CRC的发生发展是一个多基因、多因素、多阶段的过程[7]。人体免疫系统在CRC的发生发展中发挥重要作用,主要效应细胞组分包括自然杀伤细胞(natural killer cell,NK细胞)、CD4+T细胞,CD8+T细胞、巨噬细胞和树突状细胞[8]等。NK细胞是一类具有细胞毒性的固有淋巴细胞,无需预先致敏即可杀伤病毒感染细胞和肿瘤细胞[9]。同时,NK细胞通过分泌细胞因子γ干扰素(interferon-γ,IFN-γ)、肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)等促炎性细胞因子,抑制肿瘤细胞增殖和血管生成等过程,参与抗肿瘤免疫的调控[10]。研究[11]显示肿瘤组织中NK细胞的浸润水平和患者的预后相关。有研究发现CRC患者外周血[12]、肿瘤组织[13]、转移性肝肿瘤[14]中的NK细胞比例越高,均预示着患者的临床结局更好。
在CRC中,肿瘤浸润的NK细胞数量、表型和功能都会发生改变。HALAMA等人[15]发现与癌旁组织相比,实体瘤中NK细胞的浸润数量显著减少。一项体外实验的研究[16]将NK细胞和人结肠癌细胞共培养后发现,NK细胞凋亡比例增加,其表面活化性受体CD16分子表达下调。基于癌症基因组图谱(The Cancer Genome Atlas,TCGA)数据库的分析[17]显示:预后较差的CRC患者,其肿瘤浸润的NK细胞表面表达程序性死亡受体1(programmed death 1,PD-1)、程序性死亡受体-配体1(programmed death-ligand 1,PD-L1)等免疫耗竭基因显著上调。另外,JOBIN等人[18]发现CRC患者外周血中的NK细胞分泌细胞因子IFN-γ的含量降低。这些研究表明,CRC中NK细胞的活性和功能可能受损,阻碍其识别和杀伤肿瘤,介导了肿瘤的免疫逃逸。然而,现有研究对NK细胞在CRC肿瘤微环境中的表型和功能变化关注较少。
本研究使用患者的新鲜CRC肿瘤组织和癌旁组织,制备单细胞悬液,运用流式细胞术检测CRC肿瘤微环境中的免疫细胞组成,深入探索NK细胞的表型特征和功能状态,以期为精准预测和临床治疗提供参考,助力发展靶向NK细胞的治疗新策略。
1 对象与方法
1.1 研究对象
1.1.1 临床样本
选取2022—2023年在上海交通大学医学院附属瑞金医院胃肠外科就医的CRC患者为研究对象。所有样本均采集自患者手术当天。纳入标准如下:①患者既往均未接受抗肿瘤治疗。②患者过去5年未出现进展或需要治疗的其他恶性肿瘤。③术后病理诊断为CRC。
1.1.2 主要试剂与仪器
流式抗体抗人CD16/32、CD3-PE-Cy7、CD45-APC、CD19-BV650、CD56-BV650、CD19-BV605、CD158a-PE、TNF-α-APC、粒细胞-巨噬细胞集落刺激因子(granulocyte-macrophage colony-stimulating factor,GM-CSF)- BB700(BioLegend,美国),流式抗体抗人CD3-BUV805、CD56-PE-CF594、CD11c-BV510、CD11b-BV786、CD14-APC-Cy7、CD16-BUV737、CD38-BV421、人类白细胞抗原-DR(human leukocyte antigen-DR,HLA-DR)-PE-Cy5、CD69-BB790、T细胞免疫球蛋白与黏蛋白结构域3(T cell immunoglobulin domain and mucin domain-3,TIM-3)-BB750、CD27-BUV496、CD57-BV786、NKp46-PE-Cy7、CD94-PE-CF594、IFN-γ-BV711、死活细胞染料Fixable viability stain 570(BD Biosciences,美国),二硫苏糖醇(dithiothreitol,DTT)、eBioscienceTM细胞刺激试剂盒、eBioscienceTM固定破膜叉头框蛋白P3(forkhead box protein P3,FOXP3)/转录因子(transcription