
上海交通大学学报(医学版) ›› 2021, Vol. 41 ›› Issue (8): 987-998.doi: 10.3969/j.issn.1674-8115.2021.08.001
• 创新团队成果专栏 • 下一篇
出版日期:2021-08-28
发布日期:2021-08-13
通讯作者:
唐玉杰,电子信箱:yujietang@shsmu.edu.cn。作者简介:李瑞(1995―),女,硕士生;电子信箱:lirui_9512@sina.com。
基金资助:
Rui LI( )(
)( ), Yu-jie HAN, Lei ZHANG, Yu-jie TANG(
), Yu-jie HAN, Lei ZHANG, Yu-jie TANG( )
)
Online:2021-08-28
Published:2021-08-13
Contact:
TANG Yu-jie, E-mail: yujietang@shsmu.edu.cn.Supported by:摘要:
目的·从表观遗传角度寻找和鉴定弥漫内生性脑桥胶质瘤(diffuse intrinsic pontine glioma,DIPG)的单药治疗和组合靶向治疗新策略。方法·以已发表的基于8例DIPG肿瘤组织和6例正常脑组织的转录组数据为基础选择用于筛选实验的靶向小分子库。在DIPG原代细胞SU_DIPG13中进行单药筛选并寻找新的能显著抑制DIPG细胞生长的靶向小分子。通过实时定量PCR和Western blotting检测药物处理后其靶基因在mRNA和蛋白水平的表达变化。EdU和Annexin V/碘化丙啶染色后利用流式细胞术分别检测药物处理对DIPG原代细胞增殖和凋亡的影响。靶向小分子库中的药物分别与溴结构域和外端家族(bromodomain and extra terminal protein,BET)抑制剂JQ1和panobinostat组蛋白去乙酰化酶(histone deacetylase,HDAC)进行组合筛选,并体外验证对DIPG存在抑制作用的药物组合。结果·选择了包含66个小分子靶向小分子的药物库用于单药和组合筛选。单药筛选鉴定出的YM155能够显著抑制DIPG原代细胞SU_DIPG13和SU_DIPG17的生长,其靶基因BIRC5(baculoviral IAP repeat containing 5;编码survivin)在DIPG肿瘤组织中的表达高于正常组织(P=0.018)。YM155能抑制BIRC5在mRNA和蛋白水平的表达。YM155既能抑制DIPG原代细胞的增殖又能促进其凋亡。靶向小分子库中的CX4945、ABT-737分别与JQ1、panobinostat联用能在体外协同抑制DIPG细胞活性。结论·通过单药和组合药物筛选鉴定出了DIPG的靶向治疗新策略,为后续体内验证这些DIPG的新靶向治疗策略和挖掘治疗机制奠定了基础。
中图分类号:
李瑞, 韩雨洁, 张蕾, 唐玉杰. 弥漫内生性脑桥胶质瘤靶向治疗新策略的体外筛选与验证[J]. 上海交通大学学报(医学版), 2021, 41(8): 987-998.
Rui LI, Yu-jie HAN, Lei ZHANG, Yu-jie TANG. Invitro screening and validation of novel targeted therapeutic strategy against diffuse intrinsic pontine glioma[J]. JOURNAL OF SHANGHAI JIAOTONG UNIVERSITY (MEDICAL SCIENCE), 2021, 41(8): 987-998.
