1,陈楠 1,任红 1,谢静远 1. 腹膜炎是腹膜透析患者全因死亡及心血管死亡的独立危险因素[J]. 上海交通大学学报(医学版), 2019, 39(9): 1024-. |
| [7] | 周培慧,王丽. 白细胞介素 -27在顺铂所致急性肾损伤中的高表达及对细胞 凋亡的抑制作用[J]. 上海交通大学学报(医学版), 2019, 39(4): 372-. |
| [8] | 苏新玙 *,刘苗 *,俞赞喆,严豪,李振元,张贺,袁江姿,倪兆慧,方炜. 临床常用指标在腹膜透析患者容量状态评估中的价值[J]. 上海交通大学学报(医学版), 2018, 38(8): 910-. |
| [9] | 庞慧华,章海芬,车妙琳,俞赞喆,林星辉,朱铭力,陆任华,方妮娜,倪兆慧,顾乐怡. 维持性血液透析患者容量状况的评估[J]. 上海交通大学学报(医学版), 2018, 38(5): 524-. |
| [10] | 付玉玲1,胡坤1,沈艳萍1,施鑫2,乔青1. 持续非卧床腹膜透析患者成纤维生长因子 -23及可溶性klotho蛋白水平与心脏瓣膜钙化的关系[J]. 上海交通大学学报(医学版), 2018, 38(5): 541-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||