
上海交通大学学报(医学版) ›› 2021, Vol. 41 ›› Issue (6): 732-740.doi: 10.3969/j.issn.1674-8115.2021.06.005
出版日期:2021-06-28
发布日期:2021-06-29
作者简介:王 青(1993—),女,硕士生;电子信箱:基金资助:
Qing WANG( ), Wei WANG, Da-jun JIANG, Wei-tao JIA(
), Wei WANG, Da-jun JIANG, Wei-tao JIA( )
)
Online:2021-06-28
Published:2021-06-29
Supported by:摘要:
目的·研究含有钙磷涂层(CaHPO4·2H2O,DCPD)的JDBM(Mg-Nd-Zn-Zr)镁合金支架体内外促血管新生和骨缺损修复的生物学效应。方法·应用模板复制法和化学沉积法构建JDBM-DCPD和JDBM-MgF2支架,使用微型CT(micro-CT)和扫描电子显微镜检测2种支架的表征。在支架表面种植骨髓间充质干细胞(bone marrow mesenchymal stem cell, BMSC)并通过CCK-8实验及细胞黏附实验观察支架对细胞的生物相容性。通过Transwell细胞迁移实验和成管实验检测支架浸提液对内皮细胞系Ea.hy926细胞迁移和成管能力的影响,并通过免疫荧光染色进一步观察血管内皮生长因子(vascular endothelial growth factor,VEGF)的分泌情况;通过碱性磷酸酶和茜素红染色检测浸提液对BMSC成骨能力的影响。构建SD大鼠股骨髁临界性骨缺损模型并植入支架,术后8周通过Microfil血管灌注、micro-CT扫描、组织切片染色等方法评估JDBM-DCPD支架的促血管新生及促成骨能力。结果·JDBM-DCPD支架的主孔径为400~450 μm,钙磷颗粒均匀分布在孔壁上,大小为15~25 μm。BMSC能够在JDBM-DCPD支架表面黏附且生长良好。与对照组和JDBM-MgF2支架浸提液相比,JDBM-DCPD支架浸提液在体外能够显著促进Ea.hy926内皮细胞迁移、成管以及VEGF的表达,同时可显著增强BMSC早期和晚期成骨分化。体内植入8周后,JDBM-DCPD支架促进缺损区血管和新骨再生作用显著优于JDBM-MgF2支架。结论·JDBM-DCPD支架在体内外实验中展现了优良的成血管效应,特别是在体内植入后可以实现早期血管化,从而更加有效地促进骨再生。
中图分类号:
