
上海交通大学学报(医学版) ›› 2022, Vol. 42 ›› Issue (10): 1361-1374.doi: 10.3969/j.issn.1674-8115.2022.10.001
• 创新团队成果专栏 • 下一篇
姜正麟1,2,3( )(
)( ), 高云鸽1,2,3, 吴皓1,2,3(
), 高云鸽1,2,3, 吴皓1,2,3( )
)
收稿日期:2022-05-07
接受日期:2022-08-09
出版日期:2022-10-28
发布日期:2022-12-02
通讯作者:
吴 皓,电子信箱:wuhao@shsmu.edu.cn。作者简介:姜正麟(1993—),男,硕士生;电子信箱:jiangzhenglin@sjtu.edu.cn。
基金资助:
JIANG Zhenglin1,2,3( )(
)( ), GAO Yunge1,2,3, WU Hao1,2,3(
), GAO Yunge1,2,3, WU Hao1,2,3( )
)
Received:2022-05-07
Accepted:2022-08-09
Online:2022-10-28
Published:2022-12-02
Contact:
WU Hao, E-mail: wuhao@shsmu.edu.cn.Supported by:摘要:
目的·探索B细胞淋巴瘤/白血病11B(B-cell leukemia/lymphoma 11B,BCL11B)在小鼠耳蜗螺旋器(organ of Corti)发育过程中的表达。方法·选择出生前后不同时期/年龄[胚胎期14.5 d(E14.5)、E15.5、E16.5、E18.5,出生后0日龄(P0)、P2、P4、P7]、随机性别的野生型C57BL/6J小鼠24只,每个年龄3只。将其处死后,取出小鼠双侧耳蜗并固定,对左侧耳蜗解剖获得完整基底膜,右侧耳蜗经蔗糖脱水后进行包埋并制作冰冻切片。采用BCL11B抗体与肌球蛋白6(MYOSIN 6)抗体对小鼠耳蜗基底膜解剖样品、冰冻切片耳蜗样品进行免疫组化分析。采用共聚焦荧光显微镜对上述染色后的不同年龄点的组织样品进行观察,并对BCL11B阳性表达的毛细胞的计数及百分比进行统计。结果·成功制备了8个年龄点的小鼠耳蜗基底膜解剖样品及冰冻切片耳蜗样品。共聚焦荧光显微镜下显示,最先于E15.5胎鼠耳蜗螺旋器底中圈发现BCL11B的表达,其表达特异性出现在MYOSIN6阳性的外侧3排外毛细胞中;随着耳蜗发育,BCL11B在外毛细胞内的表达逐渐增强,至P2时达到顶峰,于P4时逐渐减弱,直至P7时已无法检测到。在BCL11B于外毛细胞表达的整个时期内,螺旋器中的其他细胞均未有BCL11B的表达。分别对BCL11B阳性表达毛细胞计数及百分比进行统计分析,结果显示E16.5、P0和P4这3个年龄点间的差异均无统计学意义(均P>0.05)。结论·通过免疫组化共染BCL11B与MYOSIN6证实,BCL11B是一个在耳蜗螺旋器中外毛细胞发育早期的特异性表达蛋白。
中图分类号:
姜正麟, 高云鸽, 吴皓. B细胞淋巴瘤/白血病11B在小鼠耳蜗螺旋器发育中的表达研究[J]. 上海交通大学学报(医学版), 2022, 42(10): 1361-1374.
JIANG Zhenglin, GAO Yunge, WU Hao. Study on the expression of B-cell leukemia/lymphoma 11B in the organ of Corti during mice cochlea development[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2022, 42(10): 1361-1374.
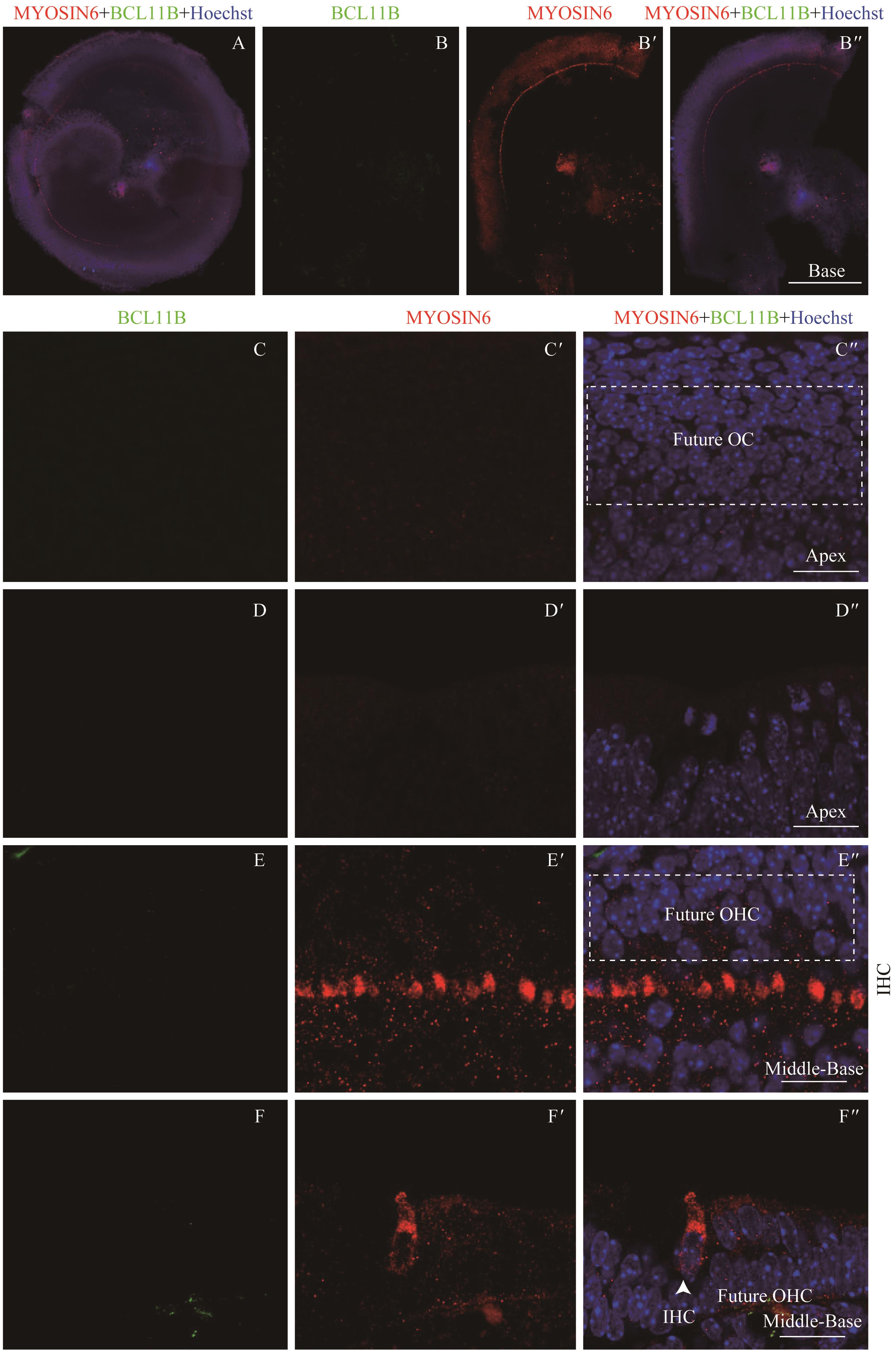
图1 共聚焦显微镜下观察E14.5时BCL11B与MYOSIN6在胎鼠耳蜗螺旋器中的表达Note:A. Expression and distribution of BCL11B and MYOSIN6 in inner hair cells under the low-magnification microscope (×10). It shows the merged image of the apical turn, middle turn and basal turn of the same cochlea, bar=200 μm. B/B'. Expression of BCL11B (B) and MYOSIN6 (B') in the hair cells of basal turn of whole mount samples. B". Merged image of B, B' and nuclear staining (×10). Bar=200 μm. C/C'. Expression of BCL11B (C) and MYOSIN6 (C') in the apical turn of whole mount samples under the high-magnification microscope (×60). C". Merged image of C, C' and nuclear staining (×60). Bar=20 μm. D/D'. Expression of BCL11B (D) and MYOSIN6 (D') in the apical turn of cross section samples under the high-magnification microscope (×60). D". Merged image of D, D' and nuclear staining (×60). Bar=20 μm. E/E'. Expression of BCL11B (E) and MYOSIN6 (E') in the middle-basal turn of whole mount samples under the high-magnification microscope (×60). E". Merged image of E, E' and nuclear staining (×60). Bar=20 μm. F/F'. Expression of BCL11B (F) and MYOSIN6 (F') in the middle-basal turn of cross section samples under the high-magnification microscope (×60). F". Merged image of F, F' and nuclear staining (×60). Bar=20 μm. Green signal represents BCL11B, red signal represents MYOSIN6, and blue signal represents nucleus; white box represents prosensory region; white arrow represents IHC; OC—organ of Corti; OHC—outer hair cell; IHC—inner hair cell.