factor)染色缓冲液试剂盒(赛默飞,美国),乙二胺四乙酸(ethylenediaminetetraacetic acid,EDTA)、4-(2-羟乙基)-1-哌嗪乙磺酸[4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid,HEPES]、RPMI1640培养基、胎牛血清(fetal bovine serum,FBS)(Gibco,美国),Ⅷ型胶原酶(type Ⅷ collagenase,Col Ⅷ)、脱氧核糖核酸酶Ⅰ(deoxyribonuclease Ⅰ,DNase Ⅰ)(Sigma Aldrich,美国),淋巴细胞分离液Lymphoprep(Serumwerk Bernburg,德国),20×磷酸盐缓冲盐溶液(phosphate buffered saline,PBS)(上海生工生物工程股份有限公司)。1300-A2生物安全柜(Thermo Fisher Scientific,美国),5810R高速离心机(Eppendorf,德国),HX-20TS智能恒温摇床(上海沪析实业有限公司),FACSymphony A3流式分析仪(BD Biosciences,美国)。
1.2 实验方法
1.2.1 人外周血单个核细胞提取
取人新鲜抗凝全血,按照1∶1比例加入1×PBS将血液进行稀释,以降低血液黏稠度,轻柔混匀后备用。向无菌15 mL离心管内加入适量的Lymphoprep溶液,将稀释后的血样按体积比(Lymphoprep∶稀释全血)1∶2的比例缓慢平铺到Lymphoprep液面上方,注意保持两液面界面分层清晰。随后室温800×g离心20 min,设置升速5,降速1。离心结束后,管内液面从上至下依次为稀释血浆层、外周血单核细胞(peripheral blood mononuclear cell,PBMC)层、分离液层和红细胞层,先吸走血浆层,再小心吸取中间白膜层即PBMC层并转移至15 mL离心管中,向离心管中加入10 mL的1×PBS重悬细胞,随后室温500×g离心5 min,弃上清液,再重复用1×PBS清洗1~2次即可用于后续试验。1 mL人外周血约能提取1×106个PBMC。
1.2.2 人结直肠癌样本消化处理
将新鲜的肿瘤组织和癌旁正常组织样本转移到含有8 mL预冷过的RPMI1640培养皿中,用剪刀和镊子去除其上的脂肪、血管和纤维组织。用镊子夹住组织,使用预冷的1×PBS冲洗2次,用湿纸巾轻轻地吸干组织,称取癌旁正常组织和肿瘤组织各1 g用于消化。用剪刀将癌旁组织切成大小约为0.3 cm3的小块,将癌旁组织放入含有20 mL癌旁组织洗液的50 mL离心管中,在恒温摇床中37 ℃,1×g洗涤60 min。用剪刀把肿瘤组织剪成两半,放入含有20 mL肿瘤组织洗液的50 mL离心管中,在恒温摇床中37 ℃,1×g洗涤15 min。洗涤完成后,对于肿瘤组织,使用涡旋仪涡旋振荡2 min。对于癌旁组织,用手用力摇晃离心管2 min,振荡摇晃后,500×g离心5 min去除上清液,再用30 mL的1×PBS冲洗至少2次,以洗去残留的DTT或EDTA。随后,分别将肿瘤组织/癌旁组织放入1.5 mL的EP管中,用剪刀剪成小块。再分别转移至含有20 mL消化液的50 mL管离心管中,放入恒温摇床中,37 ℃,1×g消化30 min。消化结束后用手剧烈摇晃离心管3~5 min,直到肿瘤组织和癌旁组织变成小块,然后用100 μm细胞过滤器滤掉残余组织,将细胞悬液收集到新的50 mL离心管中,室温下500×g离心5 min后弃去上清,加入12 mL RPMI1640完全培养基重悬细胞。为去除样本中的肿瘤细胞、红细胞和碎片等,向50 mL离心管中加入12 mL Lymphoprep,在其上层等比缓慢加入前面制备好的细胞悬液,室温下1 400×g离心25 min,升速1,降速1,离心结束后弃去上层悬液,吸取中间层的淋巴细胞,加入10 mL PBS缓冲液,室温下500×g离心5 min,去除上清,加入适量RPMI1640完全培养基重悬细胞备用。肿瘤组织洗液、癌旁组织洗液及消化液配置相关信息见表1。
