
上海交通大学学报(医学版) ›› 2024, Vol. 44 ›› Issue (9): 1069-1082.doi: 10.3969/j.issn.1674-8115.2024.09.002
• 论著 · 基础研究 • 上一篇
收稿日期:2024-05-03
接受日期:2024-05-22
出版日期:2024-09-28
发布日期:2024-10-09
通讯作者:
卞迁
E-mail:chh1142268531@163.com;wuzuo@sjtu.edu.cn;qianbian@shsmu.edu.cn
作者简介:陈怀煌(1999—),男,硕士生;电子信箱:chh1142268531@163.com基金资助:
CHEN Huaihuang( ), ZUO Wu(
), ZUO Wu( ), BIAN Qian(
), BIAN Qian( )
)
Received:2024-05-03
Accepted:2024-05-22
Online:2024-09-28
Published:2024-10-09
Contact:
BIAN Qian
E-mail:chh1142268531@163.com;wuzuo@sjtu.edu.cn;qianbian@shsmu.edu.cn
Supported by:摘要:
目的·明确CCCTC结合因子(CCCTC-binding factor,CTCF)对肝细胞脂质代谢的调控作用,并探究CTCF调控肝细胞基因表达的作用机制。方法·通过慢病毒将稳定表达Ctcf shRNA的DNA序列整合到小鼠永生化的AML12肝细胞系中,实现对Ctcf的稳定敲低。通过反转录实时荧光定量聚合酶链反应(RT-qPCR)及蛋白质印迹法(Western blotting)验证Ctcf的敲低效率。碘化丙啶(propidium iodide,PI)染色测定细胞周期,使用CCK-8法绘制细胞生长曲线,检测Ctcf敲低对细胞生长的影响。油红O染色标记细胞内脂质,检测CTCF对AML12细胞脂质代谢和脂滴累积的影响。使用CUT & Tag测序技术分析Ctcf敲低后CTCF在全基因组的结合变化。结合RNA测序(RNA-seq)的转录组数据分析CTCF结合变化后的转录组变化,使用基因本体论(GO)、京都基因和基因组数据库(KEGG)富集分析及基因集富集分析(GSEA)揭示Ctcf敲低对AML12肝细胞基因的表达影响,并探究差异基因与CTCF结合变化间的关联。结果·RT-qPCR结果表明Ctcf在 mRNA水平敲低了63.4%,Western blotting验证了CTCF在蛋白表达层面降低了57.7%(均P<0.05)。生长曲线及周期实验确定了Ctcf敲低后细胞增殖阻滞于G1/G0期。并且AML12细胞在Ctcf敲低后自发出现细胞内脂质蓄积(P<0.05)。CTCF在全基因组的结合呈现出显著变化,大多数CTCF结合差异区域表现出CTCF结合减少,但仍有部分区域CTCF结合增加。转录组数据显示Ctcf敲低导致1 344个基因出现显著的表达变化,在上调基因中富集出与脂滴堆积相关的脂质代谢通路。CTCF结合上调峰关联的差异基因富集在脂质转运与脂质定位相关通路,而CTCF结合下调峰关联的差异基因主要富集在DNA损伤修复、细胞凋亡、细胞周期等生物学过程,但CTCF在基因组的结合变化并不足以导致邻近基因的表达上调或下调。结论·CTCF通过调控脂质代谢相关基因的表达影响肝细胞的代谢功能,然而CTCF在基因组上的结合变化与邻近基因的表达缺乏显著的相关性,可能主要通过远端调控的方式对基因表达产生影响。
中图分类号:
陈怀煌, 左武, 卞迁. CTCF调控小鼠AML12肝细胞系脂质代谢功能与基因表达[J]. 上海交通大学学报(医学版), 2024, 44(9): 1069-1082.
CHEN Huaihuang, ZUO Wu, BIAN Qian. CTCF regulates lipid metabolism and gene expression in mouse AML12 liver cell line[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2024, 44(9): 1069-1082.
