
上海交通大学学报(医学版) ›› 2022, Vol. 42 ›› Issue (9): 1225-1238.doi: 10.3969/j.issn.1674-8115.2022.09.009
崔锡炜( ), 钟民衎, 热汗姑丽·艾买尔, 王智超, 李青峰(
), 钟民衎, 热汗姑丽·艾买尔, 王智超, 李青峰( )
)
收稿日期:2022-03-11
接受日期:2022-07-27
出版日期:2022-10-20
发布日期:2022-08-05
通讯作者:
李青峰,电子信箱:dr.liqingfeng@shsmu.edu.cn。作者简介:崔锡炜(1996—),男,硕士生;电子信箱:dr.cuixiwei@foxmail.com。
基金资助:
CUI Xiwei( ), CHUNG Manhon, AIMAIER Rehanguli, WANG Zhichao, LI Qingfeng(
), CHUNG Manhon, AIMAIER Rehanguli, WANG Zhichao, LI Qingfeng( )
)
Received:2022-03-11
Accepted:2022-07-27
Online:2022-10-20
Published:2022-08-05
Contact:
LI Qingfeng, E-mail: dr.liqingfeng@shsmu.edu.cn.Supported by:摘要:
目的·探究人多效蛋白(pleiotrophin,PTN)对恶性周围神经鞘瘤(malignant peripheral nerve sheath tumor,MPNST)增殖、迁移与侵袭能力的影响。方法·通过免疫组织化学与生物信息学技术检测和分析PTN在MPNST组织中的表达。通过慢病毒转染分别构建过表达PTN与敲低PTN的MPNST细胞株,分别对过表达与敲低PTN的MPNST细胞进行细胞划痕实验与Transwell细胞迁移/侵袭实验,以检测PTN对细胞迁移、侵袭能力的影响;借助CCK8细胞增殖实验、EdU细胞增殖实验与细胞克隆形成实验,检测PTN对细胞增殖与集落形成能力的影响。通过Western blotting与转录组高通量测序技术,探究PTN对信号转导通路的影响。借助鼠尾静脉肿瘤细胞注射技术,分别注射敲减PTN的MPNST细胞株(实验组)和未敲减细胞株(对照组)构建MPNST裸鼠肺转移模型,探究PTN在体内环境下的生物学功能。结果·对49例MPNST患者的肿瘤组织与3例非MPNST患者的正常神经组织样本的免疫组织化学实验表明,PTN蛋白在MPNST组织中表达显著降低。细胞划痕实验与Transwell细胞迁移/侵袭实验显示,敲减PTN可促进MPNST细胞迁移与侵袭,过表达PTN可抑制MPNST细胞迁移与侵袭。CCK8细胞增殖实验、EdU细胞增殖实验与克隆形成实验显示:过表达PTN可抑制细胞增殖,但对集落形成能力无显著影响;敲减PTN对增殖与集落形成均无显著影响。Western blotting结果表明,过表达PTN可激活促分裂原活化的蛋白激酶(MAPK)通路,而敲减PTN对MAPK通路无显著影响。高通量转录组测序结果表明,过表达PTN可调节MPNST细胞中细胞程序性死亡的相关基因。体内实验表明,相较于对照组小鼠,实验组小鼠的成瘤率无显著改变,但生成肺转移灶的数量与体积显著上升,且出现严重的肺间质病变,实验组小鼠的体质量增长速度也显著低于对照组。结论·PTN在MPNST组织中低表达;过表达PTN可在体外抑制MPNST细胞的增殖、迁移与侵袭;敲减PTN的MPNST细胞对小鼠肺部的转移能力增强。
中图分类号:
崔锡炜, 钟民衎, 热汗姑丽·艾买尔, 王智超, 李青峰. 人多效蛋白抑制恶性周围神经鞘瘤转移的机制研究[J]. 上海交通大学学报(医学版), 2022, 42(9): 1225-1238.
CUI Xiwei, CHUNG Manhon, AIMAIER Rehanguli, WANG Zhichao, LI Qingfeng. Role of human pleiotrophin in the metastasis of malignant peripheral nerve sheath tumor[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2022, 42(9): 1225-1238.
| Antibody | Lot. | Manufacturer |
|---|---|---|
| Anti-PTN primary antibody (IHC) | Sc-74443 | Santa Cruz |
| Anti-PTN primary antibody | Ab79411 | Abcam |
| Anti-ACTB primary antibody | D190826 | Sangon |
| p44/42 MAPK primary antibody | 4695 | CST |
| Phospho-p44/42 MAPK primary antibody | 4370 | CST |
| MEK1/2 primary antibody | 4694 | CST |
| Phospho-MEK1/2 primary antibody | 2338 | CST |
| AKT (pan) primary antibody | 4691 | CST |
| Phospho-AKT primary antibody | 4060 | CST |
| Anti N-cadherin primary antibody | Ab76011 | Abcam |
| Anti E-cadherin primary antibody | Ab40722 | Abcam |
| Anti-Slug primary antibody | Ab51772 | Abcam |
| Anti-Snail primary antibody | Ab53519 | Abcam |
| Anti-vimentin primary antibody | Ab92547 | Abcam |
| Anti-α-SMA primary antibody | Ab7817 | Abcam |
| Anti-FAK primary antibody | Ab40794 | Abcam |
| Anti-pFAK (Y397) primary antibody | Ab81298 | Abcam |
| Anti-SRC primary antibody | Ab109381 | Abcam |
| Anti-pSRC (Y419) primary antibody | Ab185617 | Abcam |
| DYKDDDDK (flag) primary antibody | 14793 | CST |
| Donkey to goat secondary antibody | Ab97110 | Abcam |
| Goat to rabbit secondary antibody | Ab97080 | Abcam |