Fig 1 Expression of BCL11B and MYOSIN6 in the organ of Corti at E14.5 mouse cochlea by confocal imaging
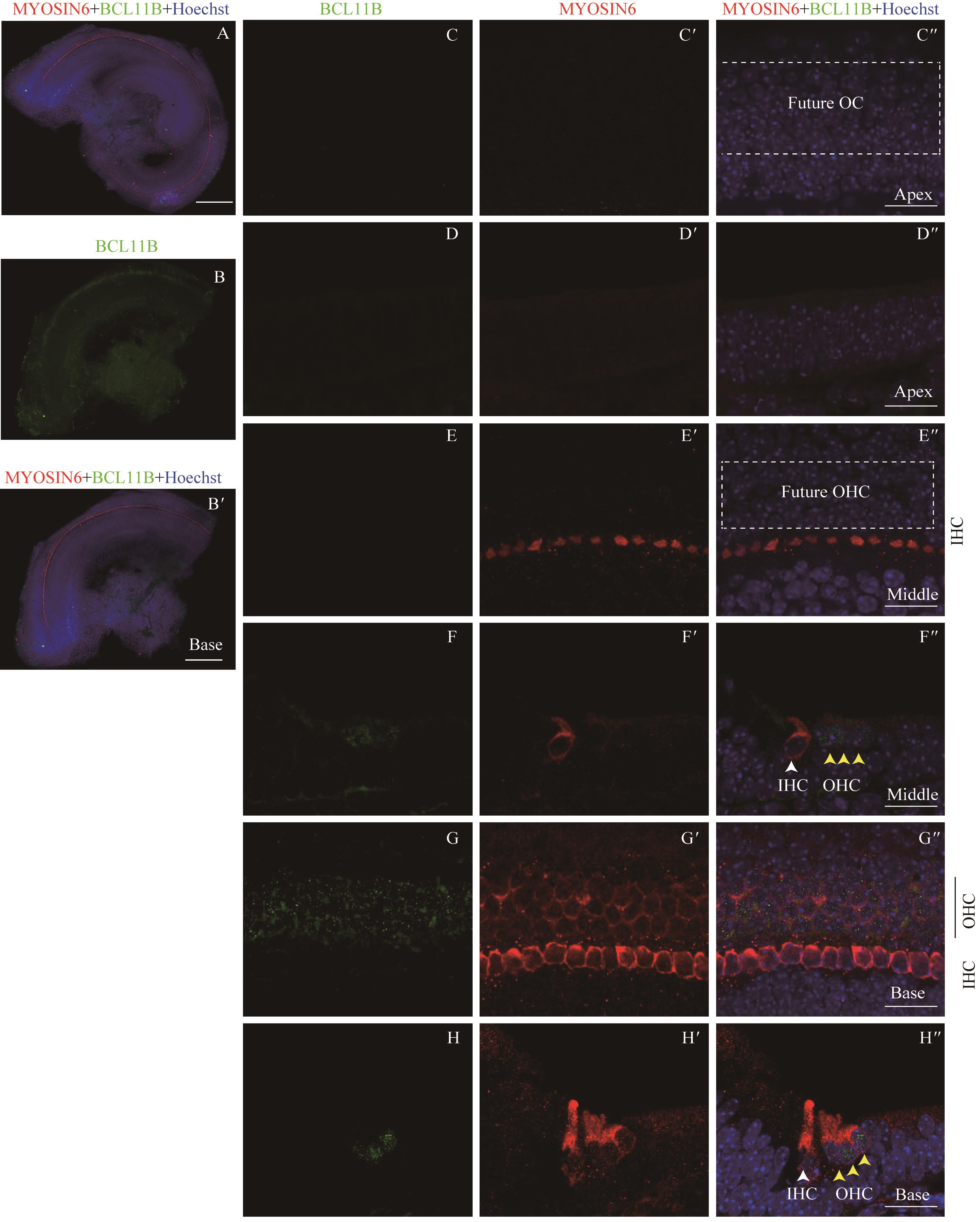
图2 共聚焦显微镜下观察E15.5时BCL11B与MYOSIN6在胎鼠耳蜗螺旋器中的表达Note:A: Expression and distribution of BCL11B and MYOSIN6 in the organ of Corti under the low-magnification microscope (×10). It shows the merged image of the apical turn, middle turn and basal turn of the same cochlea, bar=200 μm. B. Expression of BCL11B in the basal turn of organ of Corti of whole mount samples. B'. Merged image of B, MYOSIN6 and nuclear staining (×10). Bar=200 μm. C/C'. Expression of BCL11B (C) and MYOSIN6 (C') in the apical turn of whole mount samples under the high-magnification microscope (×60). C". Merged image of C, C' and nuclear staining (×60). Bar=20 μm. D/D'. Expression of BCL11B (D) and MYOSIN6 (D') in the apical turn of cross section samples under the high-magnification microscope (×60). D". Merged image of D, D' and nuclear staining (×60). Bar=20 μm. E/E'. Expression of BCL11B (E) and MYOSIN6 (E') in the middle turn of whole mount samples under the high-magnification microscope (×60). E". Merged image of E, E' and nuclear staining (×60). Bar=20 μm. F/F'. Expression of BCL11B (F) and MYOSIN6 (F') in the middle turn of cross section samples under the high-magnification microscope (×60). F". Merged image of F, F' and nuclear staining (×60). Bar=20 μm. G/G'. Expression of BCL11B (G) and MYOSIN6 (G') in the basal turn of whole mount samples under the high-magnification microscope (×60). G". Merged image of G, G' and nuclear staining (×60). Bar=20 μm. H/H'. Expression of BCL11B (H) and MYOSIN6 (H') in the basal turn of cross section samples under the high-magnification microscope (×60). H". Merged image of H, H' and nuclear staining (×60). Bar=20 μm. Green signal represents BCL11B, red signal represents MYOSIN6, and blue signal represents nucleus; white box represents prosensory region; white arrow represents IHC; black vertical line and yellow arrow represent OHC; OC—organ of Corti; IHC—inner hair cell; OHC—outer hair cell.