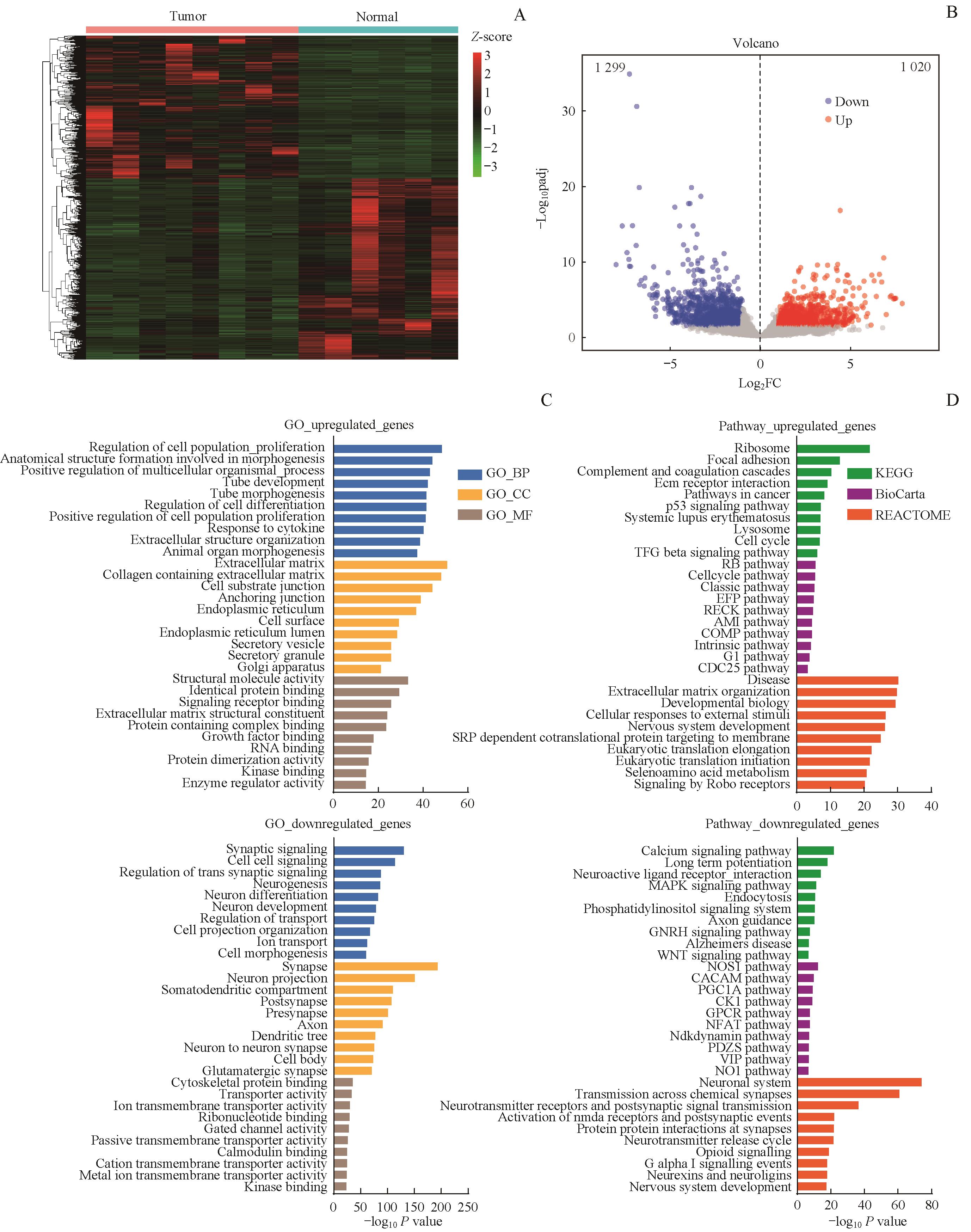
图1 DIPG肿瘤组织和正常脑组织的转录组分析Note: A/B. Heatmap (A) and volcano plot (B) showing DEGs (|Log2FC|≥1, padj≤0.05) of DIPG versus normal cerebral cortex. C/D. GO (C) and Pathway (D) analysis identifying enriched function or pathway in up- or down-regulated genes of DIPG.
