乳腺癌是女性较常见的浸润性癌症,发病率占女性恶性肿瘤的第一位[1]。绝大多数乳腺癌患者采取手术治疗。右美托咪定是一种中枢的α2-肾上腺素能受体激动剂,临床上常用于镇静和辅助全身麻醉。既往研究[2-3]发现,促炎因子、手术导致的交感应激、围术期高阿片类药物用量会促进肿瘤的发展;右美托咪定可通过调节炎症反应、减少麻醉药和阿片类药物的用量,降低与手术相关的促肿瘤因素对患者的影响,改善肿瘤患者的预后。然而,最近也有相佐证据:右美托咪定能够促进肿瘤生长复发,其主要通过直接刺激癌细胞的增殖[4-5]和改变肿瘤免疫微环境[6],促进神经胶质瘤、肺癌、结肠癌、乳腺癌的恶性发展[4-7]。因此,我们开展了这项针对乳腺癌手术患者的回顾性研究,采用倾向性评分匹配法尽量排除患者自身因素、肿瘤特征、手术因素、麻醉方式、麻醉药物、术后治疗方法等混杂因素的影响下,探讨术中使用小剂量右美托咪定对乳腺癌手术后5年内无复发生存率(recurrence-free survival,RFS)和总生存率(overall survival,OS)的影响,以期为肿瘤手术患者麻醉药物的合理选择提供理论参考。
1 资料与方法
1.1 一般资料
回顾性分析自2013年7月至2014年6月在上海交通大学医学院附属瑞金医院乳腺疾病诊治中心接受乳腺癌外科手术的患者临床资料,包括年龄,性别,体质量指数(body mass index,BMI),美国麻醉医师协会(American Society of Anesthesiologists,ASA)分级,麻醉方式,麻醉时长,麻醉药物,肿瘤分期(0、Ⅰ、Ⅱ或Ⅲ),手术类型(保乳手术或乳房切除术),是否接受放射治疗(放疗)、化学治疗(化疗)和/或激素治疗。纳入标准:①女性。②年龄18~80岁。③经空芯针穿刺活检或手术活检证实为浸润性乳腺癌。④ASA分级Ⅰ或Ⅱ级。⑤临床和随访资料完整。排除标准:①接受新辅助治疗。②被诊断为新发转移性(Ⅳ期)乳腺癌。③患严重心肺肝肾及血液系统疾病者。
1.2 麻醉方法
患者进入手术室后常规监测心率、血氧饱和度(SpO2)、无创血压、五导联心电图。2组患者依次静脉注射丙泊酚2~3 mg/kg、舒芬太尼0.3~0.5 g/kg、顺苯磺酸阿曲库铵0.2 mg/kg,进行麻醉诱导。患者气管插管后进行容量控制模式机械通气(潮气量7~10 mL/kg,呼吸频率12~15次/min),0.9~1.1 MAC(minimum alveolar concentration)的七氟醚或地氟醚、0.05~0.1 g/(kg·min)瑞芬太尼维持麻醉,术毕前根据需要追加舒芬太尼5~10 μg。根据术中是否滴注右美托咪定分为2组:右美组(滴注右美托咪定)及对照组(未滴注右美托咪定)。右美组术中滴注右美托咪定0.7~0.8 μg/kg(加至100 mL生理盐水中),在手术的前30 min内滴完;对照组滴注等量的乳酸林格液。无创收缩压低于基础值的20%者静脉注射麻黄碱5 mg,心率<45次/min者静脉注射阿托品0.25~0.5 mg。术中血压维持于80~130 mmHg/50~90 mmHg(1 mmHg=0.133 kPa)或基础值的±20%,心率维持50~100 次/min;术中采用Narcotrend(BadBramstedt公司,德国)监测麻醉深度,指数维持于35~60。
1.3 肿瘤指标检测
取病理诊断为浸润性乳腺癌的标本,用免疫组织化学(immunohistochemistry,IHC)测定样本的孕激素受体(progesterone receptor,PR)、雌激素受体(estrogen receptor,ER)、人表皮生长因子受体2(human epidermal growth factor receptor 2,HER2)和增殖细胞核抗原(Ki67)表达情况。根据2013年美国临床肿瘤学会(American Society of Clinical Oncology,ASCO)/美国病理学家协会(College of American Pathologists,CAP)指南,评估和确定所有标本的HER2表达状态。Ki67作为一种常用的乳腺癌增殖生物标志物,其表达水平越低,预后越好[8]。以20%作为Ki67高表达的分界值[9]。评估ER和PR的方法和阳性标准参考既往研究[9]。
1.4 观察指标和术后随访
分析右美组和对照组患者年龄、性别、BMI、ASA分级、肿瘤IHC指标、肿瘤TNM分期(Ⅰ、Ⅱ或Ⅲ)、手术类型(保乳手术或乳房切除术)以及接受放疗、化疗和/或激素治疗方面有无差异。采用门诊随访、住院随访或电话随访方式,对乳腺癌患者术后2年内每3个月随访1次,术后3~5年每半年随访1次,5年后每年随访1次。将末次随访或死亡时间定义为截止时间。乳腺癌复发根据临床、影像检查和/或病理活检信息进行判断。末次随访时间为2022年6月。
