弥漫大B细胞淋巴瘤(diffuse large B-cell lymphoma,DLBCL)是非霍奇金淋巴瘤(non-Hodgkin's lymphoma,NHL)中最常见的病理亚型,占所有NHL的30%~40%[1]。DLBCL在临床特点、实验室指标、免疫表型、对治疗方案的反应性等方面具有很强的异质性,影响疗效及预后。R-CHOP(利妥昔单抗+环磷酰胺+阿霉素+长春新碱/长春地辛+泼尼松)是目前治疗DLBCL的标准方案[2];然而,仍有30%~40%的患者存在耐药和复发等问题[3]。复发/难治性DLBCL通过包括自体造血干细胞移植(autologous stem cell transplantation,ASCT)在内的二线或三线治疗也许能够缓解疾病进展,但整体预后仍不佳[4],这使得该部分患者成为DLBCL治疗的难点。本研究旨在通过回顾性分析DLBCL患者的临床特征,分析这些临床指标对患者疗效及生存的影响,同时分析挽救性ASCT、巩固性ASCT及利妥昔单抗巩固维持治疗对患者总生存(overall survival,OS)的影响。
1 对象与方法
1.1 病例收集
收集上海交通大学医学院附属新华医院血液科2015年1月—2020年1月收治的198例经病理及免疫分型确诊的DLBCL患者的临床资料。除外原发中枢神经系统DLBCL 10例、合并第二肿瘤6例、基础情况差而无法耐受化学治疗(化疗)10例、临床及病理信息不完善12例,最终入组160例患者。
1.2 研究指标
分析性别、B组症状、原发部位、年龄、免疫分型、美国东部肿瘤协作组(Eastern Cooperative Oncology Group,ECOG)评分、国际预后指数(International Prognostic Index,IPI)、临床分期、乳酸脱氢酶(lactate dehydrogenase,LDH)、白蛋白、血红蛋白、淋巴细胞绝对值等对患者疗效及生存的影响。中期疗效评估依据CHESON等[5]提出的Lugona标准,包括完全缓解(complete remission,CR)、部分缓解(partial remission,PR)、疾病稳定(stable disease,SD)、疾病进展(progressive disease,PD)。同时,收集初次诊疗后2年内复发/难治的54例患者的临床数据,分析其临床特征,并评估ASCT对DLBCL患者预后的影响,54例复发/难治性患者中有4例接受了ASCT。目前,对复发/难治性DLBCL尚无明确定义。在本研究中满足以下任何一项即可诊断:①在4个或4个以上R-CHOP周期后未达到PR状态。②经过2个或2个以上周期的挽救治疗后未达到PR状态。③ASCT后12个月内疾病进展[6]。
1.3 病例分组及疗效评估
将患者IPI评分≥3分定义为高危。近期疗效指中期评估时的疗效,一般指规律化疗4~6周期后的系统评估。中期评估时疾病达到CR状态的患者有93例,其中高危组45例、低危组48例。高危组患者中接受ASCT的患者6例,接受利妥昔单抗维持治疗的患者3例,未接受进一步巩固治疗的患者36例。本研究中患者随访的截止日期为2021年12月31日,通过电话和医疗文书获得患者随访记录。总有效率定义为CR率+PR率。通过电话和医疗文书获得患者的无进展生存期(progression-free survival,PFS)和OS资料。
1.4 统计学方法
用SPSS 26.0软件分析数据。定性资料采用χ2检验或Fisher精确概率法。单因素及多因素分析采用 COX比例风险模型,PH(proportional hazards)假定用以检验各变量能否纳入COX比例风险模型。采用Kaplan-Meier方法绘制生存曲线,组间比较采用Log-rank检验。在分组分析前,对基线变量进行差异分析;如果变量的分布有显著差异,进行倾向评分匹配。P<0.05表示差异有统计学意义。
2 结果
2.1 一般资料
160例DLBCL患者的临床特征见表1。男性患者共有88例(55.0%),中位年龄是62(25~88)岁。根据IPI评分的年龄截点,将患者初治年龄分为2组,其中年龄>60岁的患者有87例(54.4%)。初治时存在发热、盗汗、消瘦等B组症状者73例(45.6%),经Hans分型为非生发中心B细胞(non germinal center B cells,Non-GCB)者104例(65%),初治时ECOG评分<2分者117例(73.1%),Ann Arbor分期Ⅰ~Ⅱ期63例(39.4%),IPI评分0~2分70例(43.8%),初治LDH水平升高者109例(68.1%),低白蛋白者37例(23.1%),淋巴细胞绝对值计数降低者56例(35.0%),贫血患者54例(33.8%),初次化疗方案以R-CHOP方案为主的患者147例(91.9%)。