Fig 1 Transcriptome analyses of DIPG specimen and normal cerebral cortex
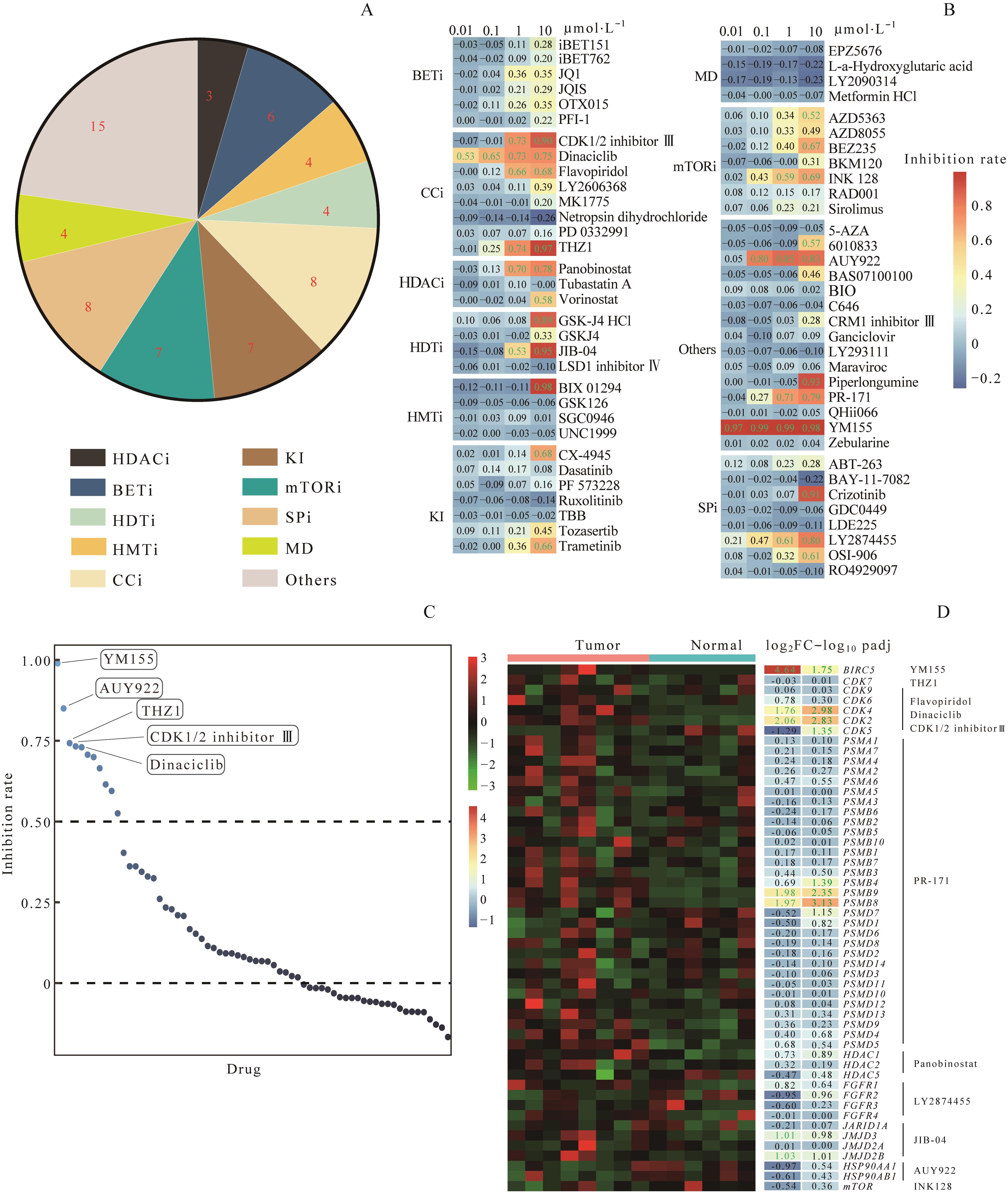
图2 SU_DIPG13细胞中小分子靶向药物的体外筛选Note: A. Classification of small molecule compounds used in drug screening. BETi—BET inhibitor; HDACi—HDAC inhibitor. B. Heatmap showing inhibition rates of cell viability by each compound at indicated concentrations in drug screening of SU_DIPG13 cells. Values up to standard (inhibition rate≥50% ) are shown in green. C. Ranks of compounds from drug screening based on inhibition rates at 1 μmol·L-1. The names of top 5 compounds are labeled. D. Heatmap showing expression levels in DIPG versus normal cerebral cortex of target genes of the top 11 compounds with inhibition rates more than 50% at 1 μmol·L-1. Values up to standards (log2FC≥1, padj≤0.05) are shown in green.HSP90AA1—heat shock protein 90 α family class A member 1; HSP90AB1—heat shock protein 90 α family class B member 1; PSMA—archaeal proteasome endopeptidase complex subunit α; PSMB—archaeal proteasome endopeptidase complex subunit β; PSMD—proteasome 26S subunit; FGFR1—fibroblast growth factor receptor 1; JARID1A—lysine demethylase 5A; JMJD3—lysine demethylase 6B; JMJD2A—lysine demethylase 4A; JMJD2B—lysine demethylase 4B.