| Rabbit to mouse secondary antibody | Ab6728 | Abcam |
表1 使用的抗体信息
Tab 1 The antibodies used in this study
| Antibody | Lot. | Manufacturer |
|---|---|---|
| Anti-PTN primary antibody (IHC) | Sc-74443 | Santa Cruz |
| Anti-PTN primary antibody | Ab79411 | Abcam |
| Anti-ACTB primary antibody | D190826 | Sangon |
| p44/42 MAPK primary antibody | 4695 | CST |
| Phospho-p44/42 MAPK primary antibody | 4370 | CST |
| MEK1/2 primary antibody | 4694 | CST |
| Phospho-MEK1/2 primary antibody | 2338 | CST |
| AKT (pan) primary antibody | 4691 | CST |
| Phospho-AKT primary antibody | 4060 | CST |
| Anti N-cadherin primary antibody | Ab76011 | Abcam |
| Anti E-cadherin primary antibody | Ab40722 | Abcam |
| Anti-Slug primary antibody | Ab51772 | Abcam |
| Anti-Snail primary antibody | Ab53519 | Abcam |
| Anti-vimentin primary antibody | Ab92547 | Abcam |
| Anti-α-SMA primary antibody | Ab7817 | Abcam |
| Anti-FAK primary antibody | Ab40794 | Abcam |
| Anti-pFAK (Y397) primary antibody | Ab81298 | Abcam |
| Anti-SRC primary antibody | Ab109381 | Abcam |
| Anti-pSRC (Y419) primary antibody | Ab185617 | Abcam |
| DYKDDDDK (flag) primary antibody | 14793 | CST |
| Donkey to goat secondary antibody | Ab97110 | Abcam |
| Goat to rabbit secondary antibody | Ab97080 | Abcam |
| Rabbit to mouse secondary antibody | Ab6728 | Abcam |
| Primer | Sequence |
|---|---|
| PPIA forward | 5'-CCCACCGTGTTCTTCGACATT-3' |
| PPIA reverse | 5'-GGACCCGTATGCTTTAGGATGA-3' |
| PTN forward | 5'-GAAGTCTGAAGCGAGCCCTG-3' |
| PTN reverse | 5'-GCTTGAGGTTTGGGCTTGGT-3' |
表2 实时荧光定量PCR引物序列
Tab 2 Primers sequences for RT-qPCR
| Primer | Sequence |
|---|---|
| PPIA forward | 5'-CCCACCGTGTTCTTCGACATT-3' |
| PPIA reverse | 5'-GGACCCGTATGCTTTAGGATGA-3' |
| PTN forward | 5'-GAAGTCTGAAGCGAGCCCTG-3' |
| PTN reverse | 5'-GCTTGAGGTTTGGGCTTGGT-3' |
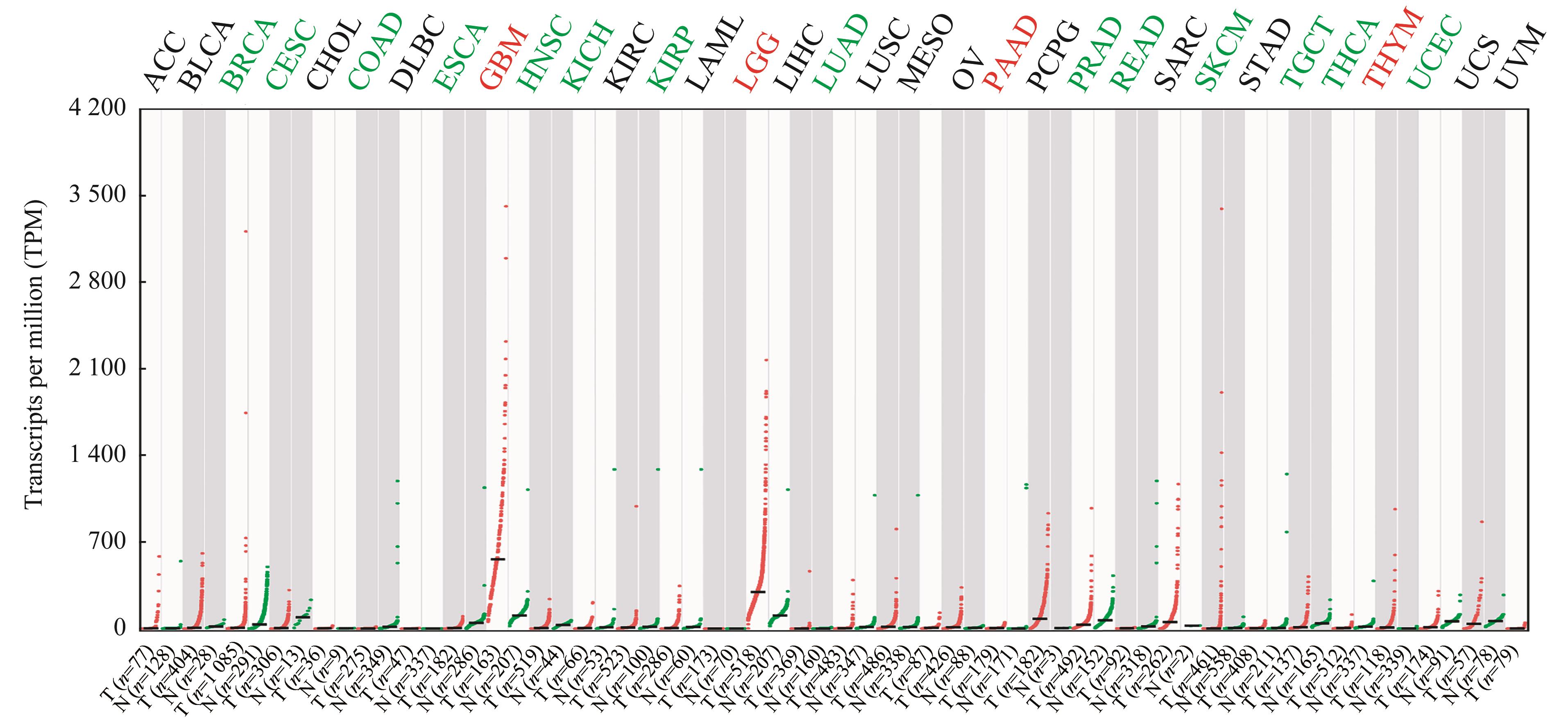
图1 PTN 在TCGA数据库33种恶性肿瘤中的表达分析Note: PTN was up-regulated in the tumors in red and down-regulated in the tumors in green. No significant differences were observed in other tumors. T—tumor tissue; N—normal tissue control; ACC—adrenocortical carcinoma; BLCA—bladder urothelial carcinoma; BRCA—breast invasive carcinoma; CESC—cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL—cholangiocarcinoma; COAD—colon adenocarcinoma; DLBC—lymphoid neoplasm diffuse large B-cell lymphoma; ESCA—esophageal carcinoma; HNSC—head and neck squamous cell carcinoma; KICH—kidney chromophobe; KIRC—kidney renal clear cell carcinoma; KIRP—kidney renal papillary cell carcinoma; LAML—acute myeloid leukemia; LIHC—liver hepatocellular carcinoma; LUAD—lung adenocarcinoma; LUSC—lung squamous cell carcinoma; MESO—mesothelioma; OV—ovarian serous cystadenocarcinoma; PAAD—pancreatic adenocarcinoma; PCPG—pheochromocytoma and paraganglioma; PRAD—prostate adenocarcinoma; READ—rectum adenocarcinoma; SARC—sarcoma; SKCM—skin cutaneous melanoma; STAD—stomach adenocarcinoma; TGCT—testicular germ cell tumors; THCA—thyroid carcinoma; THYM—thymoma; UCEC—uterine corpus endometrial carcinoma; UCS—uterine carcinosarcoma; UVM—uveal melanoma.