1.5 统计学分析
倾向性评分匹配法以是否应用右美托咪定为因变量,以年龄、BMI、化疗比例、放疗比例、激素治疗比例、ER阳性比例、PR阳性比例、Ki67评分、ASA分级、肿瘤TMN分期、手术类型、麻醉方法、麻醉时长、术中各种麻醉药用量、离开麻醉后监测治疗室(postanesthesia care unit,PACU)时视觉模拟评分法(visual analogue scale,VAS)评分等协变量为自变量,建立Logistic模型。根据倾向性评分匹配,应用最近距离匹配法对组间相同或相近的个体进行配对,创建1∶1配对(右美组与对照组)。
采用GraphPad Prism 9.0软件进行数据分析和处理。定量资料采用x±s表示,定性资料采用n(%)表示。Fisher精确检验或χ2检验评估2组患者定性资料的差异。采用Wilcoxon秩和检验评估2组患者定量资料的差异。倾向性评分匹配法卡钳值取0.02。通过绘制Kaplan-Meier曲线,统计右美组和对照组在术后第60个月无复发生存患者数和总生存患者数,与每组患者总数的比值,即为术后5年RFS和OS。Log-Rank检验评估2组患者生存曲线的差异。采用Cox比例风险回归模型评估各因素对预后的影响,行单因素分析和多因素分析,计算风险比(HR)及其95%置信区间(CI)。P<0.05表示差异有统计学意义。
2 结果
2.1 倾向性评分匹配前后2组患者基线特征及围术期资料比较
表1 倾向性评分匹配前2组患者基线特征及围术期资料比较
Tab 1
| Item | DEX group (n=385) | Control group (n=273) | P value |
|---|---|---|---|
| Age/year | 54.5±0.6 | 52.1±0.7 | 0.004 |
| BMI/(kg·m-2) | 23.2±0.2 | 23.3±0.2 | 0.002 |
| Chemotherapy/n(%) | 259 (67.3) | 180 (65.9) | 0.742 |
| Radiotherapy/n(%) | 176 (45.7) | 127 (46.5) | 0.871 |
| Hormonal therapy/n(%) | 236 (61.3) | 177 (64.8) | 0.365 |
| ER+/n(%) | 224 (58.2) | 171 (62.6) | 0.258 |
| PR+/n(%) | 155 (40.3) | 140 (51.3) | 0.011 |
| HER2+/n(%) | 94 (24.4) | 61 (22.3) | 0.576 |
| Ki67/n(%) | 209 (54.3) | 151 (55.3) | 0.812 |
| ASA classification/n(%) | 0.000 | ||
| Ⅰ | 206 (53.5) | 91 (33.3) | |
| Ⅱ | 179 (46.5) | 182 (66.7) | |
| TMN stage/n(%) | 0.937 | ||
| 0 | 50 (13.0) | 31 (11.4) | |
| Ⅰ | 155 (40.3) | 106 (38.8) | |
| ⅡA | 101 (26.2) | 77 (28.2) | |
| ⅡB | 36 (9.3) | 28 (10.3) | |
| Ⅲ | 43 (11.2) | 31 (11.4) | |
| Type of surgery/n(%) | 0.891 | ||
| Breast-conserving surgery | 111 (28.8) | 80 (29.3) | |
| Mastectomy | 274 (71.2) | 193 (70.7) | |
| Duration of anesthesia/min | 64.8±1.1 | 61.1±0.9 | 0.023 |
| Remifentanil/μg | 707.7±17.2 | 692.6±19.6 | 0.563 |
| Sufentanyl/μg | 34.5±0.5 | 34.2±0.6 | 0.638 |
| Propofol/mg | 142.0±1.2 | 138.6±1.4 | 0.074 |
| Cisatracurium/mg | 19.7±0.2 | 19.4±0.3 | 0.301 |
| Inhalable anesthetic/n(%) | 0.169 | ||
| Sevoflurane | 183 (47.5) | 115 (42.1) | |