王青, 王伟, 姜达君, 贾伟涛. 钙磷涂层的JDBM镁合金多孔支架促进血管新生及骨缺损修复的效果评估[J]. 上海交通大学学报(医学版), 2021, 41(6): 732-740.
Qing WANG, Wei WANG, Da-jun JIANG, Wei-tao JIA. Evaluation of JDBM porous scaffold coated with DCPD in promoting angiogenesis and repairing bone defects[J]. JOURNAL OF SHANGHAI JIAOTONG UNIVERSITY (MEDICAL SCIENCE), 2021, 41(6): 732-740.
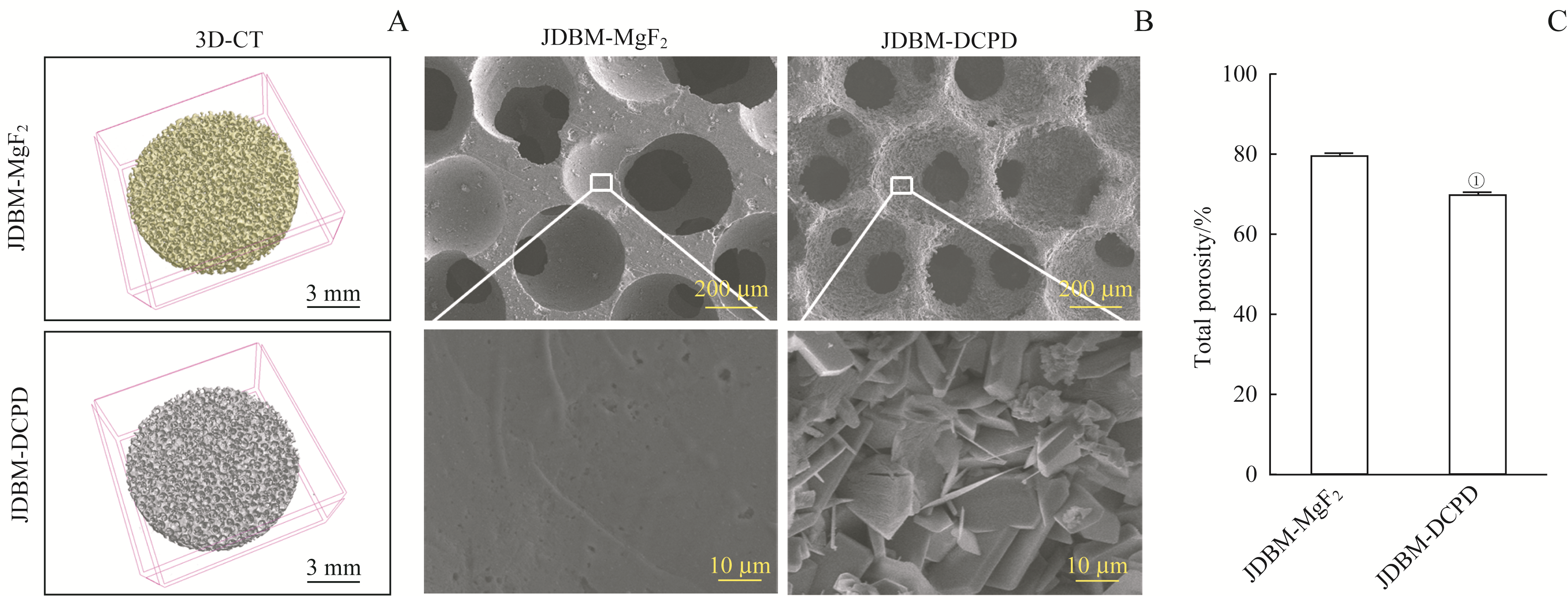
图1 JDBM-DCPD与JDBM支架表征的比较Note: A. 3D reconstruction images of JDBM-MgF2 and JDBM-DCPD porous scaffolds. B. SEM to observe the pore size and surface structure of the scaffolds (above ×100, below ×5 000). C. The porosities of JDBM-MgF2 and JDBM-DCPD porous scaffolds. ① P=0.000, compared with the JDBM-MgF2 group.
Fig 1 Comparison of JDBM-DCPD and JDBM-MgF2 porous scaffolds in characteristics
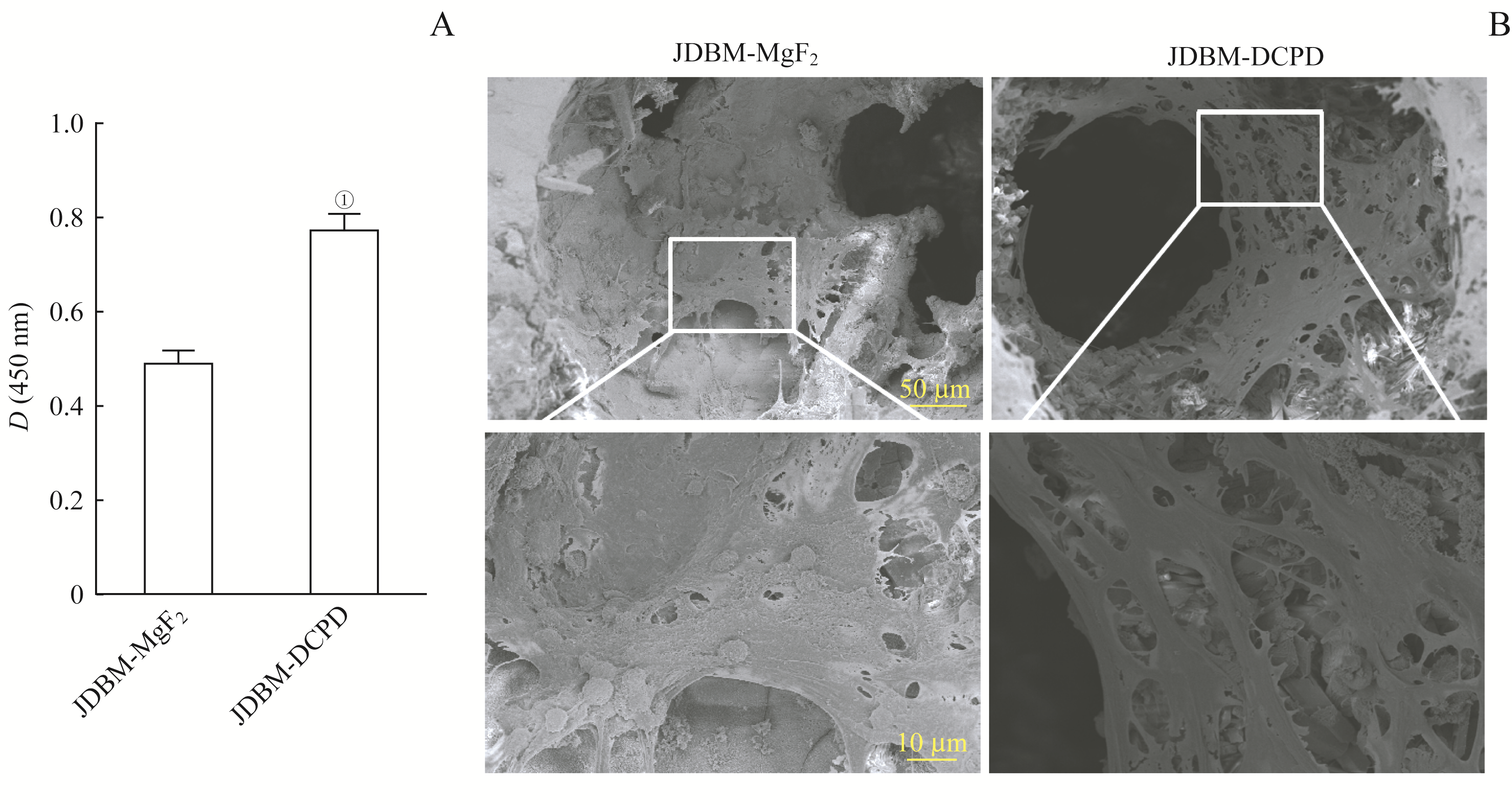
图2 JDBM-DCPD与JDBM-MgF2支架生物相容性的比较Note: A. Cell proliferation activity of BMSCs cultured on the scaffolds for 7 d detected by CCK8 kit. ①P=0.000, compared with the JDBM-MgF2 group. B. Cell adhesion of BMSCs cultured on the scaffolds for 7 d observed by SEM (above ×250, below ×1 000).