Fig 2 Drug screening in SU_DIPG13 cells with selected targeted small molecule compounds in vitro
| Drug | Inhibition rate/% | Target gene | IC50/(μmol·L-1) | Clinical trial | NCT number |
|---|---|---|---|---|---|
| YM155 | 98.97 | BIRC5 | 0.004 | Phase Ⅰ/Ⅱ | NCT01023386, NCT00818480 |
| AUY922 | 85.01 | HSP90AA1, HSP90AB1 | 0.050 | Phase Ⅱ | NCT01854034 |
| THZ1/CDK7 inhibitor | 74.26 | CDK7 | 0.280 | Phase Ⅰ | NCT04247126 |
| CDK1/2 inhibitor Ⅲ | 73.27 | CDK1, CDK2 | 0.400 | Phase Ⅰ/Ⅱ | NCT02095132 |
| Dinaciclib | 72.99 | CDK1, CDK2, CDK5, CDK9 | 0.010 | Phase Ⅰ | NCT03484520 |
| PR-171 | 70.69 | PSMA, PSMB, PSMD | 0.140 | Phase Ⅰ/Ⅱ | NCT00150462, NCT00884312 |
| Panobinostat | 69.95 | HDAC1, HDAC2, HDAC5, et al | 0.240 | Phase Ⅰ/Ⅱ/Ⅲ | NCT01496118, NCT04326764 |
| Flavopiridol | 66.49 | CDK1, CDK2, CDK4, CDK6, CDK9 | 0.200 | Phase Ⅰ/Ⅱ | NCT00445341 |
| LY2874455 | 61.49 | FGFR1, FGFR2, FGFR3, FGFR4 | 0.140 | Phase Ⅰ | NCT03125239 |
| INK 128 | 59.47 | mTOR | 0.170 | Phase Ⅰ/Ⅱ | NCT02987959, NCT03097328 |
| JIB-04 | 52.54 | JARID1A, JMJD2E, JMJD3, JMJD2A, JMJD2B, et al | 0.740 | ‒ | ‒ |
表1 在1 μmol·L-1条件下对SU_DIPG13细胞抑制率大于50%的药物
Tab 1 Drugs with growth inhibition rate over 50% at 1 μmol·L-1 in SU_DIPG13 cells
| Drug | Inhibition rate/% | Target gene | IC50/(μmol·L-1) | Clinical trial | NCT number |
|---|---|---|---|---|---|
| YM155 | 98.97 | BIRC5 | 0.004 | Phase Ⅰ/Ⅱ | NCT01023386, NCT00818480 |
| AUY922 | 85.01 | HSP90AA1, HSP90AB1 | 0.050 | Phase Ⅱ | NCT01854034 |
| THZ1/CDK7 inhibitor | 74.26 | CDK7 | 0.280 | Phase Ⅰ | NCT04247126 |
| CDK1/2 inhibitor Ⅲ | 73.27 | CDK1, CDK2 | 0.400 | Phase Ⅰ/Ⅱ | NCT02095132 |
| Dinaciclib | 72.99 | CDK1, CDK2, CDK5, CDK9 | 0.010 | Phase Ⅰ | NCT03484520 |
| PR-171 | 70.69 | PSMA, PSMB, PSMD | 0.140 | Phase Ⅰ/Ⅱ | NCT00150462, NCT00884312 |
| Panobinostat | 69.95 | HDAC1, HDAC2, HDAC5, et al | 0.240 | Phase Ⅰ/Ⅱ/Ⅲ | NCT01496118, NCT04326764 |
| Flavopiridol | 66.49 | CDK1, CDK2, CDK4, CDK6, CDK9 | 0.200 | Phase Ⅰ/Ⅱ | NCT00445341 |
| LY2874455 | 61.49 | FGFR1, FGFR2, FGFR3, FGFR4 | 0.140 | Phase Ⅰ | NCT03125239 |
| INK 128 | 59.47 | mTOR | 0.170 | Phase Ⅰ/Ⅱ | NCT02987959, NCT03097328 |