Fig 1 Bioinformatics analysis of PTN in 33 kinds of malignancies based on TCGA database
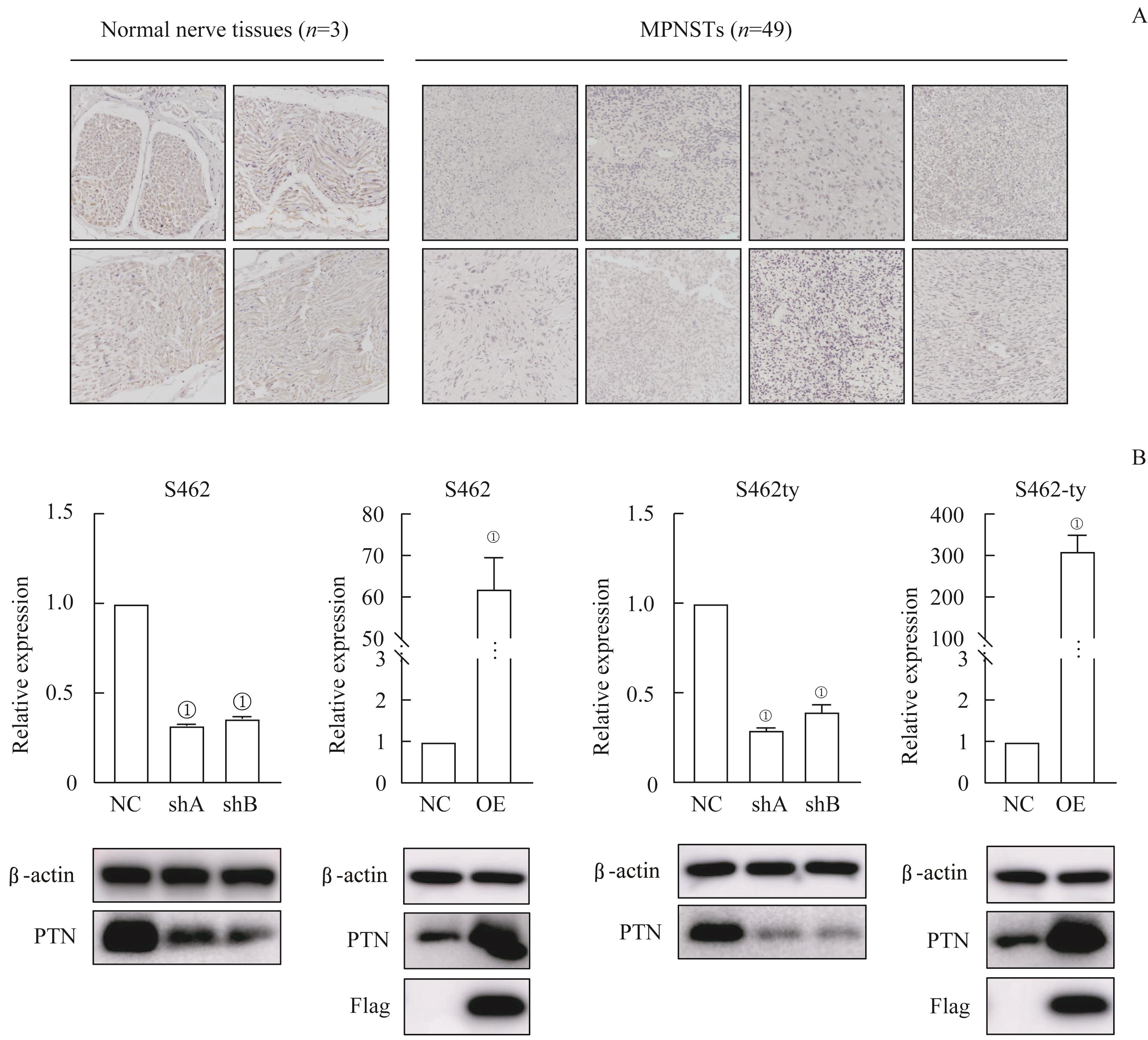
图2 PTN在MPSNT组织中的表达情况以及 PTN 过表达与 PTN 敲低的MPNST细胞系的构建Note: A. Immunohistochemistry of PTN in normal nerve tissues and MPNST tissues (×20). B. RT-qPCR and Western blotting of MPNST cell lines transfected by lentiviruses with PTN knocked-down (shA and shB) or overexpressing (OE). ①P=0.000, compared with the control group (NC).