Fig 2 Expression of BCL11B and MYOSIN6 in the organ of Corti at E15.5 mouse cochlea by confocal imaging
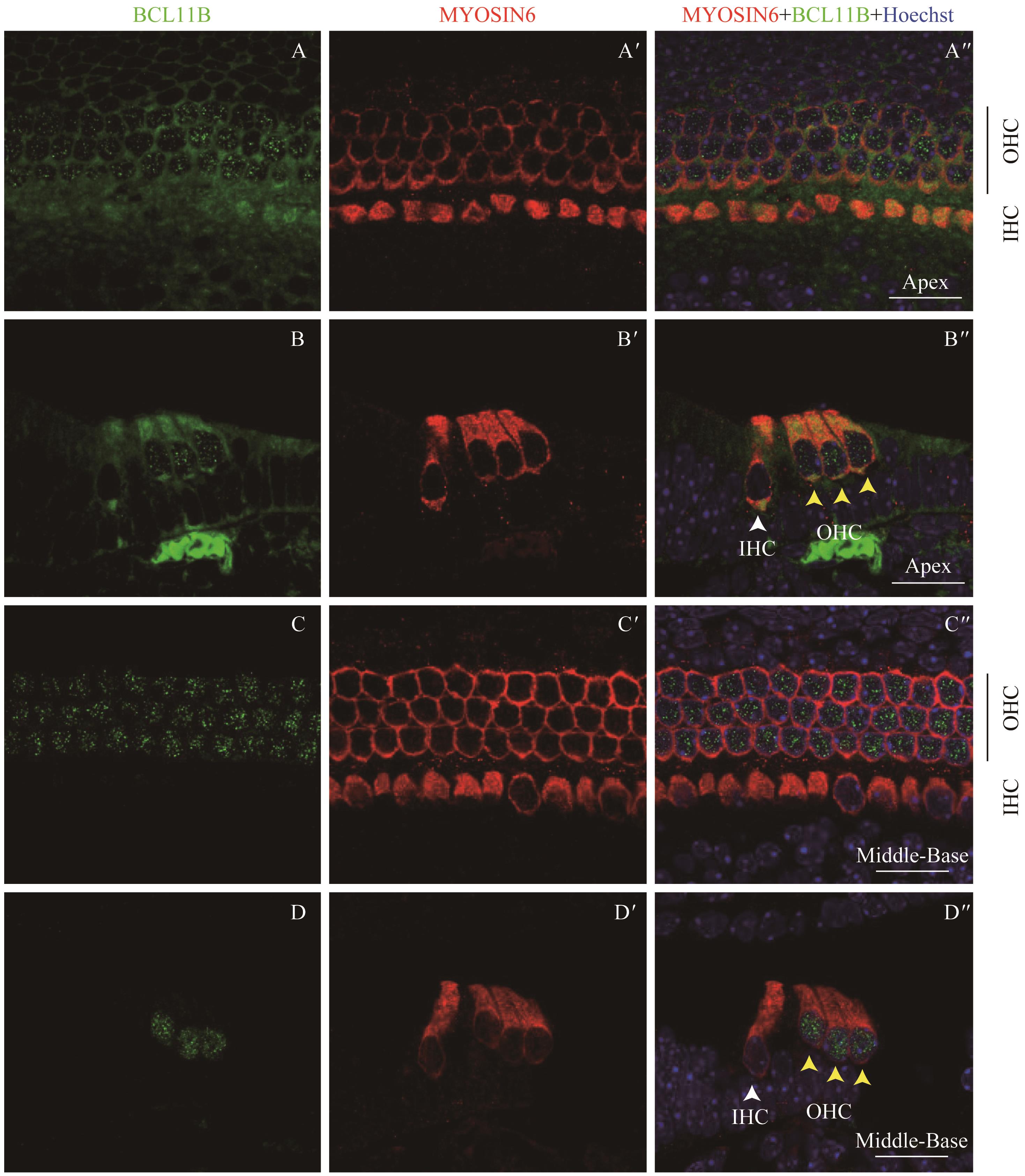
图3 共聚焦显微镜下观察E16.5时BCL11B与MYOSIN6在胎鼠耳蜗螺旋器中的表达Note:A/A'. Expression of BCL11B (A) and MYOSIN6 (A') in the apical turn of whole mount samples under the high-magnification microscope (×60). A''. Merged image of A, A' and nuclear staining (×60). B/B'. Expression of BCL11B (B) and MYOSIN6 (B') in the apical turn of cross section samples under the high-magnification microscope (×60). B''. Merged image of B, B' and nuclear staining (×60). C/C'. Expression of BCL11B (C) and MYOSIN6 (C') in the middle-basal turn of whole mount samples under the high-magnification microscope (×60). C''. Merged image of C, C' and nuclear staining (×60). D/D'. Expression of BCL11B (D) and MYOSIN6 (D') in the middle-basal turn of cross section samples under the high-magnification microscope (×60). D''. Merged image of D, D' and nuclear staining (×60). Green signal represents BCL11B, red signal represents MYOSIN6, and blue signal represents nucleus; white arrow represents IHC; black vertical line and yellow arrow represent OHC; OHC—outer hair cell; IHC—inner hair cell. Bar=20 μm.