表1 试剂配置
Tab 1
| Reagent name | System |
|---|---|
| Tumor wash buffer | |
| PBS (1×) | Up to 20 mL (for 1 g biopsies) |
| DTT (2 mol·L-1) | 65 μL (final 6.5 mmol·mL-1) |
| FBS | 2 mL (10%) |
| Normal wash buffer | |
| PBS (1×) | Up to 20 mL (for 1 g biopsies) |
| HEPES (1 mol·L-1) | 300 μL (final 15 mmol·mL-1) |
| EDTA (0.5 mol·L-1) | 200 μL (final 5 mmol·mL-1) |
| DTT (2 mol·L-1) | 10 μL (final 1 mmol·mL-1) |
| FBS | 2 mL (10%) |
| Tissue digestion buffer | |
| RPMI1640 (+10%FBS, +1%P/S) | Up to 40 mL (for 2 g biopsies) |
| Col Ⅷ | 45.7 μL (final 0.38 mg·mL-1) |
| DNase Ⅰ | 26.8 μL (final 0.1 mg·mL-1) |
1.2.3 流式细胞染色
取消化好的细胞先按体积比1∶100加入Fc端封闭剂(Anti-CD16/32),4 ℃孵育10 min。对于细胞表面染色,首先使用死活细胞染料Fixable viability stain 570室温孵育10 min来标记死细胞,细胞表面蛋白标志如CD45、CD3、CD56、CD11b、CD11c等的染色按体积比1∶200比例稀释抗体,4 ℃避光孵育30 min,然后加入1×PBS离心洗涤。对于细胞内部蛋白如IFN-γ、TNF-α及GM-CSF等的染色,先用Foxp3/转录因子染色缓冲液试剂盒将细胞4 ℃固定透化45 min,再按体积比1∶100比例稀释胞内抗体,4 ℃避光孵育1 h,1×PBS洗涤后用200 μL PBS重悬细胞等待上机检测。染色过程中离心程序均为400×g,5 min。流式细胞分析检测使用BD FACSymphonyA3流式分析仪,数据分析使用FlowJo™ v10.9.0软件。
1.3 统计学分析
本研究使用GraphPad Prism 8.0软件进行数据的统计学分析。定量资料用x±s表示,组间比较采用Student's t检验或Mann-Whitney U检验。定性资料以频数(百分比)表示。P<0.05表示差异具有统计学意义。
2 结果
2.1 患者的一般情况描述
研究共纳入CRC患者病例25例,收集临床样本包括25例肿瘤组织及配对的癌旁组织、15例匹配的外周血样本(取自前述25例患者)。患者的临床和病理特征详见表2。
表2 25例结直肠癌患者的临床和病理特征
Tab 2
| Characteristic | Colorectal cancer patient (n=25) |
|---|---|
| Age/year | 62.4±11.8 |
| Gender/n(%) | |
| Male | 9 (36.0) |
| Female | 16 (64.0) |
| Pathological T stage/n(%) | |
| T2 | 3 (12.0) |
| T3 | 18 (72.0) |
| T4 | 4 (16.0) |
| Pathological N stage/n(%) | |
| N0 | 14 (56.0) |
| N1 | 6 (24.0) |
| N2 | 5 (20.0) |
| Pathological M stage/n(%) | |
| M0 | 24 (96.0) |
| M1 | 1 (4.0) |
| TNM stage/n(%) | |
| Ⅰ | 2 (8.0) |