Fig 2 Expression of PTN in the MPNST tissues and the establishment of PTN-overexpressing/-knocked-down MPNST celllines
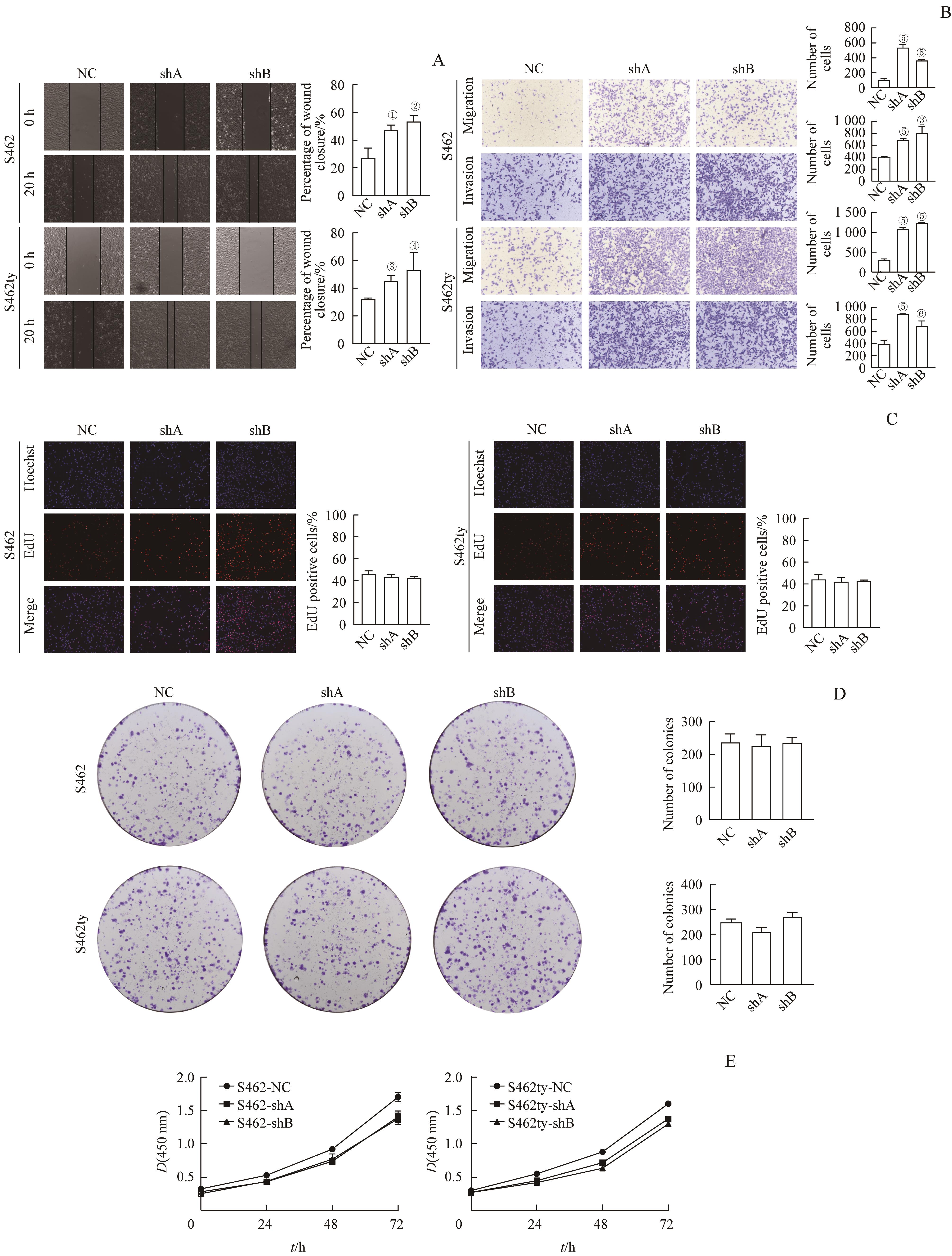
图3 敲低 PTN 基因对MPNST细胞的增殖、迁移与侵袭能力的影响Note: A. Cell wound healing assay (×20). B. Transwell migration/invasion assay (hematoxylin staining, ×20). C. EdU assay (×20). D. Colony formation assay (hematoxylin staining, ×1). E. CCK8 assay. ①P=0.011, ②P=0.005, ③P=0.003, ④P=0.044, ⑤P=0.000, ⑥P=0.008, compared with NC.
Fig 3 Effect of knocking PTN down on the proliferation, migration and invasion potential of MPNST cells
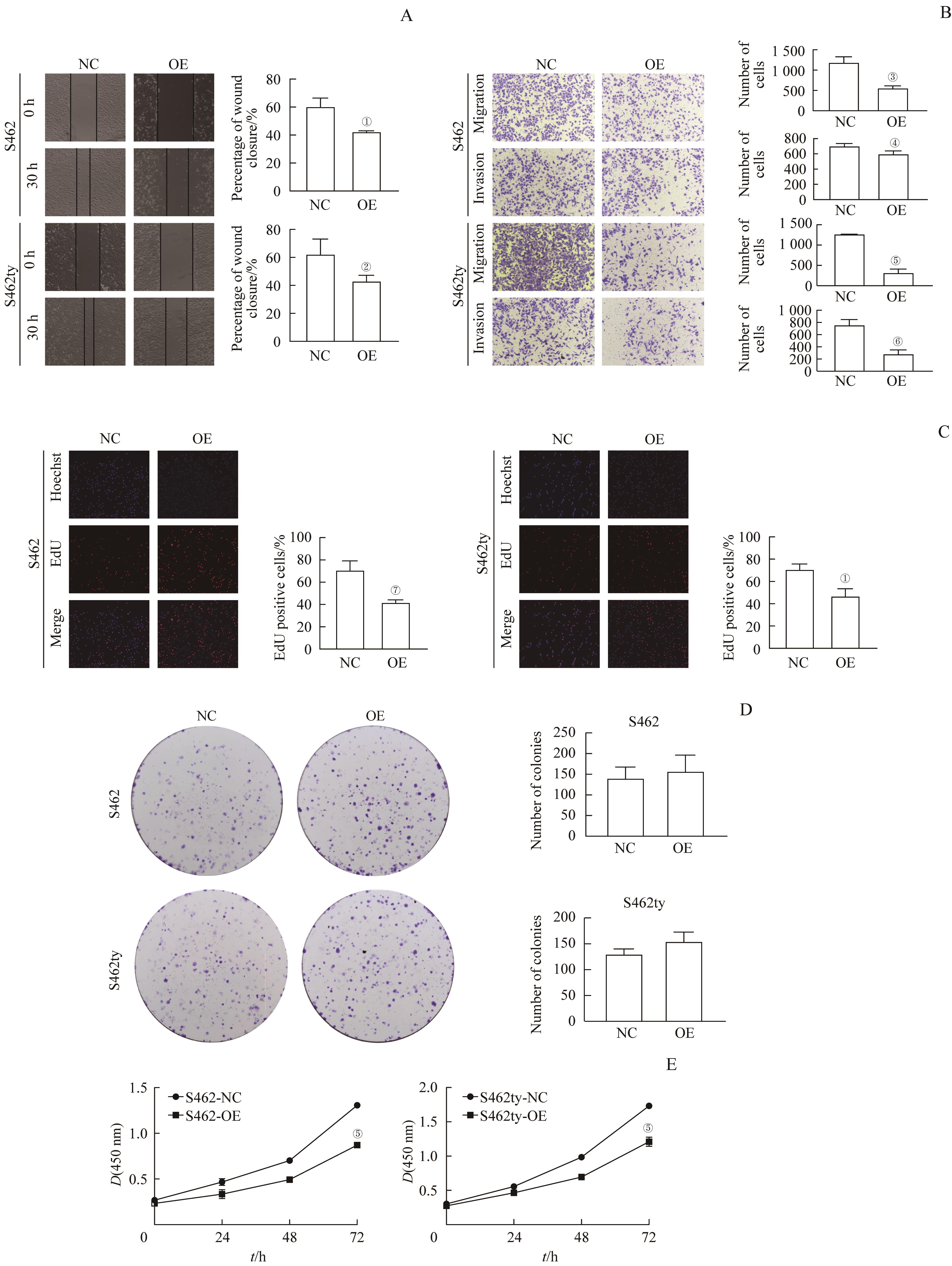
图4 过表达 PTN 基因对MPNST细胞的增殖、迁移与侵袭能力的影响Note: A. Cell wound healing assay (×20). B. Transwell migration/invasion assay (hematoxylin staining, ×20). C. EdU assay (×20). D. Colony formation assay (hematoxylin staining, ×1). E. CCK8 assay. ①P=0.007, ②P=0.047, ③P=0.003, ④P=0.044, ⑤P=0.000, ⑥P=0.002, ⑦P=0.005, compared with NC.