| Primer name | Sequence (5′→3′) |
|---|---|
| Mouse Ctcf-shRNA 1 | GGTGCAATTGAGAACATTATA CTCGAG TATAATGTTCTCAATTGCACC |
| Mouse Ctcf-shRNA 2 | TGGACGATACCCAGATCATAACTCGAGTTATGATCTGGGTATCGTCCA |
| Mouse scramble shRNA | TTCTCCGAACGTGTCACGT CTCGAG ACGTGACACGTTCGGAGAA |
表1 shRNA序列
Tab 1 shRNA sequences
| Primer name | Sequence (5′→3′) |
|---|---|
| Mouse Ctcf-shRNA 1 | GGTGCAATTGAGAACATTATA CTCGAG TATAATGTTCTCAATTGCACC |
| Mouse Ctcf-shRNA 2 | TGGACGATACCCAGATCATAACTCGAGTTATGATCTGGGTATCGTCCA |
| Mouse scramble shRNA | TTCTCCGAACGTGTCACGT CTCGAG ACGTGACACGTTCGGAGAA |
| Gene | Forward primer (5′→3′) | Reverse primer (5′→3′) |
|---|---|---|
| Ctcf | AACAGTGACCCTCCTGAGGAATC | TATAACGACGATGCCGCACCA |
| β-actin | CATTGCTGACAGGATGCAGAAGG | TGCTGGAAGGTGGACAGTGAGG |
表2 RT-qPCR引物序列
Tab 2 Primer sequences for RT-qPCR
| Gene | Forward primer (5′→3′) | Reverse primer (5′→3′) |
|---|---|---|
| Ctcf | AACAGTGACCCTCCTGAGGAATC | TATAACGACGATGCCGCACCA |
| β-actin | CATTGCTGACAGGATGCAGAAGG | TGCTGGAAGGTGGACAGTGAGG |
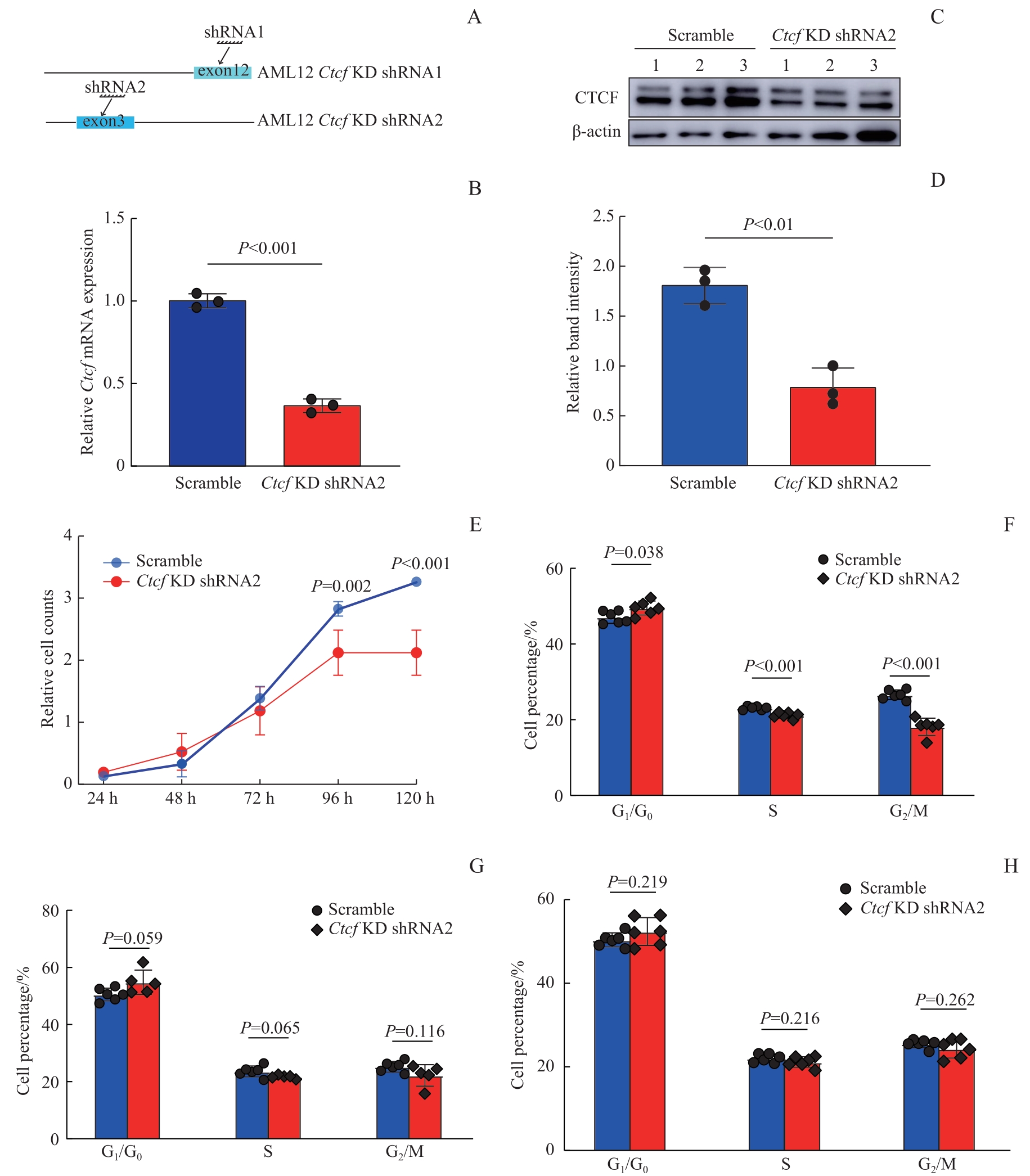
图1 Ctcf 敲低验证及细胞增殖速率检测Note: A. Schematic diagram showing the target regions of two different shRNAs used to knock down Ctcf. B. Quantification of Ctcf transcript levels in AML12 Ctcf knockdown cells and control cells (Scramble) using RT-qPCR. C. Western blotting shows efficient depletion of CTCF protein by Ctcf shRNA2. D. Quantitative analysis of the Western blotting bands shown in Fig C. E. Line graph shows cell proliferation rates of Ctcf knockdown cells and control cells measured by the CCK-8 assay. F?H. Comparative analysis of cell cycle composition of Ctcf knockdown cells and control cells at 40% (F), 60% (G), and 80% (H) confluence.