表1 DLBCL患者基本临床特征 (n=160)
Tab 1
| Variable | Patients/n(%) |
|---|---|
| Gender | |
| Male | 88 (55.0) |
| Female | 72 (45.0) |
| Age | |
| ≤60 years | 73 (45.6) |
| >60 years | 87 (54.4) |
| Group B symptoms | |
| No | 87 (54.4) |
| Yes | 73 (45.6) |
| Hans classification | |
| GCB | 56 (35.0) |
| Non-GCB | 104 (65.0) |
| ECOG score | |
| <2 scores | 117 (73.1) |
| ≥2 scores | 43 (26.9) |
| Ann Arbor stage | |
| Ⅰ‒Ⅱ | 63 (39.4) |
| Ⅲ‒Ⅳ | 97 (60.6) |
| IPI score | |
| 0‒2 scores | 70 (43.8) |
| 3‒5 scores | 90 (56.2) |
| LDH level | |
| ≤211 U·L-1 | 51 (31.9) |
| >211 U·L-1 | 109 (68.1) |
| Albumin level | |
| ≥35 g·L-1 | 123 (76.9) |
| <35 g·L-1 | 37 (23.1) |
| Absolute lymphocyte level | |
| >1.1×109·L-1 | 104 (65.0) |
| ≤1.1×109·L-1 | 56 (35.0) |
| Anemia | |
| No | 106 (66.2) |
| Yes | 54 (33.8) |
| Chemotherapy regimen | |
| R-CHOP | 147 (91.9) |
| CHOP | 13 (8.1) |
2.2 DLBCL患者近期疗效分析
160例DLBCL患者中,年龄≤60岁组患者近期总有效率为94.5%,年龄>60岁组患者为79.3%(P=0.005);IPI评分0~2分组患者近期总有效率为92.9%,IPI评分3~5分组患者为81.1%(P=0.032);白蛋白正常组患者近期总有效率为91.1%,低白蛋白组为70.3%(P=0.001);非贫血组近期总有效率为91.5%,贫血组为75.9%(P=0.007)。该结果提示,初治年龄>60岁、IPI评分3~5分、低白蛋白水平及贫血患者近期疗效不佳,而性别、初治时有无B组症状、Hans分型、ECOG评分、临床分期、LDH水平、淋巴细胞绝对值、化疗方案等因素的组间近期总有效率比较,差异无统计学意义(表2)。
表2 DLBCL患者近期疗效分析
Tab 2
| Variable | Overall response rate (CR+PR) | |
|---|---|---|
| Patients/n(%) | P value | |
| Gender | ||
| Male | 74/88 (84.1) | 0.381 |
| Female | 64/72 (88.9) | |
| Age | ||
| ≤60 years | 69/73 (94.5) | 0.005 |
| >60 years | 69/87 (79.3) | |
| Group B symptoms | ||
| No | 79/87 (90.8) | 0.068 |
| Yes | 59/73 (80.8) | |
| Hans classification | ||
| GCB | 51/56 (91.1) | 0.194 |
| Non-GCB | 87/104 (89.7) | |
| ECOG score | ||
| <2 scores | 104/117 (88.9) | 0.110 |
| ≥2 scores | 34/43 (79.1) | |
| Ann Arbor stage | ||
| Ⅰ‒Ⅱ | 57/63 (90.5) | 0.211 |
| Ⅲ‒Ⅳ | 81/97 (83.5) | |
| IPI score | ||
| 0‒2 scores | 65/70 (92.9) | 0.032 |
| 3‒5 scores | 73/90 (81.1) | |
| LDH level | ||
| ≤211 U·L-1 | 47/51 (92.2) | 0.138 |
| >211 U·L-1 | 91/109 (83.5) | |
| Albumin level | ||
| ≥35 g·L-1 | 112/123 (91.1) | 0.001 |