Fig 4 Effect of PTN overexpression on the proliferation, migration and invasion potential of MPNST cells
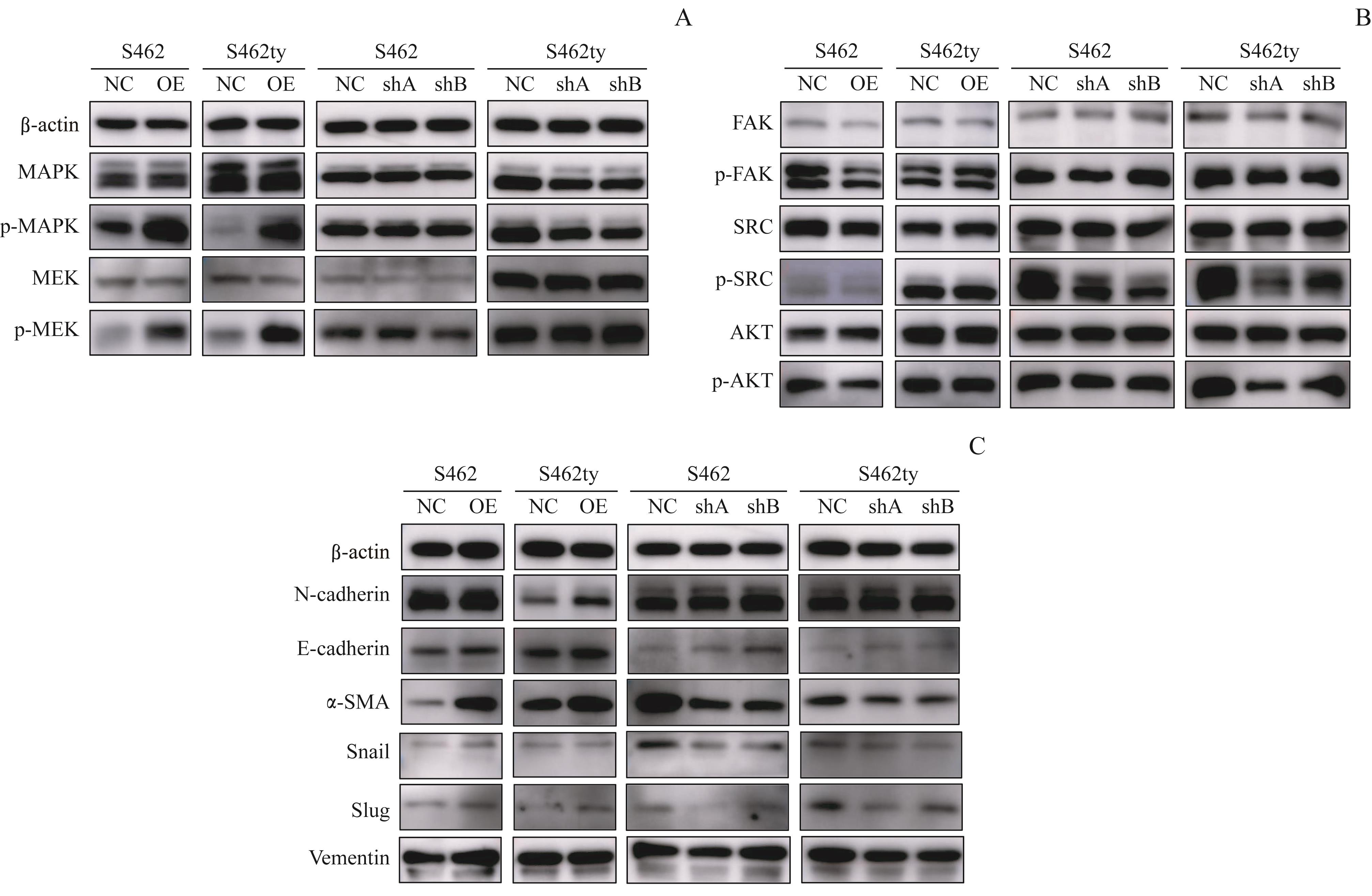
图5 Western blotting检测 PTN 过表达或敲减细胞株信号通路蛋白表达的变化Note: A. MAPK pathway-related proteins detected by Western blotting. B. EMT-related proteins detected by Western blotting. C. FAK-SRC and PI3K-AKT pathway-related proteins detected by Western blotting.