Fig 2 Comparison of JDBM-DCPD and JDBM-MgF2 in biocompatibility
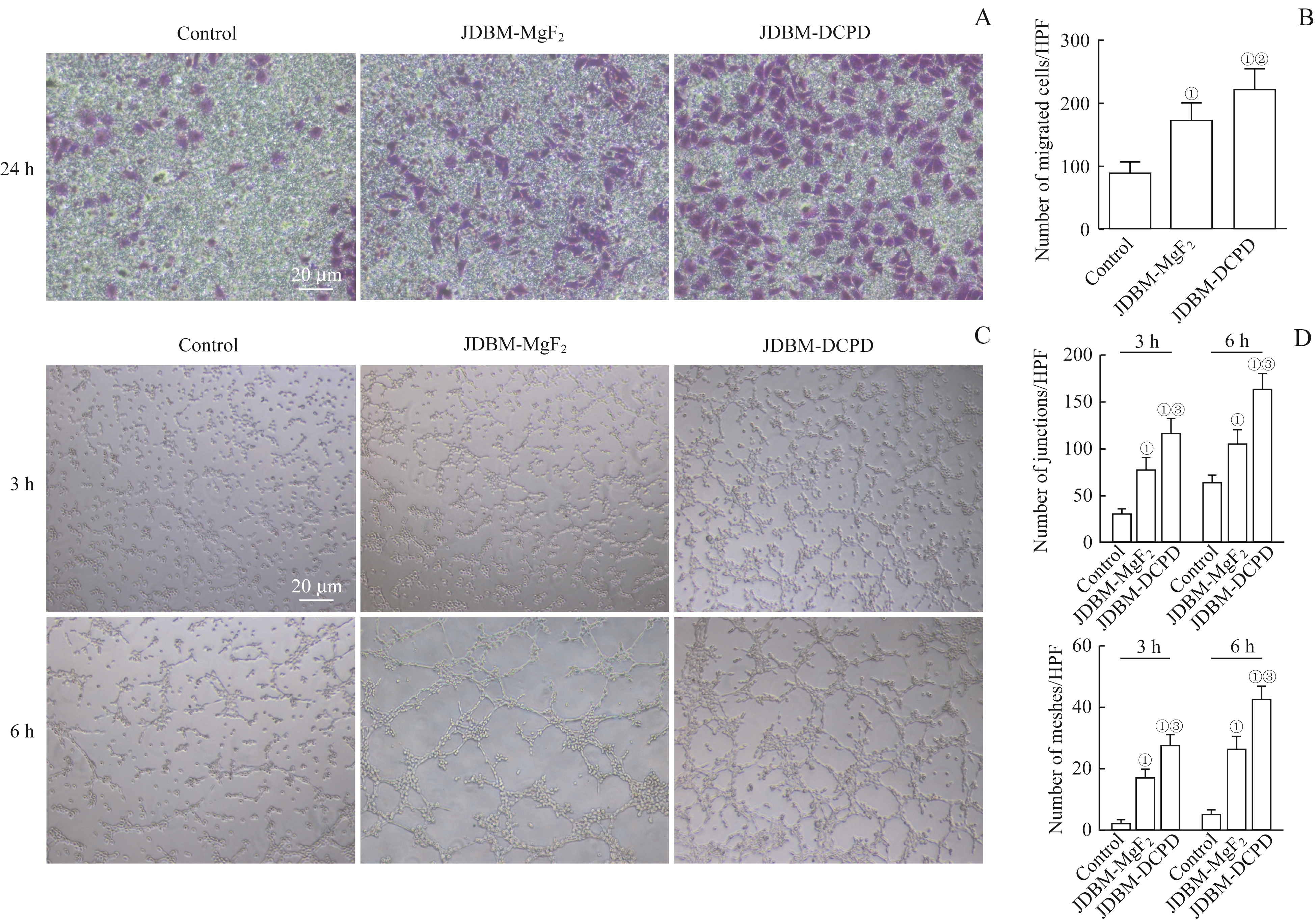
图3 JDBM-DCPD与JDBM-MgF2支架浸提液对Ea.hy926细胞迁移能力和成管情况影响的比较Note: A. Representative photographs showing the effect of the extracts of JDBM-DCPD and JDBM-MgF2 on the migration of Ea.hy926 cells after incubation for 24 h detected by Transwell migration assay (crystal violet staining, ×100). B. Quantitation of migrating Ea.hy926 cells. C. Representative photographs showing the effect of the extracts of JDBM-DCPD and JDBM-MgF2 on the tubule formation of Ea.hy926 cells after incubation for 3 h and 6 h (×100). D. Quantitation of tubule formation of Ea.hy926 cells. ①P=0.000, compared with the control group; ②P=0.007, ③P=0.000, compared with the JDBM-MgF2 group. HPF—high power field.