Fig 3 Expression of BCL11B and MYOSIN6 in the organ of Corti at E16.5 mouse cochlea by confocal imaging
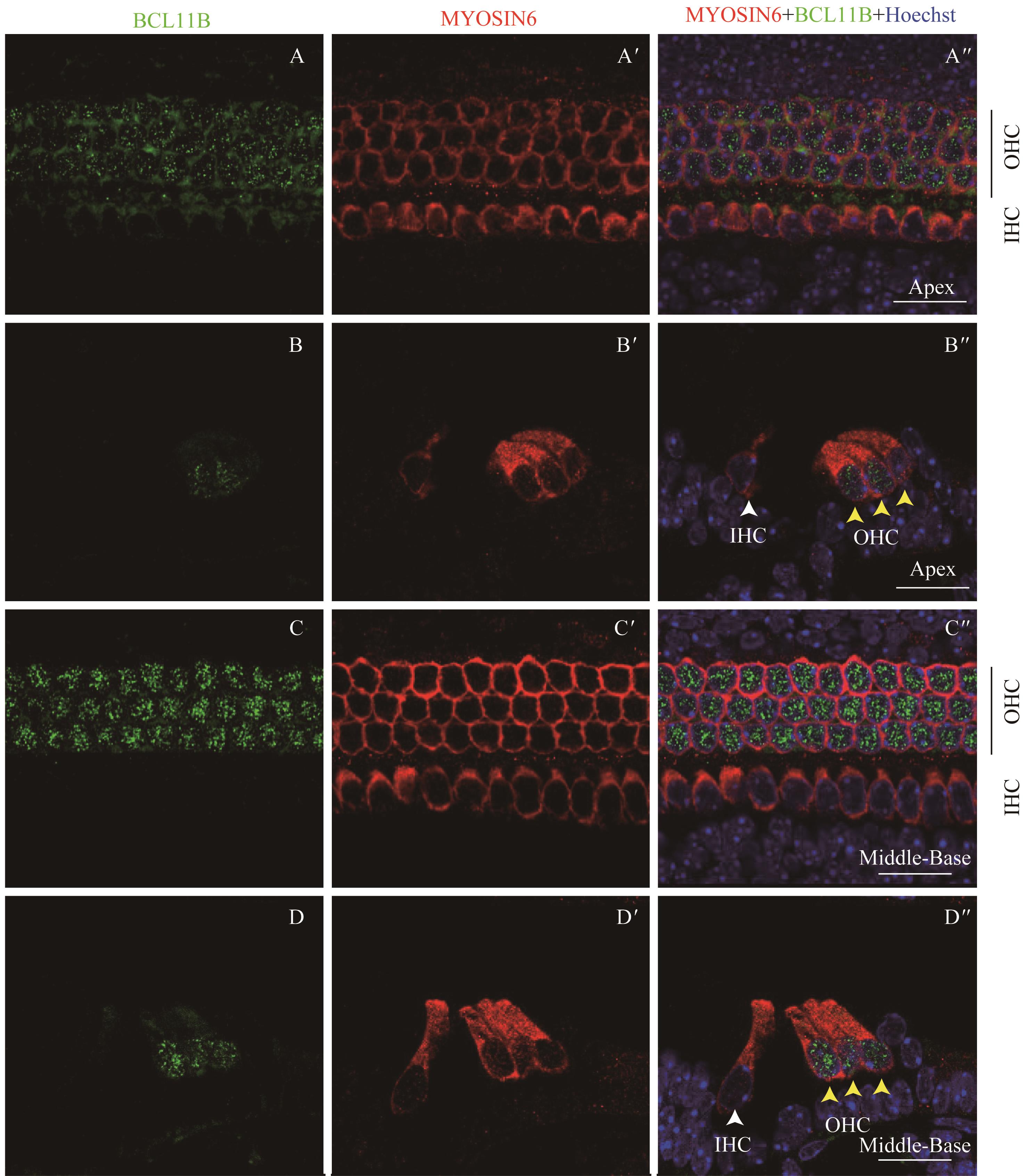
图4 共聚焦显微镜下观察E18.5时BCL11B与MYOSIN6在胎鼠耳蜗螺旋器中的表达Note:A/A'. Expression of BCL11B (A) and MYOSIN6 (A') in the apical turn of whole mount samples under the high-magnification microscope (×60). A". Merged image of A, A' and nuclear staining (×60). B/B'. Expression of BCL11B (B) and MYOSIN6 (B') in the apical turn of cross section samples under the high-magnification microscope (×60). B". Merged image of B, B' and nuclear staining (×60). C/C'. Expression of BCL11B (C) and MYOSIN6 (C') in the middle-basal turn of whole mount samples under the high-magnification microscope (×60). C". Merged image of C, C' and nuclear staining (×60). D/D'. Expression of BCL11B (D) and MYOSIN6 (D') in the middle-basal turn of cross section samples under the high-magnification microscope (×60). D". Merged image of D, D' and nuclear staining (×60). Green signal represents BCL11B, red signal represents MYOSIN6, and blue signal represents nucleus; white arrow represents IHC; black vertical line and yellow arrow represent OHC; OHC—outer hair cell; IHC—inner hair cell. Bar=20 μm.
Fig 4 Expression of BCL11B and MYOSIN6 in the organ of Corti at E18.5 mouse cochlea by confocal imaging
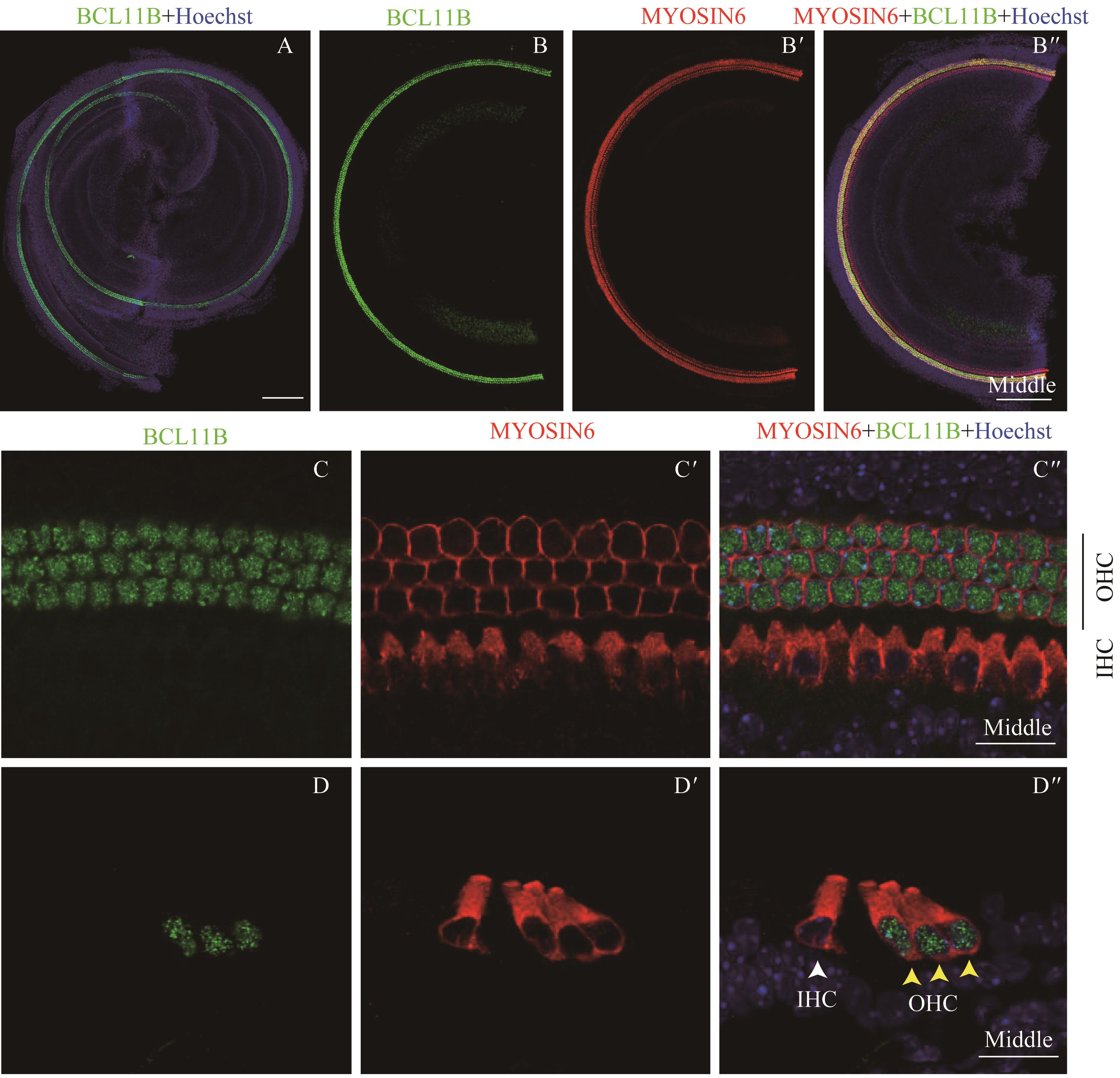
图5 共聚焦显微镜下观察P0时BCL11B与MYOSIN6在乳鼠耳蜗螺旋器中的表达Note:A. Expression and distribution of BCL11B and MYOSIN6 in inner hair cells under the low-magnification microscope (×10). It shows the merged image of the apical turn, middle turn and basal turn of the same cochlea, bar=200 