Fig 1 Validation of Ctcf knockdown and assessment of its impact on cell proliferation
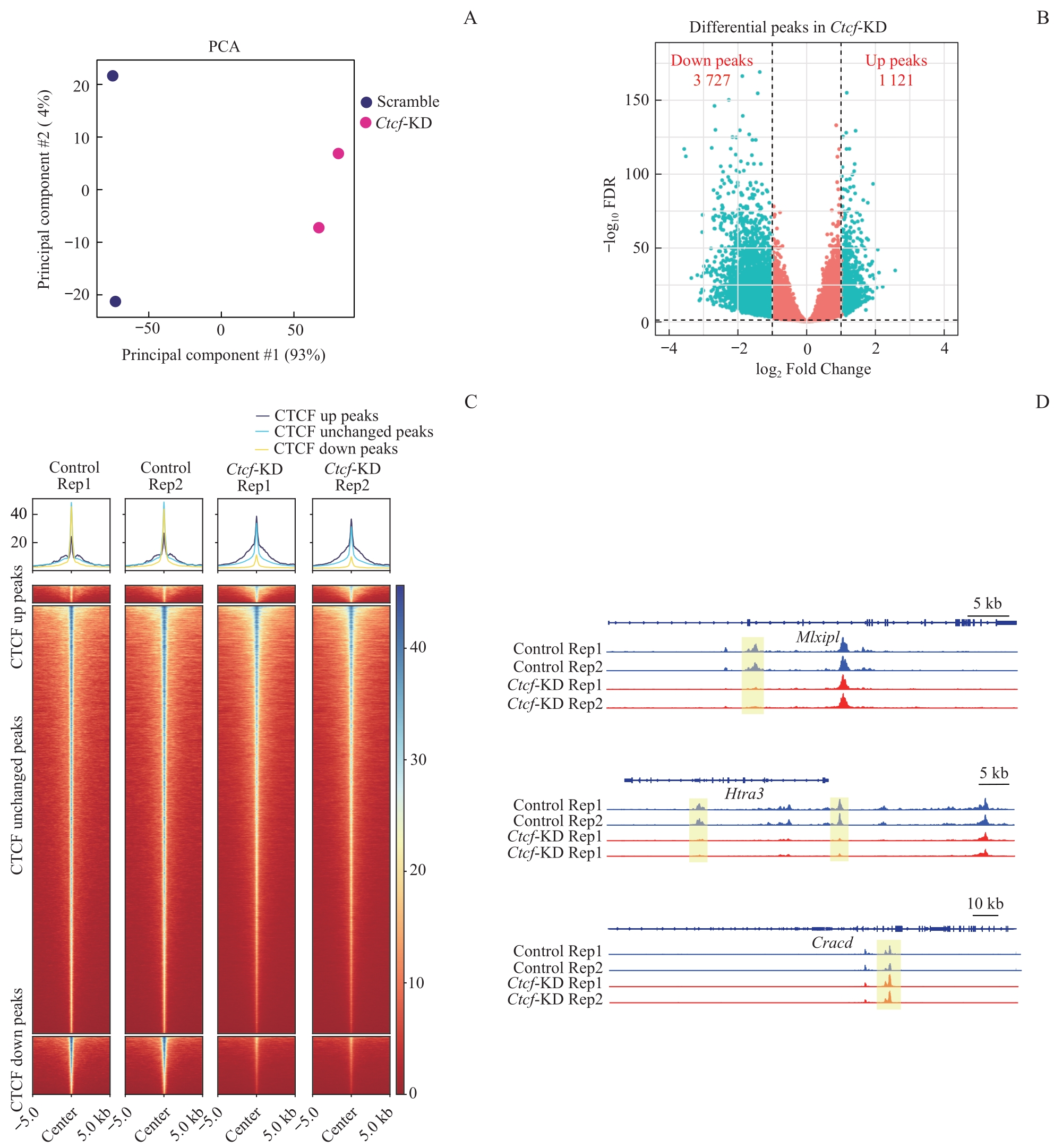
图2 Ctcf 敲低后全基因组CTCF结合变化Note: A. PCA plot of CUT & Tag data reveals significant differences in CTCF signals between Ctcf knockdown and Scramble cells. B. Volcano plot shows the changes of CTCF signals at CTCF peaks following Ctcf knockdown. C. Averaged line graph and heatmaps show the CTCF CUT&Tag signal intensities that are upregulated, unchanged, or downregulated at CTCF peaks in Ctcf knockdown cells. D. Representative regions exhibiting changes in CTCF binding after Ctcf knockdown.