Fig 3 Comparison of the effects of JDBM-DCPD and JDBM-MgF2 scaffold extracts on Ea.hy926 cell migration and tube formation
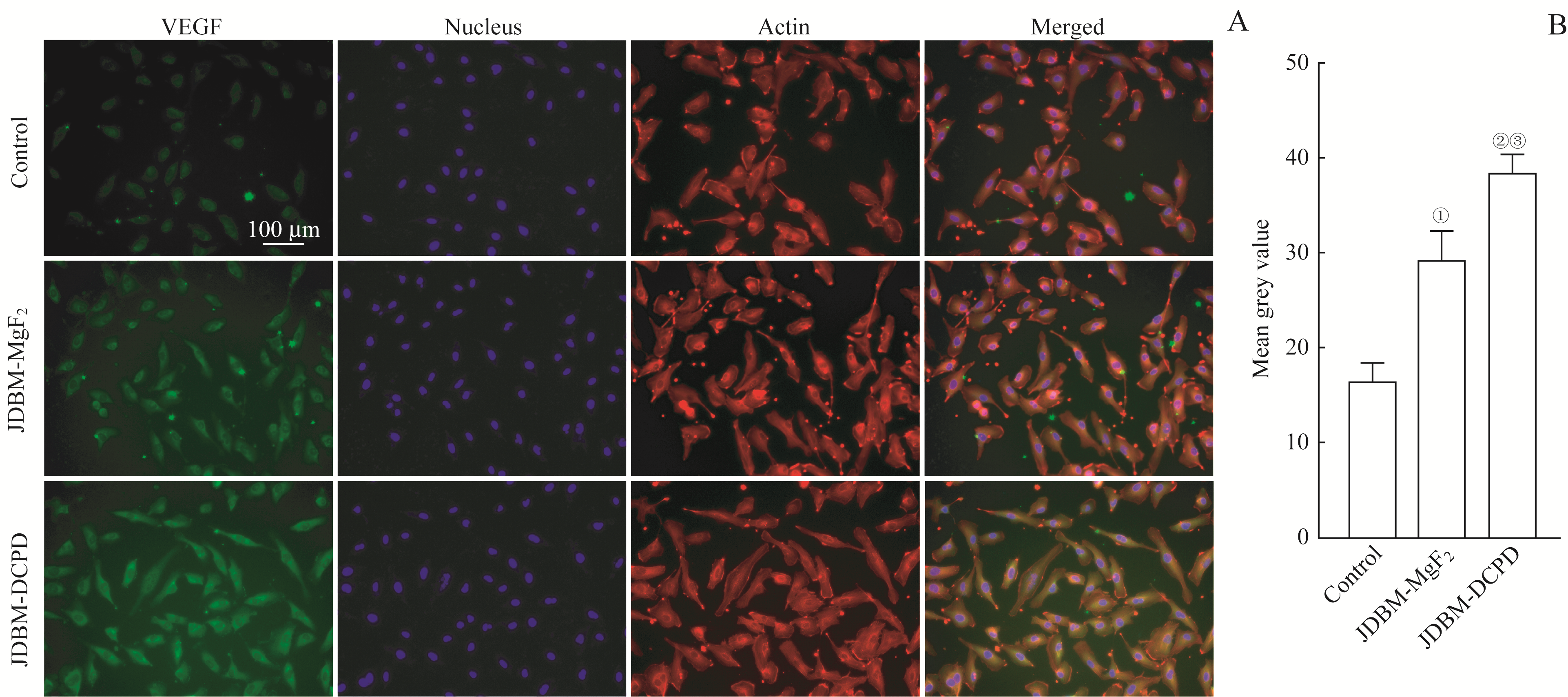
图4 JDBM-DCPD与JDBM-MgF2支架浸提液对Ea.hy926细胞VEGF表达水平影响的比较Note:A. Representative photographs showing the effect of the extracts of JDBM-DCPD and JDBM-MgF2 on the expression of VEGF in Ea.hy926 cells after incubation for 3 d detected by immunofluorescent (×200). B. Semi-quantitative analysis of fluorescence intensity. ①P=0.001, ②P=0.000, compared with the control group; ③P=0.004, compared with the JDBM-MgF2 group.