μm. B/B'. Expression of BCL11B (B) and MYOSIN6 (B') in the hair cells of middle turn of whole mount samples. B". Merged image of B, B' and nuclear staining (×10). Bar=200 μm. C/C'. Expression of BCL11B (C) and MYOSIN6 (C') in the middle turn of whole mount samples under the high-magnification microscope (×60). C". Merged image of C, C' and nuclear staining (×60). Bar=20 μm. D/D'. Expression of BCL11B (D) and MYOSIN6 (D') in the middle turn of cross section samples under the high-magnification microscope (×60). D". Merged image of D, D' and nuclear staining (×60). Bar=20 μm. Green signal represents BCL11B, red signal represents MYOSIN6, and blue signal represents nucleus; white arrow represents IHC; black vertical line and yellow arrow represent OHC; OHC—outer hair cell; IHC—inner hair cell.
Fig 5 Expression of BCL11B and MYOSIN6 in the organ of Corti at P0 mouse cochlea by confocal imaging
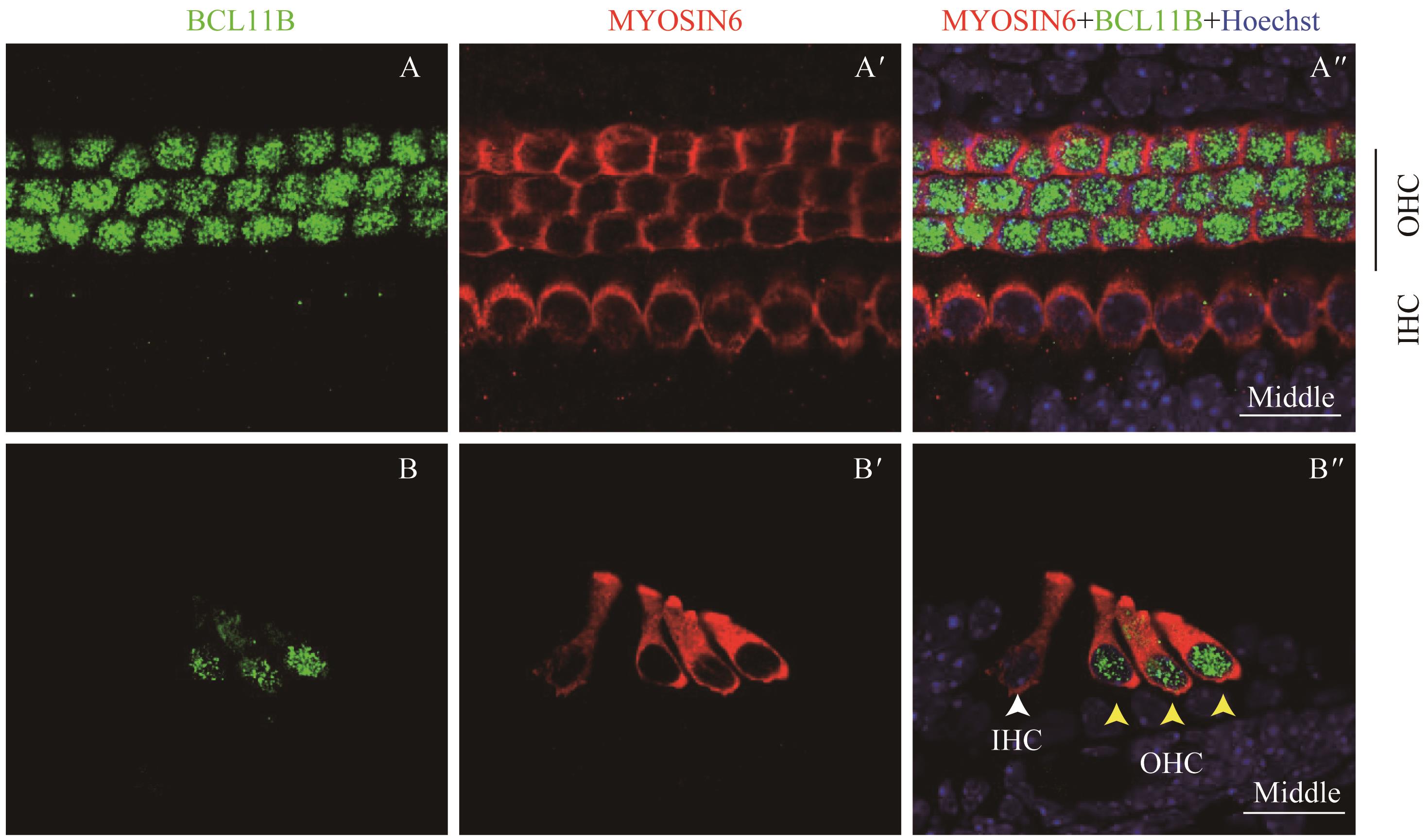
图6 共聚焦显微镜下观察P2时BCL11B与MYOSIN6在乳鼠耳蜗螺旋器中的表达Note:A/A'. Expression of BCL11B (A) and MYOSIN6 (A') in the middle turn of whole mount samples under the high-magnification microscope (×60). A". Merged image of A, A' and nuclear staining (×60). B/B'. Expression of BCL11B (B) and MYOSIN6 (B') in the middle turn of cross section samples under the high-magnification microscope (×60). B". Merged image of B, B' and nuclear staining (×60). Green signal represents BCL11B, red signal represents MYOSIN6, and blue signal represents nucleus; white arrow represents IHC; black vertical line and yellow arrow represent OHC; OHC—outer hair cell; IHC—inner hair cell. Bar=20 μm.