| JIB-04 | 52.54 | JARID1A, JMJD2E, JMJD3, JMJD2A, JMJD2B, et al | 0.740 | ‒ | ‒ |
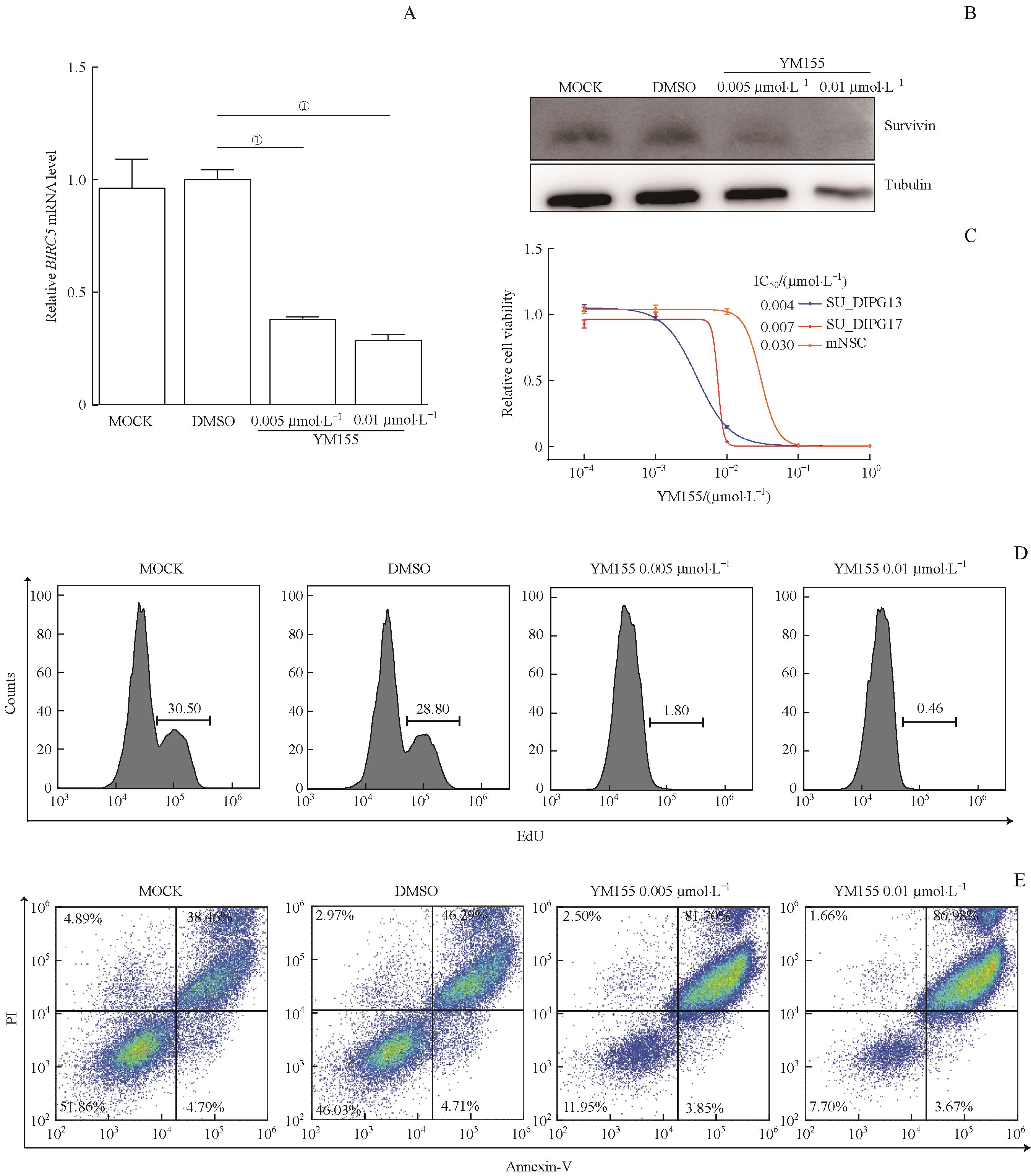
图3 靶向BIRC5的小分子药物YM155对DIPG的体外抑制作用Note: A. qRT-PCR analysis of BIRC5 in SU_DIPG13 cells treated with YM155 at indicated concentrations for 24 h. ①P=0.000. B. Western blotting detecting survivin expression in cell extracts from SU_DIPG13 cells treated with YM155 at indicated concentrations for 48 h. Tubulin is shown as loading control. C. Dosage curves of YM155 in treating SU_DIPG13 cells, SU_DIPG17 cells or mNSCs. IC50 of each line is shown. D. EdU staining detecting cell proliferation of SU_DIPG13 cells treated with YM155 at indicated concentrations for 24 h. E. Annexin V/PI staining detecting cell apoptosis of SU_DIPG13 cells treated with YM155 at indicated concentrations for 48 h.
Fig 3 Inhibitory effect of BIRC5-targeted inhibitor YM155 on DIPG in vitro

图4 DIPG的体外药物组合筛选及验证Note: A. Heatmap showing inhibition rates of combination screening at indicated concentrations. Inhibitory rates conforming to standard highlighted in green. B/C. Cell viability and synergy measurement in combinatory drug treatments. SU_DIPG13 (B) or SU_DIPG17 (C) cells were treated with panobinostat, ABT-737, JQ1 or CX4945 individually or in combination at indicated concentrations for 72 h.
Fig 4 Screening and validationof combinatory drug for DIPG in vitro
| 1 | Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021[J]. CA Cancer J Clin, 2021, 71(1): 7-33. |
| 2 | Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012—2016[J]. Neuro Oncol, 2019, 21(): v1-v100. |
| 3 | Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the international and European society for pediatric oncology DIPG registries[J]. J Clin Oncol, 2018, 36(19): 1963-1972. |
| 4 | Aziz-Bose R, Monje M. Diffuse intrinsic pontine glioma: molecular landscape and emerging therapeutic targets[J]. Curr Opin Oncol, 2019, 31(6): 522-530. |
| 5 | Zhang X, Zhang Z. Oncohistone mutations in diffuse intrinsic pontine glioma[J]. Trends Cancer, 2019, 5(12): 799-808. |
| 6 | Warren KE. Diffuse intrinsic pontine glioma: poised for progress[J]. Front Oncol, 2012, 2: 205. |
| 7 | Margueron R, Reinberg D. The polycomb complex PRC2 and its mark in life[J]. Nature, 2011, 469(7330): 343-349. |