| Ⅱ | 12 (48.0) |
| Ⅲ | 10 (40.0) |
| Ⅳ | 1 (4.0) |
2.2 结直肠癌免疫微环境的构成
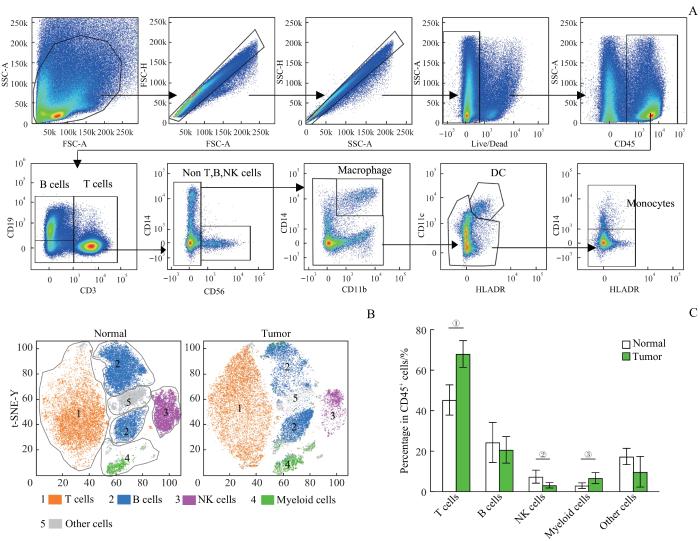
取CRC患者的新鲜肿瘤组织和配对的癌旁组织样本制备成单细胞悬液,运用流式细胞术检测肿瘤微环境中浸润的各类免疫细胞的比例。根据流式抗体组合标记将免疫细胞分为各类亚群(图1A):T淋巴细胞(CD45+CD3+ )、B淋巴细胞(CD45+CD3-CD19+ )、NK细胞(CD45+CD3-CD19-CD56+)、巨噬细胞(CD45+CD3- CD19-CD56-CD11b+CD14+)、树突状细胞(CD45+ CD3-CD19-CD56-CD11b-CD14-HLA-DR+CD11c+)、单核细胞(CD45+CD3-CD19-CD56-CD11b-CD14-HLA-DR-CD11c-CD14+),其中巨噬细胞、树突状细胞和单核细胞统称为髓系细胞。使用FlowJo软件对肿瘤组织和癌旁组织中的免疫细胞进行t-SNE(t-distributed stochastic neighbor embedding)降维分析(图1B),可见肿瘤微环境中主要的免疫细胞亚群为T淋巴细胞、B淋巴细胞、NK细胞、髓系细胞(myeloid cells)和其他细胞。对比肿瘤组织和癌旁组织中这5类细胞的比例(图1C),结果显示T淋巴细胞(P=0.000)和髓系细胞(P=0.026)在肿瘤组织中的比例明显高于癌旁组织,而NK细胞(P=0.007)在肿瘤组织中的比例明显低于癌旁组织。
图1
图1
结直肠癌免疫微环境的组成分析
Note: A. Gating strategy of T cells, B cells, NK cells, macrophages, dendritic cells and monocytes in colorectal cancer sample. B. t-SNE dimensionality reduction analysis of immune cell components in colorectal cancer samples. C. Percentage of T cells, B cells, NK cells, myeloid cells and other cells in CD45+ immune cells in tumor and normal tissues (n=25). ①P=0.000, ②P=0.007, ③P=0.026.
Fig 1
Analysis of the immune microenvironment composition in colorectal cancer
2.3 肿瘤组织中NK细胞比例下降且呈现耗竭状态
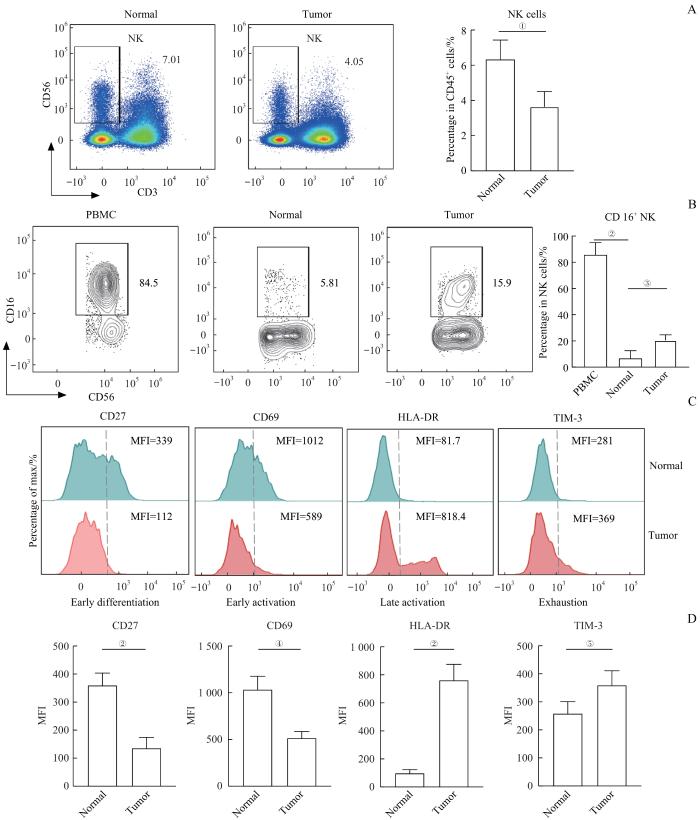
通过流式细胞术发现肿瘤组织中NK细胞的比例(P=0.007)显著低于癌旁组织(图2A)。为进一步了解NK细胞的亚群及表型特征变化,对NK细胞的表面活化分子进行检测。对比患者外周血、肿瘤组织和癌旁组织中NK细胞上CD16的表达(图2B),发现在肿瘤组织和癌旁组织中NK细胞表面CD16分子的表达(P=0.000)显著低于外周血,而肿瘤组织中NK细胞的CD16分子表达(P=0.008)显著高于癌旁组织,说明肠道组织中的NK细胞表型与外周血差异较大。此外,通过检测细胞表面早期分化标志CD27、早期活化标志CD69、晚期活化标志HLA-DR、细胞耗竭标志TIM-3分子在肿瘤组织和癌旁组织中的表达情况(图2C),发现CD27(P=0.000)和CD69(P=0.001)在肿瘤组织中表达显著下调,HLA-DR(P=0.000)和TIM-3(P=0.024)表达显著上调。检测结果提示肿瘤组织中的NK细胞呈现晚期活化和耗竭表型。
图2
图2
肿瘤组织中NK细胞比例减少且呈现耗竭表型