Fig 4 Comparison of the effects of JDBM-DCPD and JDBM-MgF2 scaffold extracts on the expression of VEGF in Ea.hy926 cells

图5 JDBM-DCPD与JDBM-MgF2支架浸提液对BMSC成骨分化影响的比较Note:A. ALP staining of BMSCs cultured in the scaffold extracts for 7 d and 14 d, respectively (×100). B. ARS staining of BMSCs cultured in the scaffold extracts for 14 d (×100).
Fig 5 Comparison of effects of JDBM-DCPD and JDBM-MgF2 scaffold extracts on osteogenic differentiation of BMSCs
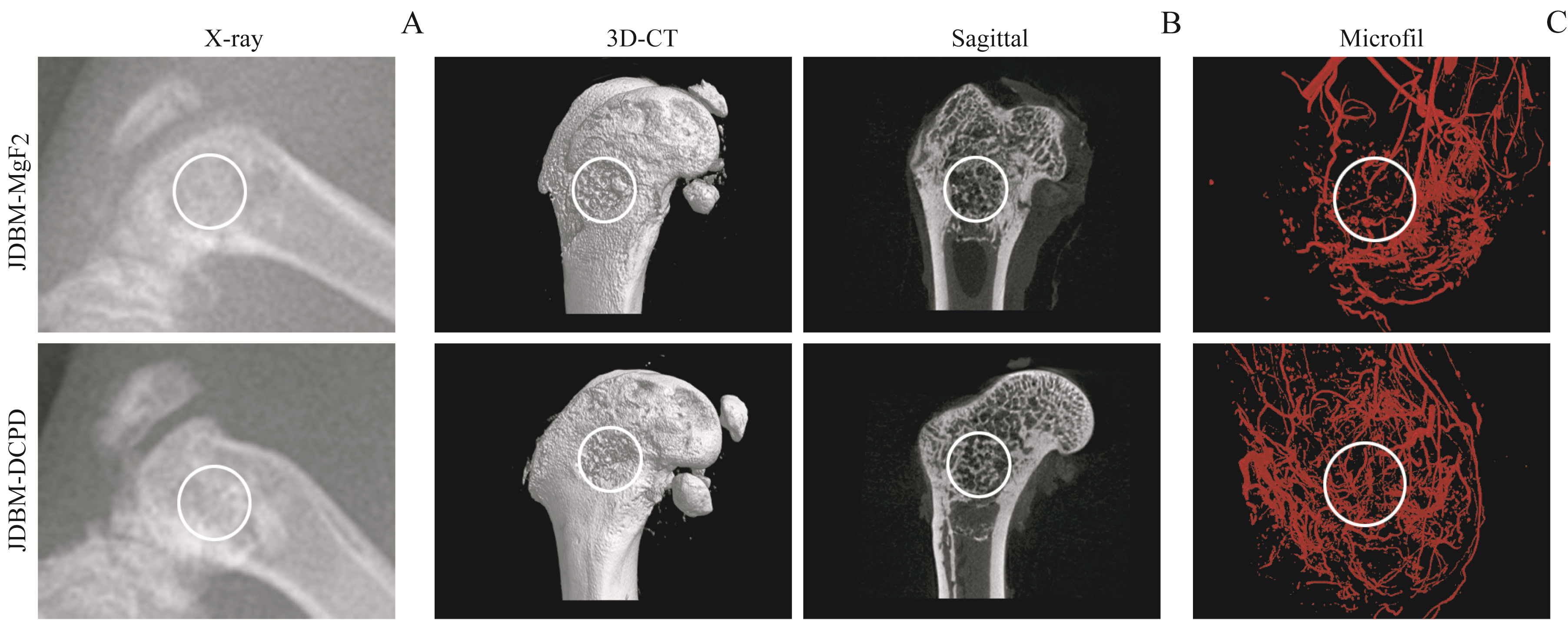
图6 植入8周后影像学检测JDBM-DCPD与JDBM-MgF2支架的体内成骨、成血管作用Note: A. X-ray photos. B. Three-dimensional reconstruction and sagittal images by CT. C. Microfil perfusion to observe the formation of new blood vessels in the defect area. The white circles indicate the bone defect area.