| 8 | Nagaraja S, Vitanza NA, Woo PJ, et al. Transcriptional dependencies in diffuse intrinsic pontine glioma[J]. Cancer Cell, 2017, 31(5): 635-652.e6. |
| 9 | Grasso C, Tang Y, Truffaux N, et al. Functionally-defined therapeutic targets in diffuse intrinsic pontine glioma[J]. Nat Med, 2015, 21(6): 555-559. |
| 10 | Borrelli EP, McGladrigan CG. Differences in safety profiles of newly approved medications for multiple myeloma in real-world settings versus randomized controlled trials[J]. J Oncol Pharm Pract, 2020: 1078155220941937. |
| 11 | Spriano F, Stathis A, Bertoni F. Targeting BET bromodomain proteins in cancer: the example of lymphomas[J]. Pharmacol Ther, 2020, 215: 107631. |
| 12 | Altieri DC. Survivin, cancer networks and pathway-directed drug discovery[J]. Nat Rev Cancer, 2008, 8(1): 61-70. |
| 13 | Kim YH, Kim SM, Kim YK, et al. Evaluation of survivin as a prognostic marker in oral squamous cell carcinoma[J]. J Oral Pathol Med, 2010, 39(5): 368-375. |
| 14 | Kelly RJ, Thomas A, Rajan A, et al. A phase Ⅰ/Ⅱ study of sepantronium bromide (YM155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer[J]. Ann Oncol, 2013, 24(10): 2601-2606. |
| 15 | Tolcher AW, Quinn DI, Ferrari A, et al. A phase Ⅱ study of YM155, a novel small-molecule suppressor of survivin, in castration-resistant taxane-pretreated prostate cancer[J]. Ann Oncol, 2012, 23(4): 968-973. |
| 16 | Dahl NA, Danis E, Balakrishnan I, et al. Super elongation complex as a targetable dependency in diffuse midline glioma[J]. Cell Rep, 2020, 31(1): 107485. |
| 17 | Chou TC. The combination index (CI<1) as the definition of synergism and of synergy claims[J]. Synergy, 2018, 7: 49-50. |
| 19 | Lin GL, Wilson KM, Ceribelli M, et al. Therapeutic strategies for diffuse midline glioma from high-throughput combination drug screening[J]. Sci Transl Med, 2019, 11(519): eaaw0064. |
| 18 | Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor[J]. Cancer Res, 2008, 68(9): 3421-3428. |
| 20 | Siddiqui-Jain A, Drygin D, Streiner N, et al. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy[J]. Cancer Res, 2010, 70(24): 10288-10298. |