| <35 g·L-1 | 26/37 (70.3) | |
| Absolute lymphocyte level | ||
| >1.1×109·L-1 | 93/104 (89.4) | 0.112 |
| ≤1.1×109·L-1 | 45/56 (80.4) | |
| Anemia | ||
| No | 97/106 (91.5) | 0.007 |
| Yes | 41/54 (75.9) | |
| Chemotherapy regimen | ||
| R-CHOP | 126/147 (85.7) | 0.508 |
| CHOP | 12/13 (92.3) | |
2.3 影响DLBCL患者预后的单因素和多因素分析
将单因素COX回归分析中P<0.05的变量经过PH假定后纳入多因素分析中。结果显示:患者初治年龄>60岁(HR=2.788,95%CI 1.575~4.936,P=0.000),non-GCB亚型(HR=2.230,95%CI 1.150~4.324,P=0.018),LDH水平升高(HR=2.064,95%CI 1.006~4.234,P=0.048),低白蛋白水平(HR=2.052,95%CI 1.169~3.602,P=0.012)是影响患者PFS的独立危险因素(表3)。患者初治年龄>60岁(HR=2.269,95%CI 1.060~4.860,P=0.035),IPI评分3~5分(HR=2.557,95%CI 1.132~5.778,P=0.024)是影响患者OS的独立危险因素(表4)。
表 3 影响DLBCL患者PFS 的单因素和多因素分析
Tab 3
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Female | 0.668 (0.394‒1.132) | 0.134 | |||
| Age>60 years | 2.744 (1.553‒4.848) | 0.001 | 2.788 (1.575‒4.936) | 0.000 | |
| Group B symptoms | 1.424 (0.850‒2.386) | 0.179 | |||
| Non-GCB | 2.398 (1.243‒4.624) | 0.009 | 2.230 (1.150‒4.324) | 0.018 | |
| ECOG score ≥2 | 2.330 (1.379‒3.937) | 0.002 | |||
| Ann Arbor stage Ⅲ‒Ⅳ | 2.496 (1.366‒4.561) | 0.003 | |||
| IPI score 3‒5 | 3.149 (1.722‒5.759) | 0.000 | |||
| Increased LDH [>211 U·L-1] | 2.796 (1.413‒5.535) | 0.003 | 2.064 (1.006‒4.234) | 0.048 | |
| Low albumin [<35 g·L-1] | 2.824 (1.657‒4.811) | 0.000 | 2.052 (1.169‒3.602) | 0.012 | |
| Lymphocytopenia (≤1.1×109·L-1) | 2.142 (1.277‒3.592) | 0.004 | |||
| Anemia | 1.894 (1.128‒3.179) | 0.016 | |||
| Chemotherapy (R-CHOP) | 0.937 (0.400‒2.195) | 0.881 | |||
表4 影响 DLBCL患者OS的单因素和多因素分析
Tab 4
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR(95%CI) | P value | HR(95%CI) | P value | ||
| Female | 0.586 (0.296‒1.158) | 0.124 | |||
| Age>60 years | 2.950 (1.416‒6.145) | 0.004 | 2.269 (1.060‒4.860) | 0.035 | |
| Group B symptoms | 1.283 (0.667‒2.468) | 0.455 | |||
| Non-GCB | 1.515 (0.712‒3.223) | 0.281 | |||
| ECOG score ≥2 | 2.122 (1.092‒4.124) | 0.027 | |||
| Ann Arbor stage Ⅲ‒Ⅳ | 2.295 (1.078‒4.888) | 0.031 | |||