Fig 6 Osteogenesis and angiogenesis of JDBM-DCPD and JDBM-MgF2 scaffolds in vivo detected by radiography 8 weeks after implantation
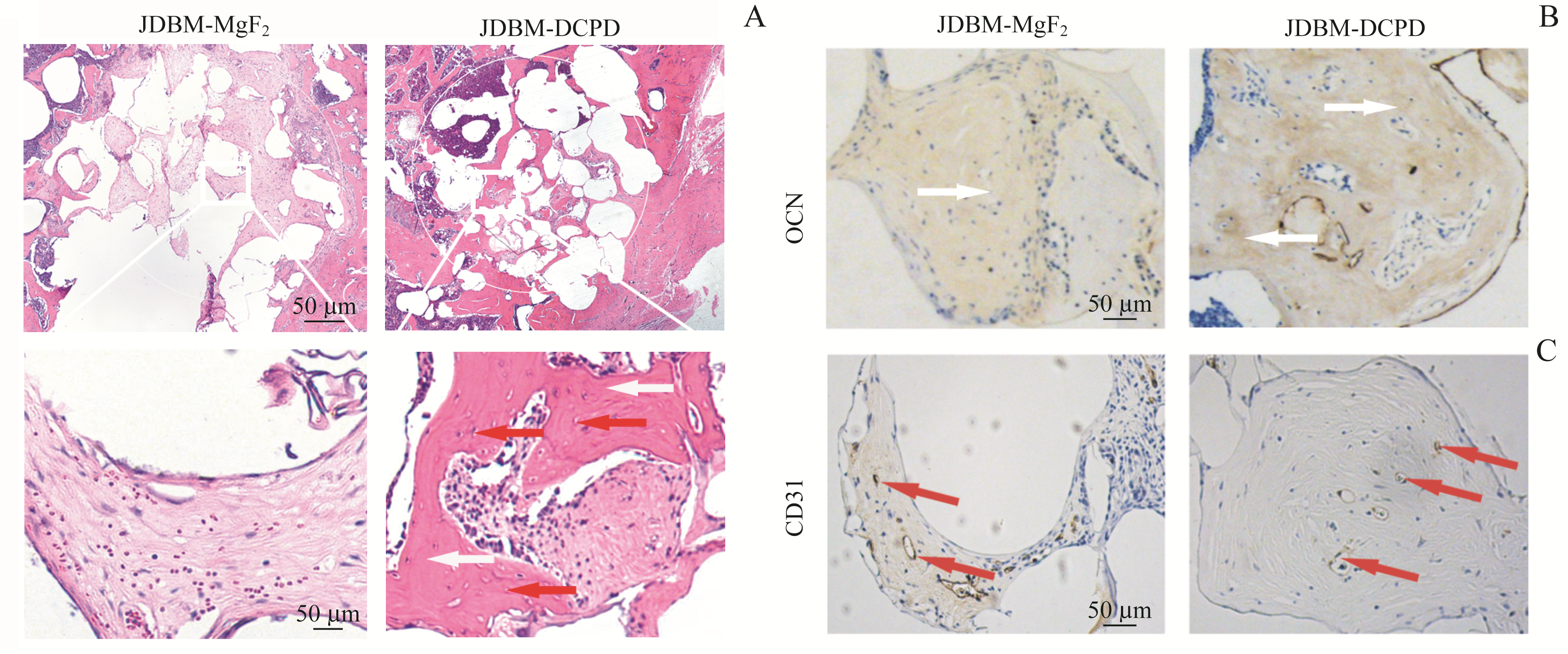
图7 植入8周后JDBM-DCPD组与JDBM-MgF2组大鼠骨缺损区的组织学观察Note:A. H-E staining of the bone defect areas (above×200, below×400). B. Expression of OCN in the defect areas (immunohistochemical staining, ×400). C. Expression of CD31 in the defect areas (immunohistochemical staining, ×400). The white arrows represent the new bone tissues and the red arrows represent the new blood vessels.
Fig 7 Histological observation of the bone defect areas in JDBM-DCPD group and JDBM-MgF2 group rats 8 weeks after implantation
| 1 | Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update[J]. Injury, 2005, 36(): S20-S27. |
| 2 | Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes[J]. ANZ J Surg, 2001, 71(6): 354-361. |
| 3 | Samartzis D, Shen FH, Goldberg EJ, et al. Is autograft the gold standard in achieving radiographic fusion in one-level anterior cervical discectomy and fusion with rigid anterior plate fixation?[J]. Spine, 2005, 30(15): 1756-1761. |
| 4 | Jin L, Li P, Wang YC, et al. Studies of superb microvascular imaging and contrast-enhanced ultrasonography in the evaluation of vascularization in early bone regeneration[J]. J Ultrasound Med, 2019, 38(11): 2963-2971. |
| 5 | Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton[J]. J Bone Miner Res, 2006, 21(2): 183-192. |
| 6 | Parfitt AM. The mechanism of coupling: a role for the vasculature[J]. Bone, 2000, 26(4): 319-323. |
| 7 | Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering[J]. Adv Drug Deliv Rev, 2011, 63(4/5): 300-311. |
| 8 | Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering[J]. Trends Biotechnol, 2008, 26(8): 434-441. |
| 9 | Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone[J]. Eur Cell Mater, 2008, 15: 100-114. |
| 10 | Thevenot P, Nair A, Dey J, et al. Method to analyze three-dimensional cell distribution and infiltration in degradable scaffolds[J]. Tissue Eng Part C Methods, 2008, 14(4): 319-331. |
| 11 | Staiger MP, Pietak AM, Huadmai J, et al. Magnesium and its alloys as orthopedic biomaterials: a review[J]. Biomaterials, 2006, 27(9): 1728-1734. |
| 12 | Velasco MA, Narváez-Tovar CA, Garzón-Alvarado DA. Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering[J]. Biomed Res Int, 2015, 2015: 729076. |
| 13 | Kunjukunju S, Roy A, Ramanathan M, et al. A layer-by-layer approach to natural polymer-derived bioactive coatings on magnesium alloys[J]. Acta Biomater, 2013, 9(10): 8690-8703. |
| 14 | Zhang J, Ma X, Lin D, et al. Magnesium modification of a calcium phosphate cement alters bone marrow stromal cell behavior via an integrin-mediated mechanism[J]. Biomaterials, 2015, 53: 251-264. |
| 15 | Qin H, Zhao Y, An Z, et al. Enhanced antibacterial properties, biocompatibility, and corrosion resistance of degradable Mg-Nd-Zn-Zr alloy[J]. Biomaterials, 2015, 53: 211-220. |