Note: A. Representative flow cytometry analysis and percentage of NK cells in normal and tumor tissues (n=25). B. Representative flow cytometry analysis and percentage of CD16+NK cells in PBMC, normal and tumor tissues (nPBMC=15, nnormal=25, and nTumor=25). C. Representative flow cytometry analysis of the expression of CD27, CD69, HLA-DR and TIM-3 on NK cells in normal and tumor tissues. D. The expression levels of CD27, CD69, HLA-DR and TIM-3 on NK cells in normal and tumor tissues (n=25). ①P=0.007, ②P=0.000, ③P=0.008, ④P=0.001, ⑤P=0.024. MFI—mean fluorenscence intensity.
Fig 2
NK cells in CRC tumor tissues were reduced and showed an exhaustion phenotype
2.4 CD38highNK细胞亚群在结直肠癌肿瘤组织中显著减少
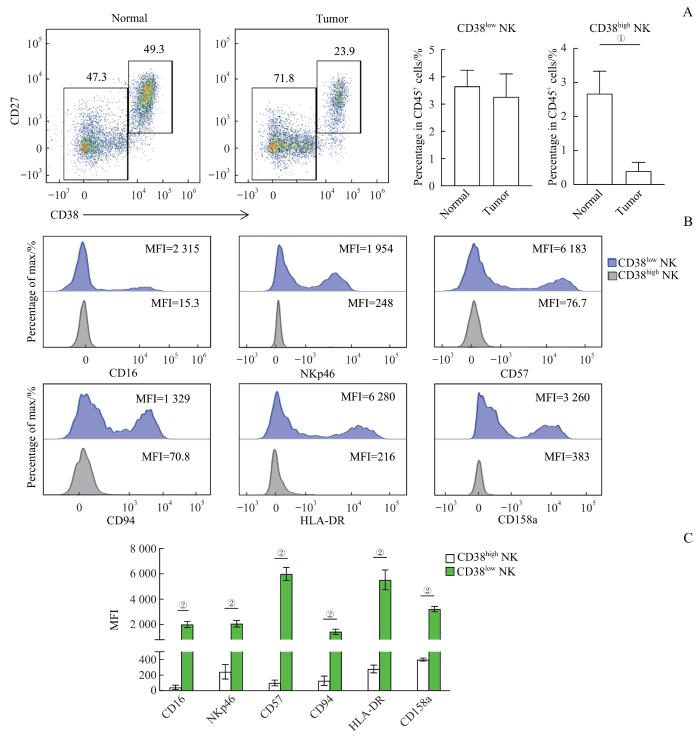
通过检测CD38分子在NK细胞上的表达水平,将NK细胞根据CD38分子的表达高低分为CD38highNK和CD38lowNK 2个亚群。对比CD38highNK细胞亚群在肿瘤组织和癌旁组织中的比例(图3A),结果可知,相比癌旁组织,肿瘤组织中的CD38highNK细胞比例明显降低(P=0.003),而CD38lowNK细胞无显著差异,同时CD38highNK相比CD38lowNK高表达早期分化标志CD27分子。为进一步探索CD38highNK细胞亚群的表型和功能特征,分别检测细胞表面标志:活化性受体CD16、活化性受体NKp46、晚期分化标志CD57、细胞表面受体CD94、晚期活化标志HLA-DR、抑制性受体CD158a在NK细胞中的表达水平(图3B)。对比上述细胞表面标志物在CD38highNK和CD38lowNK这2个亚群中的表达水平,结果可知,肿瘤组织中CD38highNK细胞亚群的CD16(P=0.000)、NKp46(P=0.000)、CD57(P=0.000)、CD94(P=0.000)、HLA-DR(P=0.000)、CD158a(P=0.000)表达都明显低于CD38lowNK细胞亚群,说明该亚群活化程度极低(图3C)。综上结果,CRC肿瘤组织中减少的NK细胞主要是CD38highNK细胞亚群,并且CD38highNK细胞是一群处于早期分化和未活化状态的NK细胞。
图3
图3
CD38highNK细胞在肿瘤组织中显著减少
Note: A. Representative flow cytometry analysis and percentage of CD38highNK cells in normal and tumor tissues (n=25). B. Representative flow cytometry analysis of the expression of CD16, NKp46, CD57, CD94, HLA-DR and CD158a in CD38highNK cells and CD38lowNK cells in tumor tissues. C. The expression levels of CD16, NKp46, CD57, CD94, HLA-DR and CD158a in CD38highNK cells and CD38lowNK cells in tumor tissues (n=25). ①P=0.003, ②P=0.000.