Fig 2 Genome-wide changes in CTCF binding after Ctcf knockdown
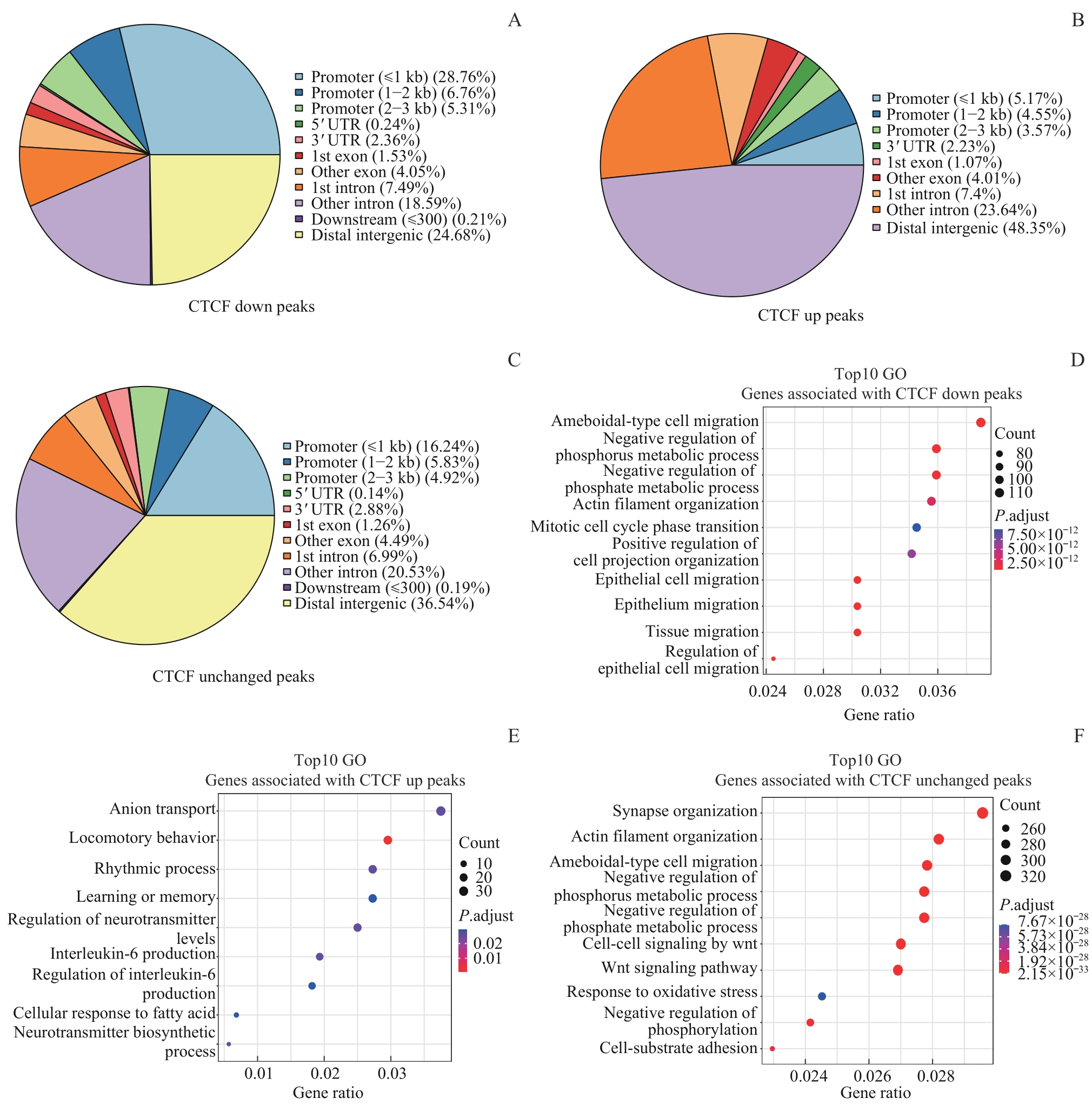
图3 Ctcf 敲低后CTCF结合变化与CTCF结合不变区域的关联基因分析Note: A?C. Pie charts show the proportions of CTCF down peaks (A), CTCF up peaks (B), and CTCF unchanged peaks (C) associated with different genomic features. D?F. Dot plots show the top 10 enriched Gene Ontology (GO) terms in genes associated with CTCF down peaks (D), CTCF up peaks (E), and CTCF unchanged peaks (F).
Fig 3 Analysis of genes associated with changed or unchanged CTCF peaks after Ctcf knockdown
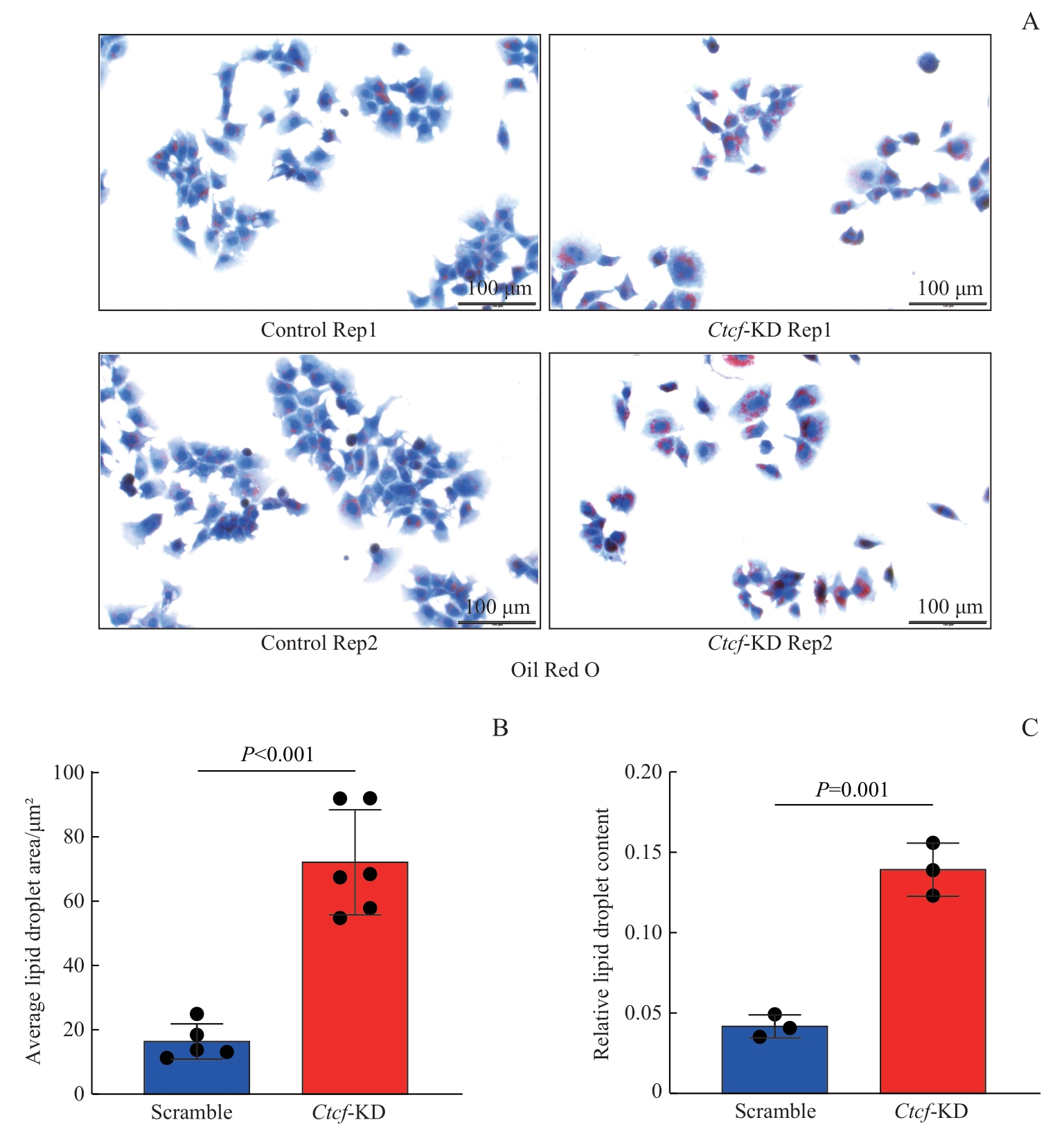
图4 Ctcf 敲低对脂滴堆积的影响Note: A. Representative Oil Red O staining images of control and Ctcf knockdown cells. B. Quantitative Image J analysis of Oil Red O staining signals shows increased lipid droplet areas in Ctcf knockdown cells. C. Quantitative spectrophotometry analysis of isopropanol-extracted Oil Red O shows increased lipid droplet content in Ctcf knockdown cells.
Fig 4 Increased lipid droplet accumulation after Ctcf knockdown
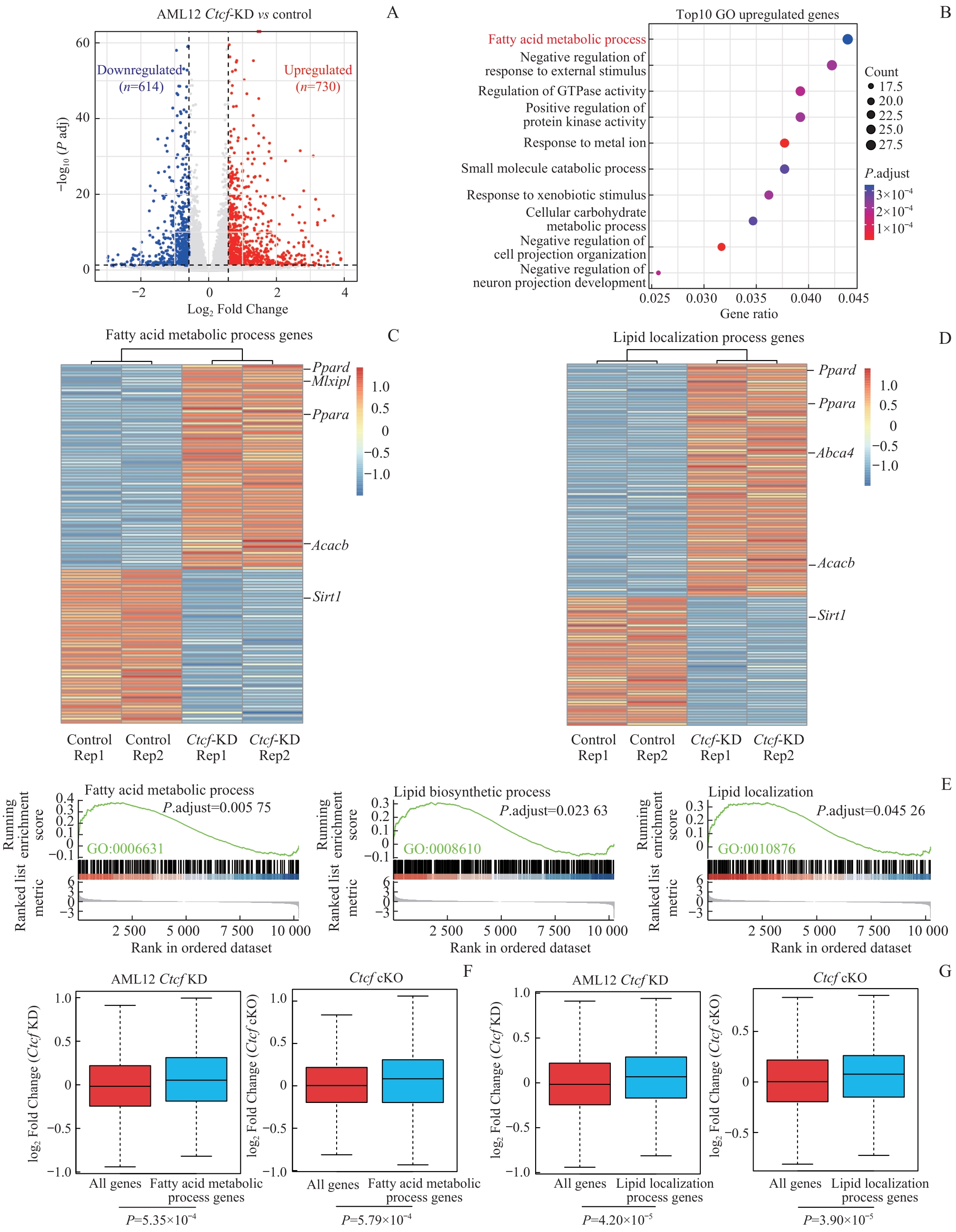
图5 Ctcf 敲低对转录组的影响Note: A. Volcano plot shows differentially expressed genes (DEGs) upon Ctcf knockdown. B. Dot plots show the top 10 enriched Gene Ontology (GO) terms in upregulated genes after Ctcf knockdown. C/D. Heatmaps show gene expression changes for DEGs involved in fatty acid metabolism (C) and lipid localization (D) processes. E. GSEA enrichment analysis shows that several gene sets related to lipid metabolism and localization are significantly upregulated in Ctcf knockdown cells. F/G. Genes involved in the fatty acid metabolic process (F) and lipid localization (G) exhibited overall upregulation in our study (Ctcf knockdown AML12 cells) and in a previously published study[19] (conditional Ctcf knockout in liver in vivo).