Fig 5 Changes of the signal pathway proteins expression in the PTN-overexpressing or -knocked-down cell lines
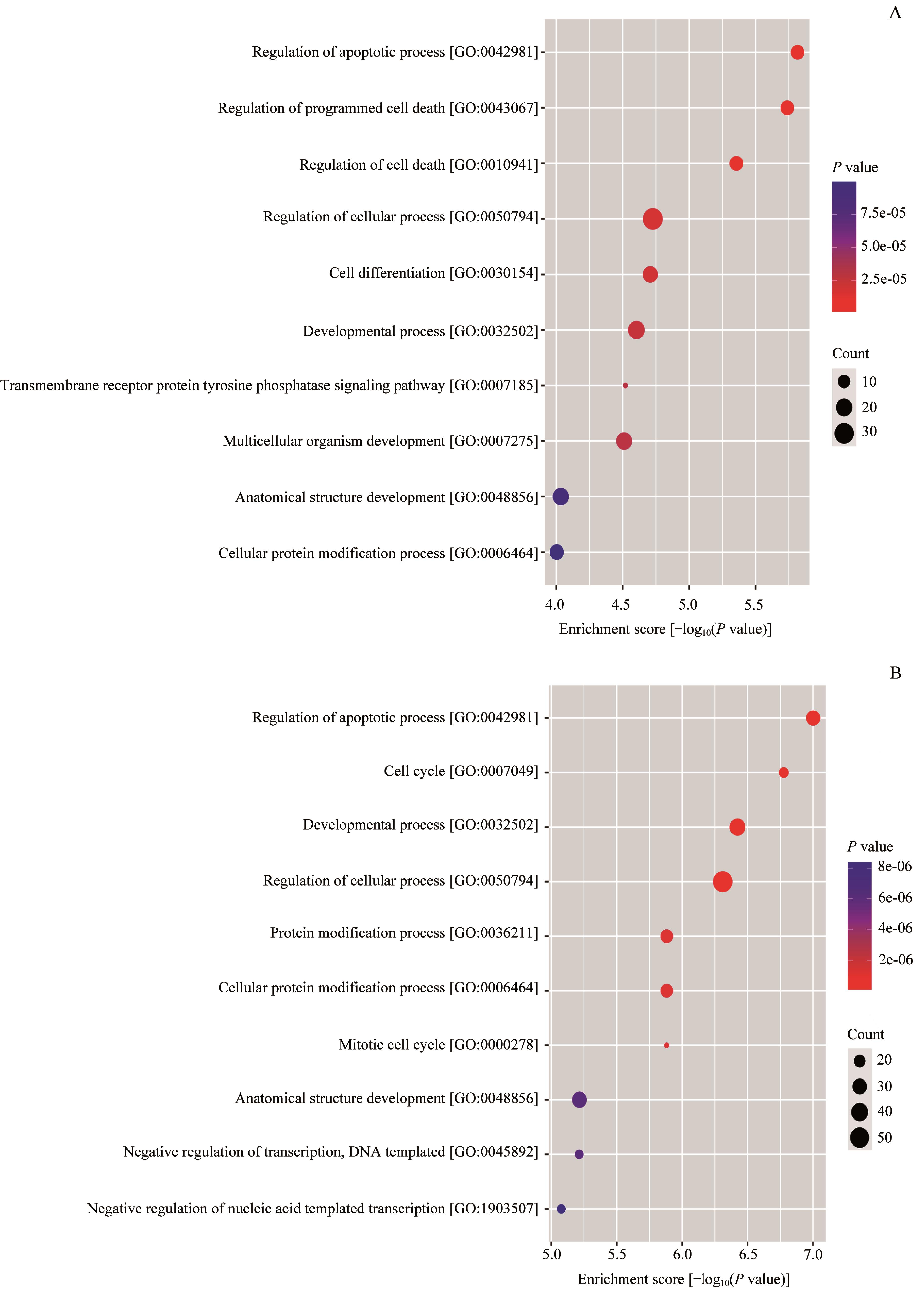
图6 过表达 PTN 的MPNST细胞株相对于对照组表达变化的基因功能富集分析Note: A. Enrichment score dot plot of the genes up-regulated in the PTN-overexpressing cells. B. Enrichment score dot plot of the genes down-regulated in the PTN-overexpressing cells.
Fig 6 Function enrichment analysis of the genes with changed expression in the MPNST cell line overexpressing PTN relative to the control cells
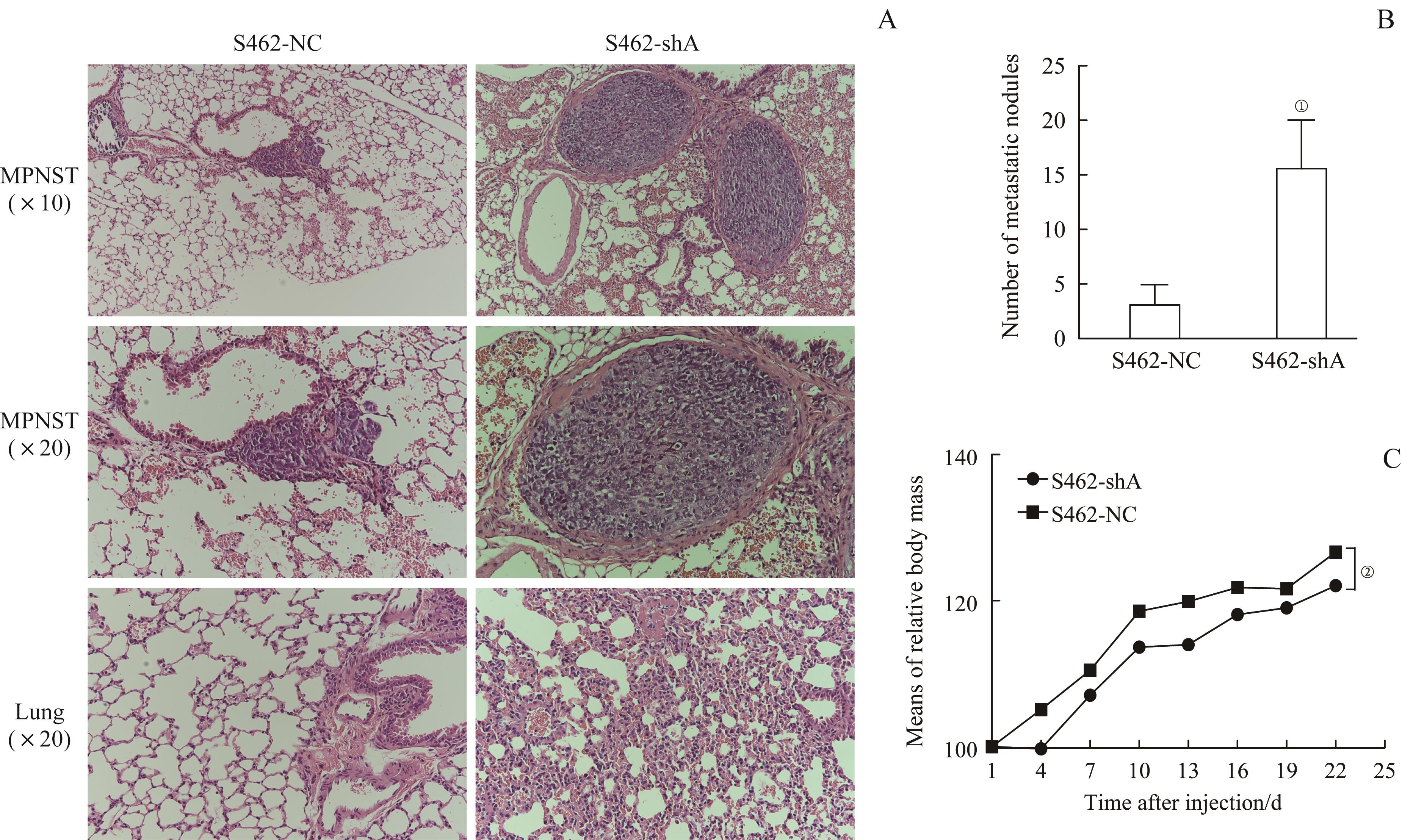
图7 S462-shA组与S462-NC组裸鼠的肺转移灶成瘤实验Note: A. H-E staining of MPNST lung metastatic lesions. B. Number of metastatic nodules in S462-shA group and S462-NC group. C. Means of relative body mass of S462-shA group and S462-NC group. ①P=0.002, ②P=0.001, compared with S462-NC group.