Fig 3
CD38highNK cells were dramatically reduced in tumor tissues
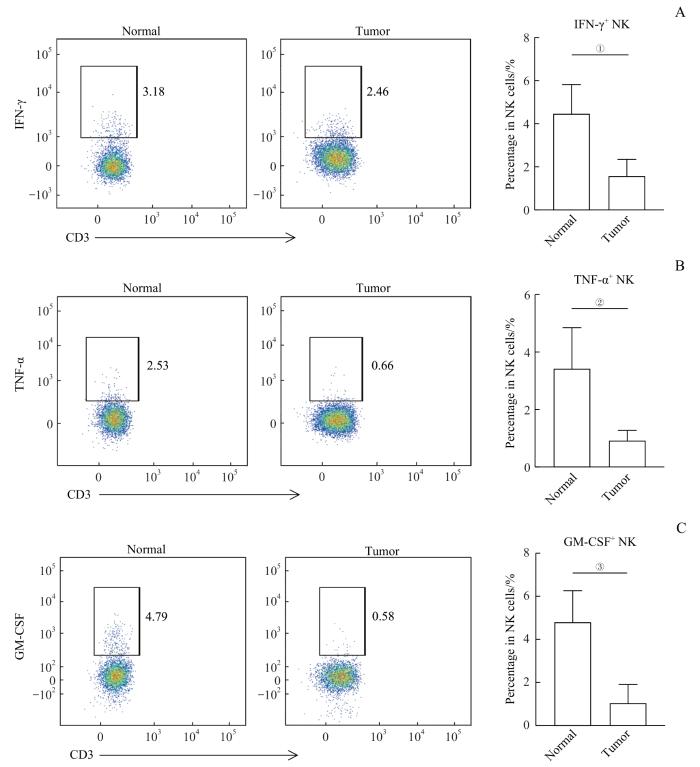
2.5 肿瘤组织中NK细胞功能受损
图4
图4
肿瘤组织中NK细胞分泌细胞因子的能力减弱
Note:A. Representative flow cytometry analysis and percentage of IFN-γ+NK cells in normal and tumor tissues (n=25). B. Representative flow cytometry analysis and percentage of TNF-α+NK cells in normal and tumor tissues (n=25). C. Representative flow cytometry analysis and percentage of GM-CSF+NK cells in normal and tumor tissues (n=25). ①P=0.032, ②P=0.042, ③P=0.019.