Fig 5 Transcriptome changes after Ctcf knockdown
| Gene | CTCF-associated gene | CTCF-non-associated gene |
|---|---|---|
| Downregulated gene | 338 | 276 |
| Unchanged gene | 5 323 | 4 921 |
| Upregulated gene | 461 | 269 |
表3 CTCF相关与不相关基因分类统计
Tab 3 Classification statistics of CTCF-associated and CTCF-non-associated genes
| Gene | CTCF-associated gene | CTCF-non-associated gene |
|---|---|---|
| Downregulated gene | 338 | 276 |
| Unchanged gene | 5 323 | 4 921 |
| Upregulated gene | 461 | 269 |

图6 CTCF结合变化与基因表达变化关联分析Note: A. Pie charts show the proportions of DEGs in genes associated or not associated with CTCF peaks. B. Pie charts show the proportions of DEGs in genes associated with CTCF down peaks, unchanged peaks, or up peaks. C/D. Box plots summarize the fold changes in expression changes for the upregulated (C) or downregulated (D) genes associated with CTCF down peaks, unchanged peaks, or up peaks. E?H. Dot plots show the top 10 enriched Gene Ontology (GO) terms in DEGs associated with CTCF up peaks (E), CTCF down peaks (F), CTCF unchanged peaks (G) and genes not associated with CTCF peaks (H).
Fig 6 Analysis of the correlation between CTCF binding changes and gene expression changes
| Gene | CTCF up peak annotation | CTCF unchanged peak annotation | CTCF down peak annotation |
|---|---|---|---|
| Downregulated gene | 14 | 338 | 117 |
| Unchange gene | 294 | 5 323 | 1 803 |
| Upregualated gene | 48 | 461 | 158 |
表4 CTCF差异结合峰注释基因和AML12细胞差异基因统计
Tab 4 Statistics of genes annotated with differential CTCF binding peaks and differentially expressed genes in AML12 cells
| Gene | CTCF up peak annotation | CTCF unchanged peak annotation | CTCF down peak annotation |
|---|---|---|---|
| Downregulated gene | 14 | 338 | 117 |