| 16 | Kong X, Wang L, Li G, et al. Mg-based bone implants show promising osteoinductivity and controllable degradation: a long-term study in a goat femoral condyle fracture model[J]. Mater Sci Eng C Mater Biol Appl, 2018, 86: 42-47. |
| 17 | Guan X, Xiong M, Zeng F, et al. Enhancement of osteogenesis and biodegradation control by brushite coating on Mg-Nd-Zn-Zr alloy for mandibular bone repair[J]. ACS Appl Mater Interfaces, 2014, 6(23): 21525-21533. |
| 18 | Tamimi F, Sheikh Z, Barralet J. Dicalcium phosphate cements: brushite and monetite[J]. Acta Biomater, 2012, 8(2): 474-487. |
| 19 | Apelt D, Theiss F, El-Warrak AO, et al. In vivo behavior of three different injectable hydraulic calcium phosphate cements[J]. Biomaterials, 2004, 25(7): 1439-1451. |
| 20 | Malhotra A, Habibovic P. Calcium phosphates and angiogenesis: implications and advances for bone regeneration[J]. Trends Biotechnol, 2016, 34(12): 983-992. |
| 21 | Wang W, Jia G, Wang Q, et al. The in vitro and in vivo biological effects and osteogenic activity of novel biodegradable porous Mg alloy scaffolds[J]. Mater Des, 2020, 189: 108514. |
| 22 | Pijuan J, Barceló C, Moreno DF, et al. In vitro cell migration, invasion, and adhesion assays: from cell imaging to data analysis[J]. Front Cell Dev Biol, 2019, 7: 107. |
| 23 | Hu H, Chen Y, Zou Z, et al. Panax notoginseng saponins prevent bone loss by promoting angiogenesis in an osteoporotic mouse model[J]. Biomed Res Int, 2020, 2020: 8412468. |
| 24 | Dai C, Guo H, Lu J, et al. Osteogenic evaluation of calcium/magnesium-doped mesoporous silica scaffold with incorporation of rhBMP-2 by synchrotron radiation-based μCT[J]. Biomaterials, 2011, 32(33): 8506-8517. |
| 25 | Griffith LG. Emerging design principles in biomaterials and scaffolds for tissue engineering[J]. Ann N Y Acad Sci, 2002, 961: 83-95. |
| 26 | Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis[J]. Biomaterials, 2005, 26(27): 5474-5491. |
| 27 | Chiesa R, Sandrini E, Santin M, et al. Osteointegration of titanium and its alloys by anodic spark deposition and other electrochemical techniques: a review[J]. J Appl Biomater Biomech, 2003, 1(2): 91-107. |
| 28 | Liang C, Wang H, Yang J, et al. Femtosecond laser-induced micropattern and Ca/P deposition on Ti implant surface and its acceleration on early osseointegration[J]. ACS Appl Mater Interfaces, 2013, 5(16): 8179-8186. |
| 29 | Ma H, Luo J, Sun Z, et al. 3D printing of biomaterials with mussel-inspired nanostructures for tumor therapy and tissue regeneration[J]. Biomaterials, 2016, 111: 138-148. |
| 30 | Xu F, Ding H, Song F, et al. Effects of preparation methods on the bone formation potential of apatite-coated chitosan microspheres[J]. J Biomater Sci Polym Ed, 2014, 25(18): 2080-2093. |
| 31 | Yu W, Zhao H, Ding Z, et al. In vitro and in vivo evaluation of MgF2 coated AZ31 magnesium alloy porous scaffolds for bone regeneration[J]. Colloids Surf B Biointerfaces, 2017, 149: 330-340. |
| 32 | Lafage-Proust MH, Prisby R, Roche B, et al. Bone vascularization and remodeling[J]. Joint Bone Spine, 2010, 77(6): 521-524. |