| 21 | Eke I, Cordes N. Focal adhesion signaling and therapy resistance in cancer[J]. Semin Cancer Biol, 2015, 31: 65-75. |
| 22 | Schramm K, Iskar M, Statz B, et al. DECIPHER pooled shRNA library screen identifies PP2A and FGFR signaling as potential therapeutic targets for diffuse intrinsic pontine gliomas[J]. Neuro Oncol, 2019, 21(7): 867-877. |
| 23 | Fortin J, Tian RX, Zarrabi I, et al. Mutant ACVR1 arrests glial cell differentiation to drive tumorigenesis in pediatric gliomas[J]. Cancer Cell, 2020, 37(3): 308-323.e12. |
| 24 | Koncar RF, Dey BR, Stanton AJ, et al. Identification of novel RAS signaling therapeutic vulnerabilities in diffuse intrinsic pontine gliomas[J]. Cancer Res, 2019, 79(16): 4026-4041. |
| 25 | Larson JD, Kasper LH, Paugh BS, et al. Histone H3.3 K27M accelerates spontaneous brainstem glioma and drives restricted changes in bivalent gene expression[J]. Cancer Cell, 2019, 35(1): 140-155.e7. |
| 26 | Batool S, Raza H, Zaidi J, et al. Synapse formation: from cellular and molecular mechanisms to neurodevelopmental and neurodegenerative disorders[J]. J Neurophysiol, 2019, 121(4): 1381-1397. |
| 27 | Friedmann-Morvinski D, Bushong EA, Ke E, et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice[J]. Science, 2012, 338(6110): 1080-1084. |
| 28 | Filbin MG, Tirosh I, Hovestadt V, et al. Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq[J]. Science, 2018, 360(6386): 331-335. |
| 29 | Iwagawa T, Watanabe S. Molecular mechanisms of H3K27me3 and H3K4me3 in retinal development[J]. Neurosci Res, 2019, 138: 43-48. |
| 30 | Deaton AM, Bird A. CpG islands and the regulation of transcription[J]. Genes Dev, 2011, 25(10): 1010-1022. |
| 31 | Lin GL, Wilson KM, Ceribelli M, et al. Therapeutic strategies for diffuse midline glioma from high-throughput combination drug screening[J]. Sci Transl Med, 2019, 11(519): eaaw0064. |
| 32 | van Tellingen O, Yetkin-Arik B, de Gooijer MC, et al. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment[J]. Drug Resist Updat, 2015, 19: 1-12. |
| 33 | Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases[J]. Nat Rev Cancer, 2020, 20(1): 26-41. |