| Desflurane | 202 (52.5) | 158 (57.9) | |
| VAS score when leaving PACU | 0.6±0.0 | 0.6±0.0 | 0.342 |
| Propensity score | 0.31±0.08 | 0.25±0.06 | 0.006 |
表2 倾向性评分匹配后2组患者基线特征及围术期资料比较
Tab 2
| Item | DEX group (n=239) | Control group (n=239) | P value |
|---|---|---|---|
| Age/year | 54.3±0.8 | 52.9±0.8 | 0.212 |
| BMI/(kg·m-2) | 23.1±0.2 | 23.0±0.2 | 0.681 |
| Chemotherapy/n(%) | 168 (70.3) | 161 (67.4) | 0.548 |
| Radiotherapy/n(%) | 119 (49.8) | 108 (45.2) | 0.357 |
| Hormonal therapy/n(%) | 116 (48.5) | 109 (45.6) | 0.582 |
| ER+/n(%) | 125 (52.3) | 141 (59.0) | 0.174 |
| PR+/n(%) | 158 (66.1) | 140 (58.6) | 0.113 |
| HER2+/n(%) | 60 (25.1) | 58 (24.3) | 0.916 |
| Ki67/n(%) | 129 (54.0) | 141 (59.0) | 0.308 |
| ASA classification/n(%) | 0.082 | ||
| Ⅰ | 109 (45.6) | 129 (54.0) | |
| Ⅱ | 130 (54.4) | 110 (46.0) | |
| TMN stage/n(%) | 0.531 | ||
| 0 | 24 (9.9) | 21 (8.5) | |
| Ⅰ | 103 (43.3) | 100 (41.9) | |
| ⅡA | 75 (31.2) | 79 (33.1) | |
| ⅡB | 10 (4.3) | 12 (5.1) | |
| Ⅲ | 27 (11.1) | 27 (11.4) | |
| Type of surgery/n(%) | 0.176 | ||
| Breast-conserving surgery | 6 (25.8) | 58 (24.3) | |
| Mastectomy | 177 (74.2) | 181 (75.7) | |
| Duration of anesthesia/min | 64.6±1.3 | 62.2±1.0 | 0.162 |
| Remifentanil/μg | 680.5±20.7 | 704.7±21.6 | 0.416 |
| Sufentanyl/μg | 34.0±0.7 | 34.2±0.7 | 0.781 |
| Propofol/mg | 141.9±1.5 | 138.2±1.4 | 0.073 |
| Cisatracurium/mg | 19.4±0.3 | 19.1±0.3 | 0.367 |
| Inhalable anesthetic/n(%) | 0.564 | ||
| Sevoflurane | 83 (34.8) | 76 (32.1) | |
| Desflurane | 156 (65.2) | 163 (67.9) | |
| VAS score when leaving PACU | 0.6±0.0 | 0.7±0.1 | 0.125 |
| Propensity score | 0.29±0.07 | 0.30±0.06 | 0.331 |
2.2 影响乳腺癌手术患者预后的单因素和多因素分析
在单因素分析中,年龄(P=0.032)、术后放疗(P=0.041)、Ki67评分(P=0.021)、肿瘤TMN分期(P=0.029)与术后5年OS显著相关。多因素分析结果显示,术后放疗、Ki67评分、肿瘤TMN分期与术后5年OS显著相关。术中滴注小剂量右美托咪定不是影响术后5年OS的危险因素(P>0.05)。结果见表3。
表3 乳腺癌患者术后生存风险影响因素分析
Tab 3
| Influencing factor | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR(95%CI) | P value | HR(95%CI) | P value | ||