| 33 | Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone[J]. Nature, 2014, 507(7492): 323-328. |
| 34 | Dhandapani R, Krishnan PD, Zennifer A, et al. Additive manufacturing of biodegradable porous orthopaedic screw[J]. Bioact Mater, 2020, 5(3): 458-467. |
| 35 | Xie H, Cui Z, Wang L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis[J]. Nat Med, 2014, 20(11): 1270-1278. |
| 36 | Liu Q, Zhou YF, Li ZB. PDGF‑BB promotes the differentiation and proliferation of MC3T3‑E1 cells through the Src/JAK2 signaling pathway[J]. Mol Med Rep, 2018, 18(4): 3719-3726. |
| 37 | Liu W, Guo S, Tang Z, et al. Magnesium promotes bone formation and angiogenesis by enhancing MC3T3-E1 secretion of PDGF-BB[J]. Biochem Biophys Res Commun, 2020, 528(4): 664-670. |
| 38 | Saghiri MA, Asatourian A, Orangi J, et al. Functional role of inorganic trace elements in angiogenesis—part Ⅰ: N, Fe, Se, P, Au, and Ca[J]. Crit Rev Oncol Hematol, 2015, 96(1): 129-142. |
| 39 | Song GL, Song SZ. A possible biodegradable magnesium implant material[J]. Adv Eng Mater, 2007, 9(4): 298-302. |
| 40 | Gu XN, Zheng YF, Chen LJ. Influence of artificial biological fluid composition on the biocorrosion of potential orthopedic Mg-Ca, Az31, Az91 alloys[J]. Biomed Mater, 2009, 4(6): 065011. |
| 41 | Fischer J, Prosenc MH, Wolff M, et al. Interference of magnesium corrosion with tetrazolium-based cytotoxicity assays[J]. Acta Biomater, 2010, 6(5): 1813-1823. |
| [1] | 黄紫晗, 黄心智. 单细胞RNA测序在骨再生研究中的应用[J]. 上海交通大学学报(医学版), 2025, 45(8): 1053-1058. |
| [2] | 许沐馨, 刘贤, 蒋立姗, 孙青. Nd:YAP激光生物刺激通过WNT/β-catenin信号通路促进人牙周韧带细胞增殖和成骨分化[J]. 上海交通大学学报(医学版), 2025, 45(5): 562-569. |
| [3] | 鲁佳艺, 刘锦喆, 郭尚春, 陶诗聪. 纳米材料通过降低活性氧水平促进骨组织再生的研究进展[J]. 上海交通大学学报(医学版), 2025, 45(4): 487-492. |
| [4] | 刘媛琪, 孙思远, 代庆刚, 江凌勇, 沈国芳. 全反式视黄酸调控颌骨骨髓间充质干细胞成骨分化双向效应的体外研究[J]. 上海交通大学学报(医学版), 2024, 44(9): 1083-1093. |
| [5] | 何姝航, 郭尚春, 陶诗聪. 神经血管耦合在骨修复中作用的研究进展[J]. 上海交通大学学报(医学版), 2024, 44(3): 373-378. |
| [6] | 高昕, 杨屹羚, 黄湘如, 代庆刚, 江凌勇. 利用CRISPR/Cas9靶向敲除Piezo1基因对小鼠间充质干细胞成骨分化的影响研究[J]. 上海交通大学学报(医学版), 2023, 43(9): 1080-1088. |
| [7] | 王千懿, 冉欣悦, 张沛灵, 慈政, 雷东, 周广东. 软骨脱细胞基质/丝素蛋白活性支架的构建及其软骨组织工程研究[J]. 上海交通大学学报(医学版), 2023, 43(7): 795-803. |
| [8] | 吴昭瑜, 许之珏, 蒲蕻吉, 王新, 陆信武. 神经损伤诱导蛋白1的生理功能及其在相关疾病中的作用[J]. 上海交通大学学报(医学版), 2023, 43(3): 358-364. |
| [9] | 刘宏强, 陆艳青, 高宇轩, 王一云, 王传东, 张晓玲. 构建高效载体OPEI沉默TRAF6促进骨关节炎软骨再生的研究[J]. 上海交通大学学报(医学版), 2022, 42(7): 846-857. |
| [10] | 吴靖, 赵正宜, 邹多宏, 杨驰, 张志愿. 帐篷钉技术在牙槽骨重度缺损修复重建中的应用:30例临床病例回顾性分析与总结[J]. 上海交通大学学报(医学版), 2022, 42(6): 768-777. |
| [11] | 陆艳青, 周兴, 李姣, 彭建平, 王传东, 张晓玲. 抗肿瘤药物依托泊苷促进间充质干细胞成骨分化的研究[J]. 上海交通大学学报(医学版), 2021, 41(7): 849-857. |
| [12] | 汤静, 于晨溪, 陈中丽, 章安迪. CTRP2与冠状动脉阻塞患者侧支循环形成的临床关联及相关作用[J]. 上海交通大学学报(医学版), 2021, 41(7): 915-919. |
| [13] | 陈 帅1,丁风华2,代 杨1,陆 林2,沈 迎2,沈卫峰2. 炎症细胞参与侧支循环形成的研究进展[J]. 上海交通大学学报(医学版), 2020, 40(3): 380-. |
| [14] | 崔雅琦,白玉冰,许怡晨,谭心辰,李梦莹,贾 浩. 脂肪来源的间充质干细胞及外囊泡促成骨分化的研究进展[J]. 上海交通大学学报(医学版), 2020, 40(12): 1672-1676. |
| [15] | 李 元,史俊宇,张 枭,赖红昌. 上颌前牙区牙槽骨缺损形态学特征与引导骨再生手术效果的相关性研究[J]. 上海交通大学学报(医学版), 2020, 40(10): 1414-1419. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||