Fig 7 Lung metastasis experiment of S462-shA group and S462-NC group in nude mice
| 1 | MOWERY A, CLAYBURGH D. Malignant peripheral nerve sheath tumors: analysis of the national cancer database[J]. Oral Oncol, 2019, 98: 13-19. |
| 2 | EVANS D G R, BASER M E, MCGAUGHRAN J, et al. Malignant peripheral nerve sheath tumours in neurofibromatosis 1[J]. J Med Genet, 2002, 39(5): 311-314. |
| 3 | FERNER R E, GUTMANN D H. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis[J]. Cancer Res, 2002, 62(5): 1573-1577. |
| 4 | ZHOU H, COFFIN C M, PERKINS S L, et al. Malignant peripheral nerve sheath tumor: a comparison of grade, immunophenotype, and cell cycle/growth activation marker expression in sporadic and neurofibromatosis 1-related lesions[J]. Am J Surg Pathol, 2003, 27(10): 1337-1345. |
| 5 | KOCHAT V, RAMAN A T, LANDERS S M, et al. Enhancer reprogramming in PRC2-deficient malignant peripheral nerve sheath tumors induces a targetable de-differentiated state[J]. Acta Neuropathol, 2021, 142(3): 565-590. |
| 6 | PEACOCK J D, PRIDGEON M G, TOVAR E A, et al. Genomic status of MET potentiates sensitivity to MET and MEK inhibition in NF1-related malignant peripheral nerve sheath tumors[J]. Cancer Res, 2018, 78(13): 3672-3687. |
| 7 | HIGHAM C S, DOMBI E, ROGIERS A, et al. The characteristics of 76 atypical neurofibromas as precursors to neurofibromatosis 1 associated malignant peripheral nerve sheath tumors[J]. Neuro Oncol, 2018, 20(6): 818-825. |
| 8 | FARID M, DEMICCO E G, GARCIA R, et al. Malignant peripheral nerve sheath tumors[J]. Oncologist, 2014, 19(2): 193-201. |
| 9 | XU Y, XU G J, LIU Z, et al. Incidence and prognosis of distant metastasis in malignant peripheral nerve sheath tumors[J]. Acta Neurochir (Wien), 2021, 163(2): 521-529. |
| 10 | KOLBERG M, HØLAND M, AGESEN T H, et al. Survival meta-analyses for >1 800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1[J]. Neuro Oncol, 2013, 15(2): 135-147. |
| 11 | HIRBE A C, COSPER P F, DAHIYA S, et al. Neoadjuvant ifosfamide and epirubicin in the treatment of malignant peripheral nerve sheath tumors[J]. Sarcoma, 2017, 2017: 3761292. |
| 12 | PASQUALI S, GRONCHI A. Neoadjuvant chemotherapy in soft tissue sarcomas: latest evidence and clinical implications[J]. Ther Adv Med Oncol, 2017, 9(6): 415-429. |
| 13 | MURAMATSU T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis[J]. J Biochem, 2002, 132(3): 359-371. |
| 14 | BLONDET B, CARPENTIER G, LAFDIL F, et al. Pleiotrophin cellular localization in nerve regeneration after peripheral nerve injury[J]. J Histochem Cytochem, 2005, 53(8): 971-977. |
| 15 | BERTRAM S, ROLL L, REINHARD J, et al. Pleiotrophin increases neurite length and number of spiral ganglion neurons in vitro[J]. Exp Brain Res, 2019, 237(11): 2983-2993. |
| 16 | FERNÁNDEZ-CALLE R, VICENTE-RODRÍGUEZ M, GRAMAGE E, et al. Pleiotrophin regulates microglia-mediated neuroinflammation[J]. J Neuroinflammation, 2017, 14(1): 46. |
| 17 | GONZÁLEZ-CASTILLO C, ORTUÑO-SAHAGÚN D, GUZMÁN-BRAMBILA C, et al. Pleiotrophin as a central nervous system neuromodulator, evidences from the hippocampus[J]. Front Cell Neurosci, 2015, 8: 443. |
| 18 | HERRADÓN G, PÉREZ-GARCÍA C. Targeting midkine and pleiotrophin signalling pathways in addiction and neurodegenerative disorders: recent progress and perspectives[J]. Br J Pharmacol, 2014, 171(4): 837-848. |
| 19 | SHI Y, PING Y F, ZHOU W C, et al. Tumour-associated macrophages secrete pleiotrophin to promote PTPRZ1 signalling in glioblastoma stem cells for tumour growth[J]. Nat Commun, 2017, 8: 15080. |
| 20 | WEI X, YANG S N, PU X, et al. Tumor-associated macrophages increase the proportion of cancer stem cells in lymphoma by secreting pleiotrophin[J]. Am J Transl Res, 2019, 11(10): 6393-6402. |
| 21 | ZHANG L, KUNDU S M, FEENSTRA T, et al. Pleiotrophin promotes vascular abnormalization in gliomas and correlates with poor survival in patients with astrocytomas[J]. Sci Signal, 2015, 8(406): ra125. |
| 22 | YAO J, HU X F, FENG X S, et al. Pleiotrophin promotes perineural invasion in pancreatic cancer[J]. World J Gastroenterol, 2013, 19(39): 6555-6558. |
| 23 | ULBRICHT U, BROCKMANN M A, AIGNER A, et al. Expression and function of the receptor protein tyrosine phosphatase zeta and its ligand pleiotrophin in human astrocytomas[J]. J Neuropathol Exp Neurol, 2003, 62(12): 1265-1275. |
| 24 | CHEN H M, GORDON M S, CAMPBELL R A, et al. Pleiotrophin is highly expressed by myeloma cells and promotes myeloma tumor growth[J]. Blood, 2007, 110(1): 287-295. |
| 25 | CHOUDHURI R, ZHANG H T, DONNINI S, et al. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis[J]. Cancer Res, 1997, 57(9): 1814-1819. |
| 26 | GRZELINSKI M, STEINBERG F, MARTENS T, et al. Enhanced antitumorigenic effects in glioblastoma on double targeting of pleiotrophin and its receptor ALK[J]. Neoplasia, 2009, 11(2): 145-156. |