Fig 4
Impaired cytokine production of NK cells in tumors
3 讨论
人类NK细胞可以根据CD56分子的表达分为CD56bright和CD56dim 2个亚群[23]。近年来的研究[24]发现,NK细胞在不同组织中可以分化发育为不同的亚群。肠道、肝脏、肾脏等不同组织部位驻留的NK细胞表型呈现出高度的异质性[25]。本研究发现,CRC患者的癌旁组织和肿瘤组织中表达CD16分子的NK细胞比例均明显少于外周血,说明肠道组织中NK细胞的表型与外周循环NK细胞相比差异较大。CD16分子是NK细胞的经典活化性表面受体,参与抗体依赖细胞介导的细胞毒作用(antibody-dependent cell-mediated cytotoxicity,ADCC)[26]。研究同时发现,CRC肿瘤组织中CD16+NK细胞比例明显高于癌旁组织,猜测可能是肿瘤中浸润的NK细胞受到肿瘤微环境信号刺激,激活程度更高。
CD38是一种Ⅱ型跨膜糖蛋白,相对分子质量为45 000,同时具有胞外酶活性和细胞受体功能。在激活钙信号,调控免疫细胞的成熟、分化、激活和免疫耐受等过程中发挥关键作用[29]。CD38分子被认为是T淋巴细胞的经典活化标志,但在NK细胞上的作用还不甚明确。本研究根据CD38分子的表达量将NK细胞分为CD38highNK细胞和CD38lowNK细胞2个亚群。统计学分析结果发现相比于癌旁组织,肿瘤组织中CD38highNK细胞的比例显著减少,而CD38lowNK细胞的比例无差异,说明肿瘤组织中减少的NK细胞主要为CD38highNK细胞亚群。进一步分析CD38highNK细胞亚群后发现,其细胞表面一系列表型和功能标志:活化性受体CD16、活化性受体NKp46、晚期分化标志CD57、细胞表面受体CD94、晚期活化标志HLA-DR、抑制性受体CD158a的表达均显著下调,而早期活化分子CD27表达显著上调,表明此群细胞正处于早期分化且未活化的状态。有体外实验[30]发现,CD38分子参与了NK细胞与靶细胞之间免疫突触的形成。因此CRC中CD38highNK细胞亚群的减少,可能导致了肿瘤浸润NK细胞杀伤能力的下降。
NK细胞作为人体免疫系统发挥抗肿瘤免疫监视的重要组分之一,不仅可以快速识别和杀伤肿瘤细胞,还可以通过分泌IFN-γ、TNF-α、GM-CSF等细胞因子和趋化因子,招募其他免疫细胞,并有效促进T细胞和B细胞发生二次免疫应答[28]。本研究通过检测上述3个主要细胞因子的表达水平,发现CRC肿瘤浸润NK细胞分泌IFN-γ、TNF-α、GM-CSF的能力对比癌旁组织均显著降低。证明CRC患者的NK细胞在肿瘤微环境中存在功能受损。综上所述,CRC肿瘤微环境中NK细胞的一系列变化可能促进了肿瘤的发生发展。
通过整理25例CRC患者的临床病理分期信息,发现大部分患者都处在TNM分期[31]的Ⅱ期和Ⅲ期:Ⅱ期(12例)、Ⅲ期(10例)。经过分析后发现不同病理分期患者的肿瘤组织和癌旁组织中NK细胞的表型和功能特征没有明显的差异,可能是由于大部分患者处在相近的疾病发展阶段。未来研究中将纳入更多Ⅰ期和Ⅳ期的患者样本,以期通过比较不同分期患者获得更多有关CRC发生发展的发现。
本研究发现CD38highNK亚群在肿瘤组织中比例降低,但CD38分子除在多发性骨髓瘤等肿瘤细胞上高表达外,还在T细胞、B细胞、髓源抑制细胞等免疫细胞中存在高表达亚群,并发挥不同功能[32]。还需要对CD38high免疫细胞亚群进行更深入的研究,以阐明CD38在CRC实体瘤中的具体作用。
综上所述,本研究通过探索CRC患者肿瘤组织NK细胞的表型和功能特征,阐述肿瘤微环境中NK细胞呈现的功能受损和耗竭状态,揭示肿瘤细胞免疫逃逸的可能原因,以期为临床治疗和发展靶向NK细胞的免疫治疗提供新思路。
作者贡献声明
冯昫皎、刘健悦参与实验实施和数据分析。沈蕾、冯昫皎、刘健悦、戚炀炀、孙晶参与论文写作。沈蕾负责课题设计与实验指导。所有作者均阅读并同意最终稿件的提交。
AUTHOR's CONTRIBUTIONS
FENG Xujiao and LIU Jianyue performed the experiments and analyzed the data. SHEN Lei, FENG Xujiao, LIU Jianyue, QI Yangyang, and SUN Jing wrote the manuscript. SHEN Lei conceived, designed and supervised the project. All authors have read the last version of paper and consented for submission.
利益冲突声明
所有作者声明不存在利益冲突。
COMPETING INTERESTS
All authors disclose no relevant conflict of interests.
参考文献