| Unchange gene | 294 | 5 323 | 1 803 |
| Upregualated gene | 48 | 461 | 158 |
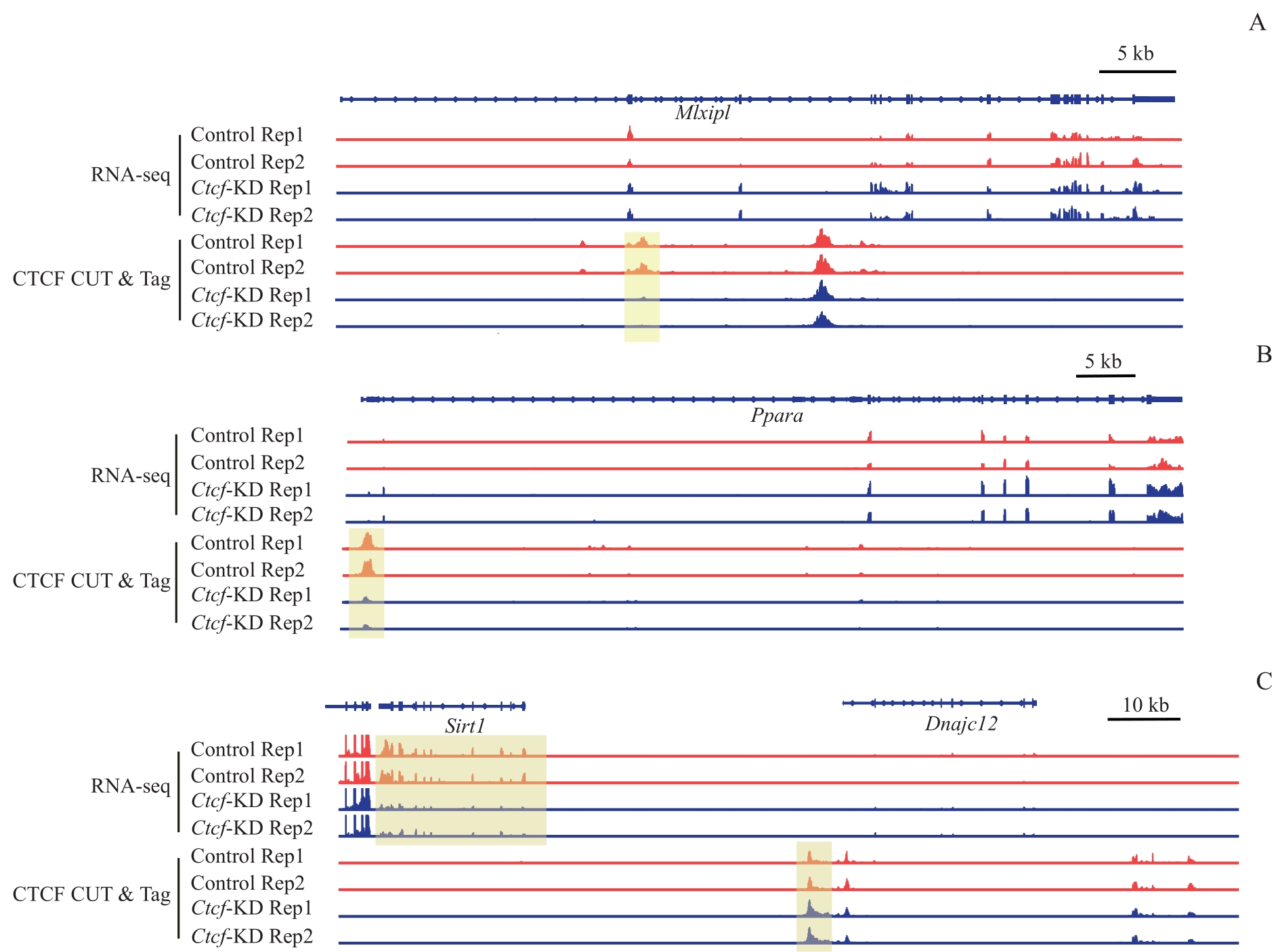
图7 CTCF结合变化调控基因表达的复杂性Note: A?C. CTCF binding and RNA-seq tracks of representative regions show that the changes in CTCF signals do not necessarily correlate with changes in gene expression direction. A. Mlxipl gene; B. Ppara gene; C. Sirt1 gene.
Fig 7 Complex effects of CTCF binding changes on gene expression
| 1 | ARZATE-MEJÍA R G, RECILLAS-TARGA F, CORCES V G. Developing in 3D: the role of CTCF in cell differentiation[J]. Development, 2018, 145(6): dev137729. |
| 2 | KUBO N, ISHII H, XIONG X, et al. Promoter-proximal CTCF binding promotes distal enhancer-dependent gene activation[J]. Nat Struct Mol Biol, 2021, 28(2): 152-161. |
| 3 | LIU Y T, WAN X, LI H, et al. CTCF coordinates cell fate specification via orchestrating regulatory hubs with pioneer transcription factors[J]. Cell Rep, 2023, 42(10): 113259. |
| 4 | BISSERIER M, MATHIYALAGAN P, ZHANG S H, et al. Regulation of the methylation and expression levels of the BMPR2 gene by SIN3a as a novel therapeutic mechanism in pulmonary arterial hypertension[J]. Circulation, 2021, 144(1): 52-73. |
| 5 | NUEBLER J, FUDENBERG G, IMAKAEV M, et al. Chromatin organization by an interplay of loop extrusion and compartmental segregation[J]. Proc Natl Acad Sci U S A, 2018, 115(29): E6697-E6706. |
| 6 | XIANG J F, CORCES V G. Regulation of 3D chromatin organization by CTCF[J]. Curr Opin Genet Dev, 2021, 67: 33-40. |
| 7 | DAVIDSON I F, BARTH R, ZACZEK M, et al. CTCF is a DNA-tension-dependent barrier to cohesin-mediated loop extrusion[J]. Nature, 2023, 616(7958): 822-827. |
| 8 | ROWLEY M J, CORCES V G. Organizational principles of 3D genome architecture[J]. Nat Rev Genet, 2018, 19(12): 789-800. |