| 27 | LI F Q, TIAN F, WANG L, et al. Pleiotrophin (PTN) is expressed in vascularized human atherosclerotic plaques: IFN‑γ/JAK/STAT1 signaling is critical for the expression of PTN in macrophages[J]. FASEB J, 2010, 24(3): 810-822. |
| 28 | FERNÁNDEZ-CALLE R, VICENTE-RODRÍGUEZ M, PASTOR M, et al. Pharmacological inhibition of receptor protein tyrosine phosphatase β/ζ (PTPRZ1) modulates behavioral responses to ethanol[J]. Neuropharmacology, 2018, 137: 86-95. |
| 29 | HIMBURG H A, YAN X, DOAN P L, et al. Pleiotrophin mediates hematopoietic regeneration via activation of RAS[J]. J Clin Invest, 2014, 124(11): 4753-4758. |
| 30 | HERRADON G, RAMOS-ALVAREZ M P, GRAMAGE E. Connecting metainflammation and neuroinflammation through the PTN-MK-RPTPβ/ζ axis: relevance in therapeutic development[J]. Front Pharmacol, 2019, 10: 377. |
| 31 | WIDEMANN B C, ITALIANO A. Biology and management of undifferentiated pleomorphic sarcoma, myxofibrosarcoma, and malignant peripheral nerve sheath tumors: state of the art and perspectives[J]. J Clin Oncol, 2018, 36(2): 160-167. |
| 32 | KROEP J R, OUALI M, GELDERBLOM H, et al. First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: an EORTC soft tissue and bone sarcoma group study[J]. Ann Oncol, 2011, 22(1): 207-214. |
| 33 | MIAO R Y, WANG H T, JACOBSON A, et al. Radiation-induced and neurofibromatosis-associated malignant peripheral nerve sheath tumors (MPNST) have worse outcomes than sporadic MPNST[J]. Radiother Oncol, 2019, 137: 61-70. |
| 34 | KADOMATSU K, KISHIDA S, TSUBOTA S. The heparin-binding growth factor midkine: the biological activities and candidate receptors[J]. J Biochem, 2013, 153(6): 511-521. |
| 35 | MITSIADIS T A, SALMIVIRTA M, MURAMATSU T, et al. Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interactions during fetal development and organogenesis[J]. Development, 1995, 121(1): 37-51. |
| 36 | MURAMATSU T. Structure and function of midkine as the basis of its pharmacological effects[J]. Br J Pharmacol, 2014, 171(4): 814-826. |
| 37 | MASHOUR G A, RATNER N, KHAN G A, et al. The angiogenic factor midkine is aberrantly expressed in NF1-deficient Schwann cells and is a mitogen for neurofibroma-derived cells[J]. Oncogene, 2001, 20(1): 97-105. |
| 38 | MASHOUR G A, WANG H L, CABAL-MANZANO R, et al. Aberrant cutaneous expression of the angiogenic factor midkine is associated with neurofibromatosis type-1[J]. J Invest Dermatol, 1999, 113(3): 398-402. |
| 39 | FRIEDRICH C, HOLTKAMP N, CINATL J, et al. Overexpression of midkine in malignant peripheral nerve sheath tumor cells inhibits apoptosis and increases angiogenic potency[J]. Int J Oncol, 2005, 27(5): 1433-1440. |
| 40 | LEMBERG K M, WANG J W, PRATILAS C A. From genes to-omics: the evolving molecular landscape of malignant peripheral nerve sheath tumor[J]. Genes, 2020, 11(6): 691. |
| 41 | MARTIN E, ACEM I, GRÜNHAGEN D J, et al. Prognostic significance of immunohistochemical markers and genetic alterations in malignant peripheral nerve sheath tumors: a systematic review[J]. Front Oncol, 2020, 10: 594069. |
| 42 | WANG W P, CHEN J X, LIAO R, et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence[J]. Mol Cell Biol, 2002, 22(10): 3389-3403. |
| 43 | KYJACOVA L, SAUP R, RÖNSCH K, et al. IER2-induced senescence drives melanoma invasion through osteopontin[J]. Oncogene, 2021, 40(47): 6494-6512. |
| 44 | FERBEYRE G, DE STANCHINA E, LIN A W, et al. Oncogenic ras and p53 cooperate to induce cellular senescence[J]. Mol Cell Biol, 2002, 22(10): 3497-3508. |
| 45 | ZHU J, WOODS D, MCMAHON M, et al. Senescence of human fibroblasts induced by oncogenic Raf[J]. Genes Dev, 1998, 12(19): 2997-3007. |
| 46 | REN J Y, GU Y H, CUI X W, et al. Protein tyrosine phosphatase receptor S acts as a metastatic suppressor in malignant peripheral nerve sheath tumor via profilin 1-induced epithelial-mesenchymal transition[J]. Front Cell Dev Biol, 2020, 8: 582220. |
| [1] | 肖蓉, 陶双芬, 陈思宇, 郑磊贞, 朱美玲. 幽门螺杆菌参与胃癌侵袭转移的研究进展[J]. 上海交通大学学报(医学版), 2023, 43(4): 495-499. |
| [2] | 唐雷, 徐迎春, 张凤春. 胶原蛋白在肿瘤发生和发展中的作用综述[J]. 上海交通大学学报(医学版), 2023, 43(12): 1577-1584. |
| [3] | 张修齐, 沈柏用. 胰腺导管腺癌神经侵袭的细胞学机制研究进展[J]. 上海交通大学学报(医学版), 2022, 42(6): 833-838. |
| [4] | 刘梦珂, 纪濛濛, 程林, 黄金艳, 孙晓建, 赵维莅, 王黎. 黄芩苷抗肿瘤作用机制的研究进展[J]. 上海交通大学学报(医学版), 2021, 41(2): 246-250. |
| [5] | 徐忠匀,吴书其,王少雁,王丹阳,傅宏亮. 131I治疗分化型甲状腺癌伴肺转移的效果评价及其影响因素分析[J]. 上海交通大学学报(医学版), 2019, 39(4): 412-. |
| [6] | 汪成,洪晏,余燕民,等. 肿瘤转移相关蛋白3与乳腺癌预后的相关性研究[J]. 上海交通大学学报(医学版), 2016, 36(2): 195-. |
| [7] | 刘博,邱纯,李鹏,等. 靶向敲除JARID2基因对葡萄膜黑色素瘤细胞生长与转移的影响[J]. 上海交通大学学报(医学版), 2015, 35(5): 642-. |
| [8] | 史浩骏,王志刚. 长链非编码RNA肺腺癌转移相关转录本1及其在肿瘤转移中的作用[J]. 上海交通大学学报(医学版), 2014, 34(8): 1254-. |
| [9] | 徐 枫,席 云,张 敏,等. 18F-FDG PET/CT对腹部增强CT术前评估胰腺癌远处转移的增益价值[J]. 上海交通大学学报(医学版), 2014, 34(12): 1762-. |
| [10] | 马玉波, 王 忠, 顾爱春, 等. SPECT/CT图像融合鉴别诊断疑似骨转移灶良恶性的价值[J]. , 2010, 30(10): 1246-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||