| IPI score 3‒5 | 3.261 (1.484‒7.166) | 0.003 | 2.557 (1.132‒5.778) | 0.024 | |
| Increased LDH [>211 U·L-1] | 3.422 (1.329‒8.808) | 0.011 | |||
| Low albumin [<35 g·L-1] | 2.423 (1.238‒4.745) | 0.010 | |||
| Lymphocytopenia (≤1.1×109·L-1) | 1.760 (0.911‒3.402) | 0.093 | |||
| Anemia | 1.720 (0.891‒3.320) | 0.106 | |||
| Chemotherapy (R-CHOP) | 1.422 (0.431‒4.699) | 0.563 | |||
2.4 复发/难治性DLBCL患者死亡的危险因素分析
160例初次诊断及治疗的DLBCL患者,2年内复发/难治患者有54例(33.8%),至随访终止时出现死亡事件的有34例。54例复发/难治性患者中位年龄为64岁(29~88岁),初治年龄>60岁(70.4%)、non-GCB亚型(81.5%)、Ann Arbor Ⅲ~Ⅳ期(77.8%)、IPI评分3~5分(77.8%)及LDH升高者(85.2%)居多。其中,初治年龄>60岁患者中死亡病例25例(65.8%),non-GCB亚型患者中死亡病例26例(59.1%),Ann Arbor分期Ⅲ~Ⅳ期患者中死亡病例26例(61.9%),IPI评分3~5分患者中死亡病例27例(64.3%),初治LDH水平升高患者中死亡病例30例(65.2%)。不同临床指标水平的患者间比较,死亡率的差异无统计学意义(P>0.05),见表5。
表5 复发/难治性DLBCL患者死亡的危险因素分析
Tab 5
| Variable | Death rate | |
|---|---|---|
| Patients/n(%) | P value | |
| Gender | ||
| Male | 23/35 (65.7) | 0.570 |
| Female | 11/19 (57.9) | |
| Age | ||
| ≤60 years | 9/16 (56.3) | 0.507 |
| >60 years | 25/38 (65.8) | |
| Group B symptoms | ||
| No | 17/25 (68.0) | 0.477 |
| Yes | 17/29 (58.6) | |
| Hans classification | ||
| GCB | 8/10 (80.0) | 0.383 |
| Non-GCB | 26/44 (59.1) | |
| ECOG score | ||
| <2 scores | 20/31 (64.5) | 0.784 |
| ≥2 scores | 14/23 (60.9) | |
| Ann Arbor stage | ||
| Ⅰ‒Ⅱ | 8/12 (66.7) | 1.000 |
| Ⅲ‒Ⅳ | 26/42 (61.9) | |
| IPI score | ||
| 0‒2 scores | 7/12 (58.3) | 0.970 |
| 3‒5 scores | 27/42 (64.3) | |
| LDH level | ||
| ≤211 U·L-1 | 4/8 (50.0) | 0.670 |
| >211 U·L-1 | 30/46 (65.2) | |
| Albumin level | ||
| ≥35 g·L-1 | 20/32 (62.5) | 0.932 |
| <35 g·L-1 | 14/22 (63.6) | |
| Absolute lymphocyte level | ||
| >1.1×109·L-1 | 19/27 (70.4) | 0.260 |
| ≤1.1×109·L-1 | 15/27 (55.6) | |
| Anemia | ||
| No | 19/29 (65.5) | 0.675 |
| Yes | 15/25 (60.0) | |
| ASCT | ||
| Yes | 0/4 (0) | 0.030 |
| No | 34/50 (68.0) | |
在病情复发进展的患者中,有4例接受了挽救性ASCT,随访3年内未出现死亡事件,提示接受ASCT是复发/难治性DLBCL患者死亡事件发生的保护性因素(P=0.030),见表5。
2.5 ASCT和利妥昔单抗维持治疗对DLBCL患者OS的影响
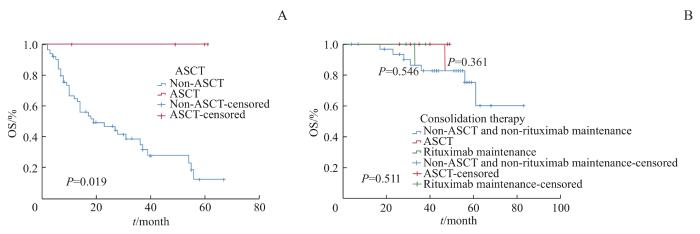