| 9 | DEHINGIA B, MILEWSKA M, JANOWSKI M, et al. CTCF shapes chromatin structure and gene expression in health and disease[J]. EMBO Rep, 2022, 23(9): e55146. |
| 10 | HYLE J, ZHANG Y, WRIGHT S, et al. Acute depletion of CTCF directly affects MYC regulation through loss of enhancer-promoter looping[J]. Nucleic Acids Res, 2019, 47(13): 6699-6713. |
| 11 | RAHME G J, JAVED N M, PUORRO K L, et al. Modeling epigenetic lesions that cause gliomas[J]. Cell, 2023, 186(17): 3674-3685.e14. |
| 12 | POULOS R C, THOMS J A I, GUAN Y F, et al. Functional mutations form at CTCF-cohesin binding sites in melanoma due to uneven nucleotide excision repair across the motif[J]. Cell Rep, 2016, 17(11): 2865-2872. |
| 13 | RIBEIRO DE ALMEIDA C, STADHOUDERS R, DE BRUIJN M J, et al. The DNA-binding protein CTCF limits proximal Vκ recombination and restricts κ enhancer interactions to the immunoglobulin κ light chain locus[J]. Immunity, 2011, 35(4): 501-513. |
| 14 | HIRAYAMA T, TARUSAWA E, YOSHIMURA Y, et al. CTCF is required for neural development and stochastic expression of clustered Pcdh genes in neurons[J]. Cell Rep, 2012, 2(2): 345-357. |
| 15 | CHRISTOV M, CLARK A R, CORBIN B, et al. Inducible podocyte-specific deletion of CTCF drives progressive kidney disease and bone abnormalities[J]. JCI Insight, 2018, 3(4): e95091. |
| 16 | GOMEZ-VELAZQUEZ M, BADIA-CAREAGA C, LECHUGA-VIECO A V, et al. CTCF counter-regulates cardiomyocyte development and maturation programs in the embryonic heart[J]. PLoS Genet, 2017, 13(8): e1006985. |
| 17 | DUBOIS-CHEVALIER J, OGER F, DEHONDT H, et al. A dynamic CTCF chromatin binding landscape promotes DNA hydroxymethylation and transcriptional induction of adipocyte differentiation[J]. Nucleic Acids Res, 2014, 42(17): 10943-10959. |