| Age | 1.02 (1.00, 1.03) | 0.032 | 1.01 (0.99, 1.04) | 0.062 | |
| BMI | 1.01 (0.98, 1.03) | 0.891 | 1.00 (0.99, 1.01) | 0.603 | |
| Chemotherapy | 0.98 (0.95, 1.00) | 0.063 | 0.98 (0.95, 1.11) | 0.062 | |
| Radiotherapy | 0.54 (0.24, 1.47) | 0.041 | 0.55 (0.26, 1.54) | 0.033 | |
| Hormonal therapy | 1.10 (0.56, 1.48) | 0.732 | 0.93 (0.65, 1.33) | 0.562 | |
| ER+ | 0.98 (0.45, 1.54) | 0.087 | 1.12 (0.66, 1.69) | 0.118 | |
| PR+ | 1.09 (0.53, 1.44) | 0.228 | 1.22 (0.64, 1.73) | 0.215 | |
| HER2+ | 1.02 (0.51, 1.49) | 0.198 | 1.12 (0.58, 1.63) | 0.115 | |
| Ki67 | 1.15 (1.01, 1.22) | 0.021 | 1.13 (1.09, 1.18) | 0.033 | |
| ASA classification | 0.97 (0.95, 1.01) | 0.067 | 1.01 (0.97, 1.04) | 0.632 | |
| TMN stage | 0.029 | 0.032 | |||
| 0 | 0.99 (0.95, 1.05) | 1.25 (0.74, 2.09) | |||
| Ⅰ | 1.15 (0.64, 1.71) | 1.24 (0.46, 2.12) | |||
| ⅡA | 1.09 (0.52, 1.51) | 0.96 (0.62, 1.38) | |||
| ⅡB | 1.16 (0.88, 1.23) | 1.21 (1.01, 1.58) | |||
| Ⅲ | 1.23 (0.74, 1.74) | 1.26 (0.87, 1.84) | |||
| Type of surgery | 0.781 | 0.593 | |||
| Breast-conserving surgery | 1.09 (0.84, 1.52) | 1.08 (0.73, 1.59) | |||
| Mastectomy | 0.98 (0.91, 1.15) | 1.02 (0.79, 1.69) | |||
| Duration of anesthesia | 1.02 (0.85, 1.36) | 0.232 | 0.99 (0.69, 1.23) | 0.312 | |
| Remifentanil | 0.69 (0.34, 1.89) | 0.538 | 0.74 (0.49, 1.09) | 0.381 | |
| Sufentanyl | 0.87 (0.39, 1.41) | 0.667 | 0.98 (0.71, 1.29) | 0.769 | |
| Propofol | 1.01 (0.88, 1.29) | 0.517 | 1.10 (0.89, 1.31) | 0.487 | |
| Cisatracurium | 1.05 (0.91, 1.25) | 0.473 | 1.08 (0.88, 1.27) | 0.388 | |
| Inhalable anesthetic | 0.94 (0.73, 1.27) | 0.081 | 0.92 (0.87, 1.08) | 0.057 | |
| VAS score when leaving PACU | 1.02 (0.81, 1.19) | 0.790 | 1.10 (0.81, 1.19) | 0.682 | |
| Dexmedetomidine | 1.15 (0.62, 2.16) | 0.626 | 1.17 (0.62, 2.20) | 0.673 | |
2.3 右美组和对照组Kaplan-Meier生存曲线分析
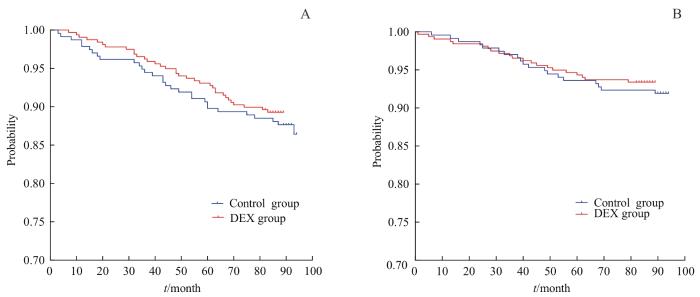
Kaplan-Meier生存曲线(图1)表明,右美组和对照组患者术后5年的RFS分别为93.8%(95%CI 90.7%~95.9%)和90.2%(95%CI 86.0%~93.2%),差异无统计学意义(P>0.05)。右美组和对照组患者术后5年的OS分别是94.9%(95%CI 92.0%~96.8%)和94.4%(95%CI 90.9%~96.6%),差异无统计学意义(P>0.05)。