| 34 | Minematsu T, Sonoda T, Hashimoto T, et al. Pharmacokinetics, distribution and excretion of YM155 monobromide, a novel small-molecule survivin suppressant, in male and pregnant or lactating female rats[J]. Biopharm Drug Dispos, 2012, 33(3): 160-169. |
| 35 | Cheng ZX, Gong YY, Ma YF, et al. Inhibition of BET bromodomain targets genetically diverse glioblastoma[J]. Clin Cancer Res, 2013, 19(7): 1748-1759. |
| 36 | Matzuk MM, McKeown MR, Filippakopoulos P, et al. Small-molecule inhibition of BRDT for male contraception[J]. Cell, 2012, 150(4): 673-684. |
| 37 | Allard E, Passirani C, Benoit JP. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors[J]. Biomaterials, 2009, 30(12): 2302-2318. |
| 38 | Vogelbaum MA, Aghi MK. Convection-enhanced delivery for the treatment of glioblastoma[J]. Neuro Oncol, 2015, 17(): ii3-ii8. |
| 39 | Souweidane MM, Kramer K, Pandit-Taskar N, et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: a single-centre, dose-escalation, phase 1 trial[J]. Lancet Oncol, 2018, 19(8): 1040-1050. |
| 40 | Luther N, Zhou ZP, Zanzonico P, et al. The potential of theragnostic ¹²⁴I-8H9 convection-enhanced delivery in diffuse intrinsic pontine glioma[J]. Neuro Oncol, 2014, 16(6): 800-806. |
| [1] | 刘美伶, 周亚兵, 王晓强. 儿童神经纤维瘤病1型颅内肿瘤性病变的治疗进展[J]. 上海交通大学学报(医学版), 2024, 44(3): 399-406. |
| [2] | 梅艳青, 韩雨洁, 翁文筠, 张蕾, 唐玉杰. 靶向抑制CDK12/13在高级别胶质瘤中的体外治疗效果和作用分子机制探究[J]. 上海交通大学学报(医学版), 2023, 43(5): 545-559. |
| [3] | 徐艳丽, 管文斌, 茅伟伟, 朱传营, 张勤, 袁晓军, 谈珍. 52例颅内非典型畸胎样/横纹肌样瘤的临床特征及生存分析[J]. 上海交通大学学报(医学版), 2021, 41(9): 1222-1227. |
| [4] | 那迪娜·帕尔哈提null, 严妍, 车千纪, 罗菁, 刘鑫男, 李斌. 嵌合抗原受体T细胞疗法在胶质母细胞瘤中的应用与展望[J]. 上海交通大学学报(医学版), 2021, 41(7): 982-986. |
| [5] | 赵晓军, 乔志刚, 梁挺宇, 安艳玲. 乳腺癌易感基因1蛋白在脑胶质瘤中的表达及对替莫唑胺敏感性的影响[J]. 上海交通大学学报(医学版), 2021, 41(1): 118-122. |
| [6] | 张明达,冯海忠. 组蛋白变体macroH2A1调控肿瘤增殖的研究进展[J]. 上海交通大学学报(医学版), 2020, 40(9): 1283-1287. |
| [7] | 徐艳丽, 管文斌, 茅伟伟, 朱传营, 张勤, 袁晓军, 谈珍. 52例颅内非典型畸胎样/横纹肌样瘤的临床特征及生存分析[J]. 上海交通大学学报(医学版), 0, (): 1-6. |
| [8] | 刘景景, 胡慧洁, 刘子楷, 宋明柯. 钠钙交换体阻滞剂CB-DMB对人胶质母细胞瘤细胞生长的抑制作用[J]. 上海交通大学学报(医学版), 0, (): 50-56. |
| [9] | 刘子豪, 唐文芳, 王辉. 儿童脑肿瘤氨基酸PET显像的研究进展[J]. 上海交通大学学报(医学版), 0, (): 1-4. |
| [10] | 那迪娜·帕尔哈提, 严妍, 车千纪, 罗菁, 刘鑫男, 李斌. 嵌合抗原受体T细胞疗法在胶质母细胞瘤中的应用与展望[J]. 上海交通大学学报(医学版), 0, (): 1-5. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||