| 18 | WANG R R, QIU X Y, PAN R, et al. Dietary intervention preserves β cell function in mice through CTCF-mediated transcriptional reprogramming[J]. J Exp Med, 2022, 219(7): e20211779. |
| 19 | CHOI Y, SONG M J, JUNG W J, et al. Liver-specific deletion of mouse CTCF leads to hepatic steatosis via augmented PPARγ signaling[J]. Cell Mol Gastroenterol Hepatol, 2021, 12(5): 1761-1787. |
| 20 | WANG W, REN G, HONG N, et al. Exploring the changing landscape of cell-to-cell variation after CTCF knockdown via single cell RNA-seq[J]. BMC Genomics, 2019, 20(1): 1015. |
| 21 | PINTO P B, DOMSCH K, LOHMANN I. Hox function and specificity: a tissue centric view[J]. Semin Cell Dev Biol, 2024, 152/153: 35-43. |
| 22 | AITKEN S J, IBARRA-SORIA X, KENTEPOZIDOU E, et al. CTCF maintains regulatory homeostasis of cancer pathways[J]. Genome Biol, 2018, 19(1): 106. |
| 23 | DAVIDSON I F, PETERS J M. Genome folding through loop extrusion by SMC complexes[J]. Nat Rev Mol Cell Biol, 2021, 22(7): 445-464. |
| [1] | 江全鑫, 陈素贞, 刘军力. 铜蓝蛋白在脂质代谢稳态调控中作用的研究进展[J]. 上海交通大学学报(医学版), 2024, 44(1): 124-130. |
| [2] | 胡婵婵, 范毅, 徐源, 胡志坚, 曾奕明. 脂质代谢在肺癌发生发展及诊疗领域中的研究进展[J]. 上海交通大学学报(医学版), 2022, 42(12): 1766-1771. |
| [3] | 顾鹏, 孙星. 超级增强子驱动致癌转录机制的研究进展[J]. 上海交通大学学报(医学版), 2021, 41(10): 1378-1383. |
| [4] | 刘舒婷,姚玉峰,倪进婧. 鼠伤寒沙门菌中假定转录调控因子的筛选[J]. 上海交通大学学报(医学版), 2018, 38(10): 1174-. |
| [5] | 闵雪洁,赵 丽,赵小平. SREBP在肿瘤中的研究进展[J]. 上海交通大学学报(医学版), 2016, 36(8): 1256-. |
| [6] | 刘锦燕,史册,王影,等. 白假丝酵母菌唑类耐药相关的转录调控研究进展[J]. 上海交通大学学报(医学版), 2016, 36(2): 291-. |
| [7] | 孙珍珍,彭川,郑金英,等. 甲硫氨酸负荷致同型半胱氨酸轻度升高对大鼠肝脏脂质代谢的影响及机制研究[J]. 上海交通大学学报(医学版), 2015, 35(7): 983-. |
| [8] | 罗澜,陈艳娟,万远方. 急性早幼粒细胞白血病患者BAX表达水平及意义[J]. 上海交通大学学报(医学版), 2015, 35(6): 799-. |
| [9] | 杨科峰, 房玥晖, 张 雄, 等. 植物甾醇对大鼠脂质代谢紊乱的预防作用[J]. , 2010, 30(1): 13-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||