Fig 6 Expression of BCL11B and MYOSIN6 in the organ of Corti at P2 mouse cochlea by confocal imaging
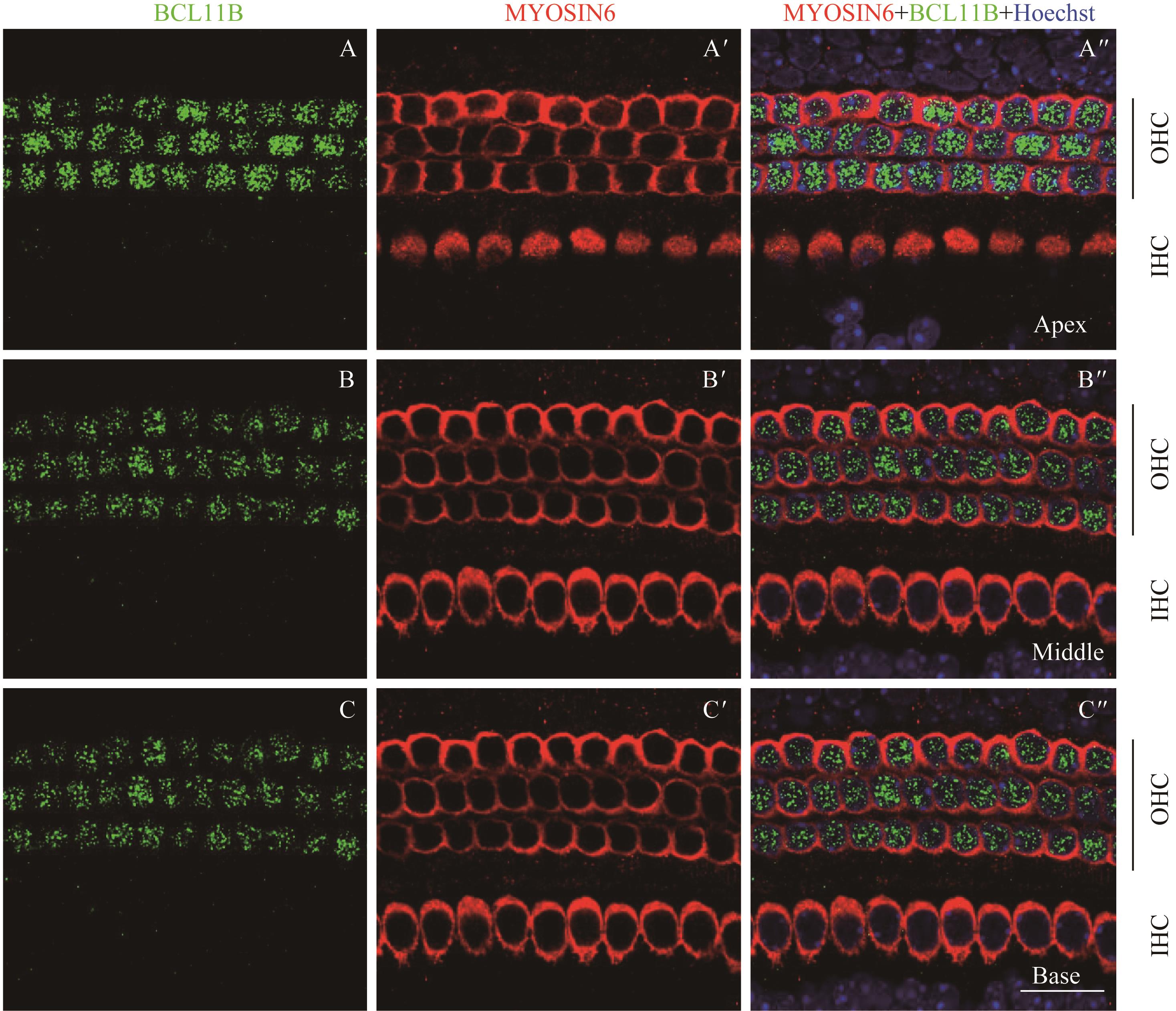
图7 共聚焦显微镜下观察P4时BCL11B与MYOSIN6在乳鼠耳蜗螺旋器中的表达Note:A/A'. Expression of BCL11B (A) and MYOSIN6 (A') in the apical turn of whole mount samples under the high-magnification microscope (×60). A". Merged image of A, A' and nuclear staining (×60). B/B'. Expression of BCL11B (B) and MYOSIN6 (B') in the middle turn of whole mount samples under the high-magnification microscope (×60). B". Merged image of B, B' and nuclear staining (×60). C/C'. Expression of BCL11B (C) and MYOSIN6 (C') in the basal turn of whole mount samples under the high-magnification microscope (×60). C". Merged image of C, C' and nuclear staining (×60). Green signal represents BCL11B, red signal represents MYOSIN6, and blue signal represents nucleus; black vertical line represents OHC; IHC—inner hair cell; OHC—outer hair cell. Bar=20 μm. As whole mount samples and cross section samples showed the same conclusion, only whole mount samples are presented here.
Fig 7 Expression of BCL11B and MYOSIN6 in the organ of Corti at P4 mouse cochlea by confocal imaging
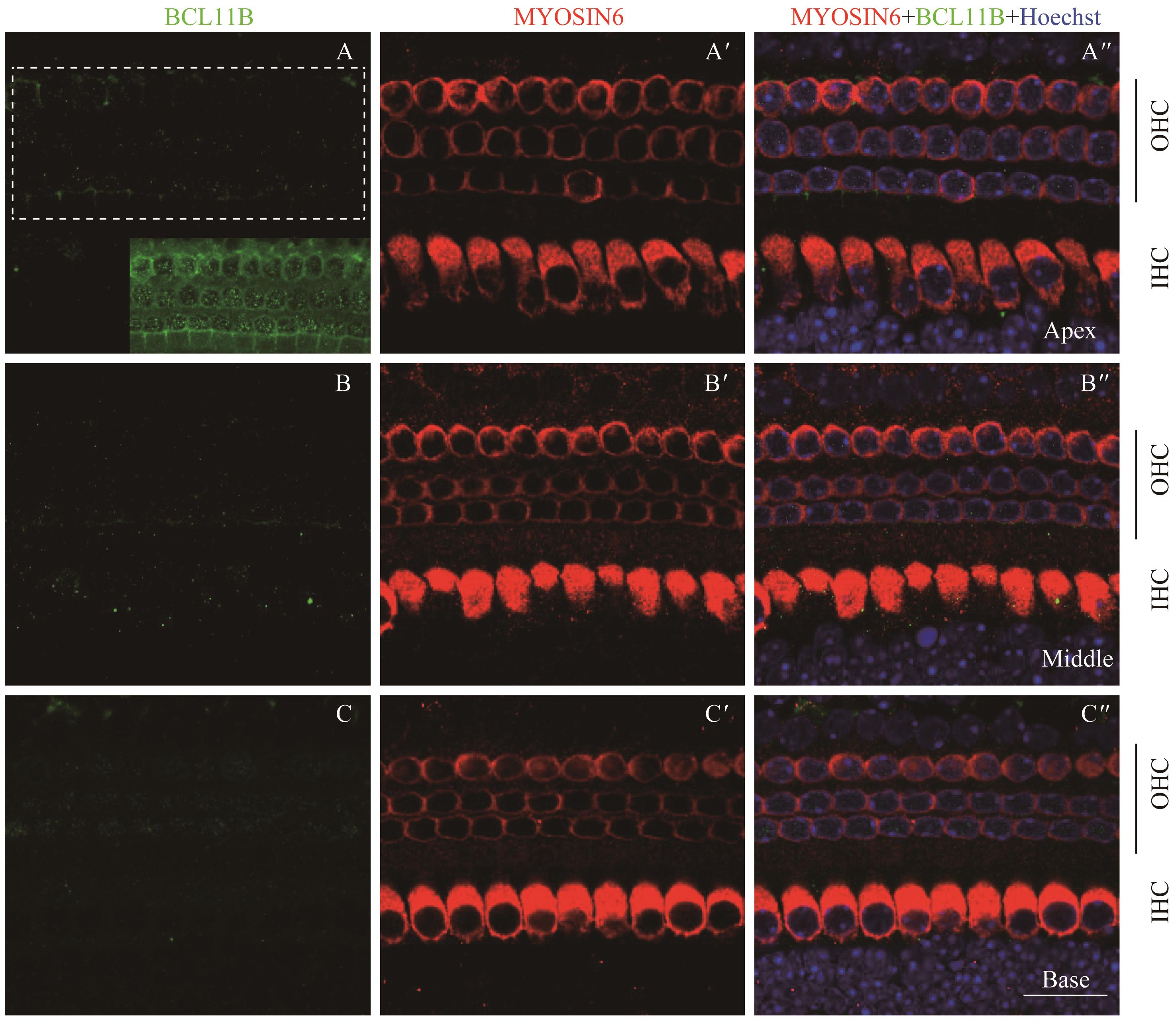