图1
图1
2组患者RFS(A)和OS(B)的比较
Fig 1
Comparison of RFS (A) and OS (B) between the two groups of patients
3 讨论
在我国乳腺癌是引起45岁以下女性死亡的最常见肿瘤[10]。鉴于外科手术是治疗乳腺癌的主要手段,因此有必要探明常用的麻醉镇痛药物是否会对乳腺癌手术患者的预后造成不良影响。
右美托咪定对α2-肾上腺素能受体具有较高的选择性,具有独特的镇静、抗焦虑、镇痛和阻滞交感神经、抗炎作用[11]。人乳腺癌细胞系已被证明表达α2-肾上腺素能受体[12]。曲马多通过抑制α2-肾上腺素能受体信号通路可抑制人乳腺癌细胞的增殖、迁移和侵袭[13],提示α2-肾上腺素能受体可能是一种人乳腺癌发病的关键调节因子。一项针对胃癌胃切除术患者的随机对照试验表明,术中给予右美托咪定可以降低应激反应,调节免疫功能[14],提示右美托咪定可抑制肿瘤的生长。然而,近年来有研究表明,右美托咪定通过激活癌细胞或宿主细胞的α2-肾上腺素受体诱导肿瘤生长和转移[6];非小细胞肺癌患者术中使用58~140 μg右美托咪定虽不影响术后5年RFS,但与术后5年OS降低有关[3];术中使用右美托咪定剂量累积超过100 μg可能降低非小细胞肺癌患者术后5年OS[15]。
鉴于这些争议,我们进行了这项基于大型综合医院单中心连续手术病例的回顾性研究,旨在分析术中使用小剂量右美托咪定是否影响乳腺癌手术患者的术后5年RFS和OS。本中心使用右美托咪定剂量较低,有助于维持术中血流动力学稳定[16]、减少苏醒期躁动和拔除气管插管时的应激反应[17],同时心血管不良反应少。入组的乳腺癌患者均进行完整的随访,手术医师、麻醉医师为同一团队人员,因此术者操作手法及手术时间较为均一,麻醉方法均为全身麻醉,麻醉药物为丙泊酚、舒芬太尼、瑞芬太尼、七氟醚或异氟醚,以及肌肉松弛药顺式阿曲库铵。通过倾向性评分匹配法以是否应用右美托咪定为因变量,年龄、BMI、化疗比例、放疗比例、激素治疗比例、ER阳性比例、PR阳性比例、Ki67评分、ASA分级、肿瘤TMN分期、手术类型、麻醉方法、麻醉时长、术中各种麻醉药用量、离开PACU时VAS评分等协变量为自变量进行1∶1匹配,匹配后2组患者的基线资料无明显差异,排除了个体差异、肿瘤分期差异、治疗方法差异、麻醉方式及麻醉药物差异等因素的影响。ER、PR、HER2与乳腺癌的发生和病情进展有紧密联系,其中HER2与癌细胞转化密切相关,乳腺癌患者出现癌变后HER2水平会在短时间内异常升高,诱导癌细胞进行大量增殖,促进病灶扩大,加剧病情;而乳腺癌患者出现癌变后PR与ER则会出现下降的情况[18]。倾向性评分匹配后对照组与右美组在这些指标上差异无统计学意义,排除了肿瘤类型对该研究结果的影响。
本研究为回顾性研究,存在局限性。不同剂量的右美托咪定对患者预后可能产生不同的影响,更高剂量或更长时程使用右美托咪定的影响仍需进一步研究。大样本量多中心前瞻性研究将有助于探明不同剂量右美托咪定对肿瘤患者预后的影响。
综上所述,采用倾向性评分匹配法在尽量排除患者因素(年龄、BMI)、肿瘤特征、手术因素、麻醉方式、麻醉药物、术后治疗方法(放疗、化疗、激素治疗)等混杂因素的影响下,发现术中小剂量(0.7~0.8 μg/kg)使用右美托咪定不影响乳腺癌手术患者术后5年RFS和OS。本研究可为肿瘤手术患者麻醉药物的合理选择提供理论参考。更高剂量右美托咪定的作用需进行前瞻性的多中心随机对照研究进一步确认。
作者贡献声明
杨笑萱、朱珊、钱程收集临床资料,杨笑萱分析数据并撰写文章,储晓英指导课题设计、修改文章。所有作者均阅读并同意了最终稿件的提交。
AUTHOR's CONTRIBUTIONS
YANG Xiaoxuan, ZHU Shan and QIAN Cheng collected the clinical data. YANG Xiaoxuan analysed the data and wrote the manuscript. CHU Xiaoying designed the study and revised the manuscript. All the authors have read the last version of paper and consented for submission.
利益冲突声明
所有作者声明不存在利益冲突。
COMPETING INTERESTS
All authors disclose no relevant conflict of interests.
参考文献