160例初次诊断及治疗的DLBCL患者中,2年内复发或快速进展的患者有54例(33.8%),经过CHOP/R-CHOP为主的规律化疗,中期经PET-CT评估达到CR的患者有93例(58.1%),其中IPI评分≥3分的高危患者有45例。为了进一步了解ASCT和利妥昔单抗维持巩固治疗对高危患者生存的影响,我们对复发/难治性患者接受和未接受挽救性ASCT与患者OS的关系进行分析。结果显示:至随访结束,接受挽救性ASCT的患者尚未出现死亡事件;而未接受ASCT的患者3年OS率为35.1%,5年OS率为12.3%,中位生存期18个月;2组间差异有统计学意义(P=0.019),见图1A。同时分析中期评估达到CR的高危组患者中接受巩固性ASCT的患者(n=6)、接受利妥昔单抗维持巩固治疗患者(n=3)和未接受ASCT及利妥昔单抗维持治疗的患者(n=36)的OS,结果显示:接受巩固性ASCT及利妥昔单抗维持治疗的患者,至随访结束尚未出现死亡事件;未接受巩固治疗组患者3年OS率为82.8%,5年OS率为60.2%;3组间差异无统计学意义(图1B)。
图1
图1
ASCT及利妥昔单抗维持治疗的DLBCL患者的生存曲线与生存分析
Note: A. OS curve of relapsed/refractory DLBCL patients with ASCT (Log-rank P=0.019). B. OS curve of ASCT or rituximab maintenance therapy in patients in the high-risk group with CR who had achieved interim assessment (ASCT vs no consolidation therapy, Log-rank P=0.361; rituximab maintenance vs no consolidation therapy, Log-rank P=0.546. There was no significant difference among the three groups, P=0.511).
Fig1
Survival curves and survival analysis of patients with ASCT or rituximab maintenance therapy
3 讨论
DLBCL患者在临床特征、基因表型、对治疗方案的反应性等方面具有很强的异质性。虽然R-CHOP方案的使用极大改善了患者的总体预后,但仍有部分复发/难治性患者预后不佳,是目前DLBCL治疗的难点。影响DLBCL预后的因素很多,本研究发现患者初治年龄>60岁和IPI评分3~5分是影响患者OS的独立危险因素。这些危险因素在复发/难治性DLBCL患者的临床特征中也得到验证;但对于复发/难治性患者,这些临床指标的预后价值明显下降。
近年来,随着基因测序与细胞免疫的发展,越来越多的治疗方法应用于复发/难治性DLBCL患者。CART(chimeric antigen receptor T-cell)疗法基于自体 T 细胞的基因修饰,以表达嵌合受体,靶向CD19等在DLBCL中高度表达的抗原,可使部分的复发/难治性DLBCL患者达到CR,有效提高患者总生存率[9-11]。WANG等[12]研究发现ASCT联合CART组与ASCT组相比,表现出更好的3年PFS,但3年OS未见明显差异。细胞免疫疗法是目前针对复发/难治性DLBCL患者较有前景的新型疗法,多项免疫治疗相关临床试验均取得较好的结果[13-16]。因本研究是回顾性分析,采用新型疗法的复发/难治性DLBCL患者较少,故暂未评估CART疗法及细胞免疫疗法在复发/难治性DLBCL患者中的治疗效果。这也是本研究的不足之处。
作者贡献声明
所有作者均参与研究设计。赵洁、姜言负责材料准备、数据收集和分析、论文撰写。赵洁、郝思国负责论文的修订。所有作者均阅读并同意了最终稿件的提交。
All the authors contributed to the study design. Material preparation, data collection and analysis,and paper writing were performed by ZHAO Jie and JIANG Yan. ZHAO Jie and HAO Siguo were responsible for the revision of the paper. All the authors have read the last version of paper and consented for submission.
利益冲突声明
所有作者声明不存在利益冲突。
All authors disclose no relevant conflict of interests.
参考文献