图8 共聚焦显微镜下观察P7时BCL11B与MYOSIN6在乳鼠耳蜗螺旋器中的表达Note:A/A'. Expression of BCL11B (A) and MYOSIN6 (A') in the apical turn of whole mount samples under the high-magnification microscope (×60). A". Merged image of A, A' and nuclear staining (×60). B/B'. Expression of BCL11B (B) and MYOSIN6 (B') in the middle turn of whole mount samples under the high-magnification microscope (×60). B". Merged image of B, B' and nuclear staining (×60). C/C'. Expression of BCL11B (C) and MYOSIN6 (C') in the basal turn of whole mount samples under the high-magnification microscope (×60). C". Merged image of C, C' and nuclear staining (×60). Green area at the bottom right of figure A is the overexposed image of the white dotted box area. Green signal represents BCL11B, red signal represents MYOSIN6, and blue signal represents nucleus; black vertical line represents OHC; IHC—inner hair cell; OHC—outer hair cell. Bar=20 μm. As whole mount samples and cross section samples showed the same conclusion, only whole mount samples are presented here.
Fig 8 Expression of BCL11B and MYOSIN6 in the organ of Corti at P7 mouse cochlea by confocal imaging
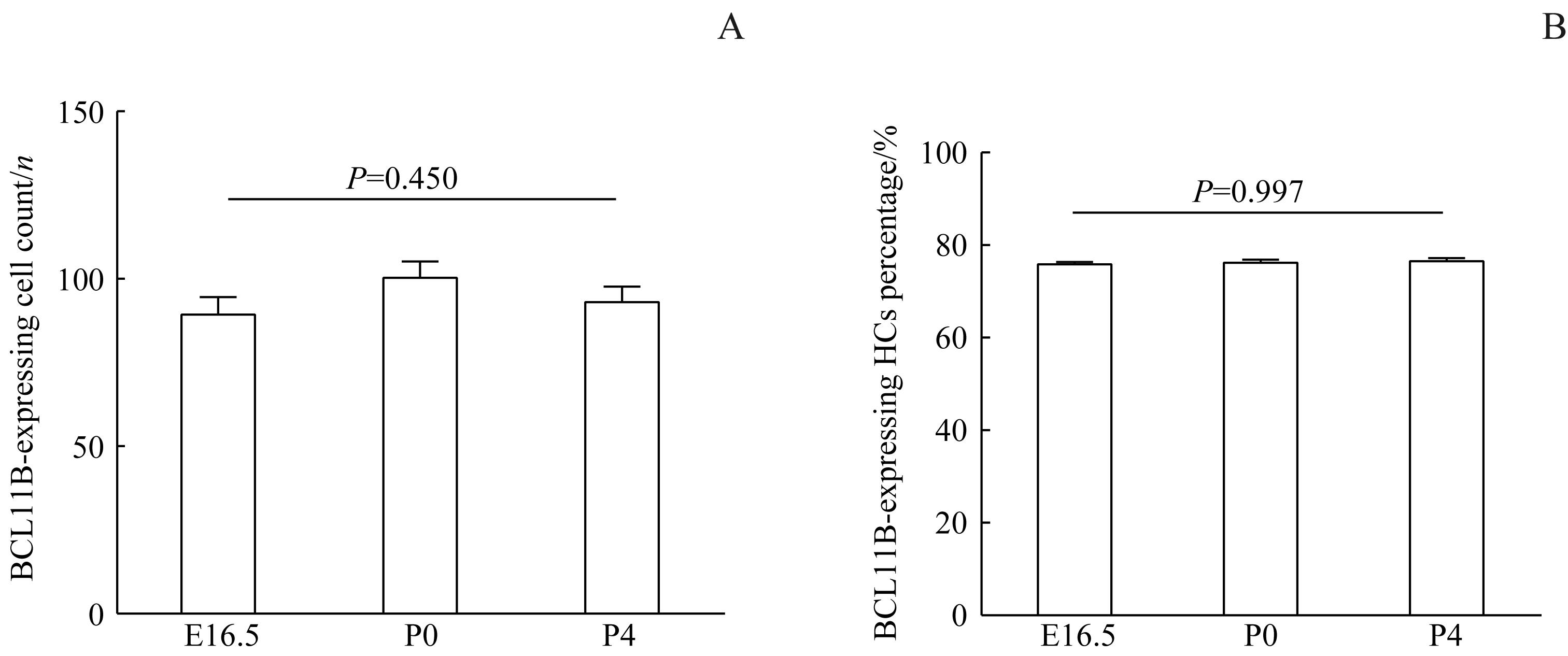
图9 E16.5、P0及P4时小鼠螺旋器中BCL11B阳性表达的毛细胞的计数及百分比统计Note:A. Statistics on the count of BCL11B-expressing HCs in mice. HCs—hair cells. B. Percentage statistics of BCL11B-expressing HCs in mice.
Fig 9 Count and percentage statistics of BCL11B-expressing HCs in the organ of Corti of mice at E16.5, P0 and P4
| 1 | BASCH M L, BROWN R M 2nd, JEN H I, et al. Where hearing starts: the development of the mammalian cochlea[J]. J Anat, 2016, 228(2): 233-254. |
| 2 | FRITZSCH B, PAN N, JAHAN I, et al. Inner ear development: building a spiral ganglion and an organ of Corti out of unspecified ectoderm[J]. Cell Tissue Res, 2015, 361(1): 7-24. |
| 3 | DRIVER E C, KELLEY M W. Development of the cochlea[J]. Development, 2020, 147(12): dev162263. |
| 4 | WALTERS B J, COAK E, DEARMAN J, et al. In vivo interplay between p27Kip1, GATA3, ATOH1, and POU4F3 converts non-sensory cells to hair cells in adult mice[J]. Cell Rep, 2017, 19(2): 307-320. |
| 5 | CHEN P, SEGIL N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti[J]. Development, 1999, 126(8): 1581-1590. |
| 6 | SATTERWHITE E, SONOKI T, WILLIS T G, et al. The BCL11 gene family: involvement of BCL11A in lymphoid malignancies[J]. Blood, 2001, 98(12): 3413-3420. |
| 7 | LI P, BURKE S, WANG J X, et al. Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion[J]. Science, 2010, 329(5987): 85-89. |
| 8 | SOTTILE R, PANJWANI M K, LAU C M, et al. Human cytomegalovirus expands a CD8+ T cell population with loss of BCL11B expression and gain of NK cell identity[J]. Sci Immunol, 2021, 6(63): eabe6968. |
| 9 | WANG C Y, SUN Y T, FANG K M, et al. Function of B-cell CLL/lymphoma 11B in glial progenitor proliferation and oligodendrocyte maturation[J]. Front Mol Neurosci, 2018, 11: 4. |
| 10 | LENNON M J, JONES S P, LOVELACE M D, et al. Bcl11b-A critical neurodevelopmental transcription factor-roles in health and disease[J]. Front Cell Neurosci, 2017, 11: 89. |
| 11 | WIWATPANIT T, LORENZEN S M, CANTÚ J A, et al. Trans-differentiation of outer hair cells into inner hair cells in the absence of INSM1[J]. Nature, 2018, 563(7733): 691-695. |
| 12 | OKUMURA H, MIYASAKA Y, MORITA Y, et al. Bcl11b heterozygosity leads to age-related hearing loss and degeneration of outer hair cells of the mouse cochlea[J]. Exp Anim, 2011, 60(4): 355-361. |
| 13 | AVRAHAM K B, HASSON T, STEEL K P, et al. The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells[J]. Nat Genet, 1995, 11(4): 369-375. |
| 14 | SUN S H, LI S T, LUO Z N, et al. Dual expression of Atoh1 and Ikzf2 promotes transformation of adult cochlear supporting cells into outer hair cells[J]. eLife, 2021, 10: e66547. |
| 15 | CHESSUM L, MATERN M S, KELLY M C, et al. Helios is a key transcriptional regulator of outer hair cell maturation[J]. Nature, 2018, 563(7733): 696-700. |
| 16 | LIBERMAN M C, GAO J G, HE D Z, et al. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier[J]. Nature, 2002, 419(6904): 300-304. |
| 17 | DALLOS P, FAKLER B. Prestin, a new type of motor protein[J]. Nat Rev Mol Cell Biol, 2002, 3(2): 104-111. |
| 18 | ZHENG J, TAKAHASHI S, ZHOU Y, et al. Prestin and electromotility may serve multiple roles in cochlear outer hair cells[J]. Hear Res, 2022, 423: 108428. |
| 19 | SHU Y, LI W, HUANG M, et al. Renewed proliferation in adult mouse cochlea and regeneration of hair cells[J]. Nat Commun, 2019, 10(1): 5530. |
| 20 | LEE S, SONG J J, BEYER L A, et al. Combinatorial Atoh1 and Gfi1 induction enhances hair cell regeneration in the adult cochlea[J]. Sci Rep, 2020, 10(1): 21397. |
| 21 | CHEN Y, GU Y Y, LI Y G, et al. Generation of mature and functional hair cells by co-expression of Gfi1, Pou4f3, and Atoh1 in the postnatal mouse cochlea[J]. Cell Rep, 2021, 35(3): 109016. |
| 22 | FANG J, ZHANG W C, YAMASHITA T, et al. Outer hair cell-specific prestin-CreERT2 knockin mouse lines[J]. Genesis, 2012, 50(2): 124-131. |
| [1] | 张钦杰, 黄穗, 谭皓月, 周祥, 王君怡, 刘雨滋, 文雯, 郭嘉, 吴皓, 贾欢. 听觉脑干植入声码器模型的开发及验证[J]. 上海交通大学学报(医学版), 2024, 44(10): 1279-1286. |
| [2] | 周卫军, 刘思迪, 蔡瑞捷, 刘宏超, 王美建, 吴皓, 刘辉辉, 汪照炎. 星形胶质细胞在噪声损伤后小鼠耳蜗核突触修复中的作用[J]. 上海交通大学学报(医学版), 2024, 44(4): 454-461. |
| [3] | 杨璐, 黄美萍, 周嵌, 李进, 李蕴, 黄治物. 蜗神经发育不良先天性耳聋儿童人工耳蜗植入干预的听觉及言语能力长效评估研究[J]. 上海交通大学学报(医学版), 2023, 43(7): 890-897. |
| [4] | 洪晗馨, 王龙昊, 刘辉辉, 彭浒, 吴皓, 杨涛. 线粒体内膜转位酶8A基因敲除小鼠的构建及其内耳功能研究[J]. 上海交通大学学报(医学版), 2023, 43(3): 261-268. |
| [5] | 黄治物, 吴皓. 年龄相关性听力损失研究进展与临床干预策略[J]. 上海交通大学学报(医学版), 2022, 42(9): 1182-1187. |
| [6] | 成桢哲, 金晨曦, 冯宝怡, 郑晓飞, 刘祎晴, 吴皓, 陶永. 基于腺相关病毒血清型8型介导的Gjb2基因c.109G>A纯合突变耳聋小鼠的基因治疗[J]. 上海交通大学学报(医学版), 2022, 42(6): 735-741. |
| [7] | 周祥, 潘金锡, 张钦杰, 李蕴, 陈颖, 谭皓月, 彭飞, 黄穗, 谭治平, 吴皓, 贾欢. 听觉脑干植入豚鼠模型构建的标准化步骤及评价[J]. 上海交通大学学报(医学版), 2022, 42(5): 583-590. |
| [8] | 周佳蕾, 盛海斌, 王皓煜, 鲁岩, 王方方, 吴皓, 华云峰. 三维电镜在脑干耳蜗核神经元形态学研究中的应用[J]. 上海交通大学学报(医学版), 2022, 42(2): 142-149. |
| [9] | 姚俊吉, 陈见清, 谭皓月, 汪照炎, 张治华, 吴皓, 贾欢. 听神经瘤自然生长规律与症状演变的初步分析:56例患者回顾[J]. 上海交通大学学报(医学版), 2021, 41(7): 898-902. |
| [10] | 贾 欢1, 2*,陈 颖1, 2*,张治华1, 2,李静洁3,李 蕴1, 2,陈见清1, 2,李 孛1, 2,谭皓月1, 2,汪照炎1, 2,吴 皓1, 2. 人工听觉脑干植入在先天性耳聋低龄儿童中的应用探索[J]. 上海交通大学学报(医学版), 2020, 40(10): 1324-1329. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||