
上海交通大学学报(医学版) ›› 2022, Vol. 42 ›› Issue (10): 1490-1497.doi: 10.3969/j.issn.1674-8115.2022.10.016
收稿日期:2022-05-07
接受日期:2022-08-28
出版日期:2022-10-28
发布日期:2022-10-17
通讯作者:
房静远,电子信箱:jingyuanfang@sjtu.edu.cn。作者简介:蒋 怡(1996—),女,硕士生;电子信箱:jiangyi1501@163.com。
基金资助:
JIANG Yi1( ), JIANG Ping2, ZHANG Mingming1, FANG Jingyuan1(
), JIANG Ping2, ZHANG Mingming1, FANG Jingyuan1( )
)
Received:2022-05-07
Accepted:2022-08-28
Online:2022-10-28
Published:2022-10-17
Contact:
FANG Jingyuan, E-mail: jingyuanfang@sjtu.edu.cn.Supported by:摘要:
嗜黏蛋白阿克曼菌(Akkermansia muciniphila,A. muciniphila)是人类肠道中的常见共生菌之一,可利用黏蛋白作为唯一的碳、氮来源,在肠道内定植和生长。肠道稳态对于维持人体正常生理功能至关重要,肠道功能发生紊乱与代谢疾病、免疫疾病、感染性疾病和肿瘤等的发生、发展密切相关。肠道微生物群是影响肠道健康的关键因素,作为肠道微生物的一员,A. muciniphila在肠道炎症、肠道肿瘤、受肠道紊乱影响的疾病(如肝病、代谢性疾病等)中的作用已被证实,且作用机制亦逐渐被阐明,被认为是一种很有前景的候选益生菌。该文针对A. muciniphila的特征、在肠道中的分布、与肠道相关疾病的关联及作用机制进行综述。
中图分类号:
蒋怡, 江平, 张明明, 房静远. 嗜黏蛋白阿克曼菌在肠道相关疾病中作用的研究进展[J]. 上海交通大学学报(医学版), 2022, 42(10): 1490-1497.
JIANG Yi, JIANG Ping, ZHANG Mingming, FANG Jingyuan. Research progress in the role of Akkermansia muciniphila in gut-related diseases[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2022, 42(10): 1490-1497.
| Disease | Mouse model | Determinant | Effect (+/-) |
|---|---|---|---|
| Colitis | DSS induced colitis | Bacteria | -/+[ |
| AmEV | -[ | ||
| Amuc_1100 | -[ | ||
| Colitis | Salmonella typhimurium-infected colitis | Bacteria | +[ |
| Colitis | Il10-/- mice spontaneous colitis | Bacteria | +[ |
| CAC | AOM/DSS induced CAC | Amuc_1100 | -[ |
| CRC | ApcMin/+ mice spontaneous CRC | Bacteria | -[ |
| CRC | HCT116/CT26 subcutaneously transplantation tumor | Bacteria | -[ |
| ALD | Alcohol induced ALD | Bacteria | -[ |
| Liver injury | ConA induced liver injury | Bacteria | -[ |
| Obesity/T2DM | High-fat diet induced obesity/T2DM | Bacteria | -[ |
| Amuc_1100 | -[ |
表 1 A. muciniphila 及其菌体成分或分泌物在不同疾病中发挥的作用
Tab 1 Effect of A. muciniphila and its components or secretion on different diseases
| Disease | Mouse model | Determinant | Effect (+/-) |
|---|---|---|---|
| Colitis | DSS induced colitis | Bacteria | -/+[ |
| AmEV | -[ | ||
| Amuc_1100 | -[ | ||
| Colitis | Salmonella typhimurium-infected colitis | Bacteria | +[ |
| Colitis | Il10-/- mice spontaneous colitis | Bacteria | +[ |
| CAC | AOM/DSS induced CAC | Amuc_1100 | -[ |
| CRC | ApcMin/+ mice spontaneous CRC | Bacteria | -[ |
| CRC | HCT116/CT26 subcutaneously transplantation tumor | Bacteria | -[ |
| ALD | Alcohol induced ALD | Bacteria | -[ |
| Liver injury | ConA induced liver injury | Bacteria | -[ |
| Obesity/T2DM | High-fat diet induced obesity/T2DM | Bacteria | -[ |
| Amuc_1100 | -[ |
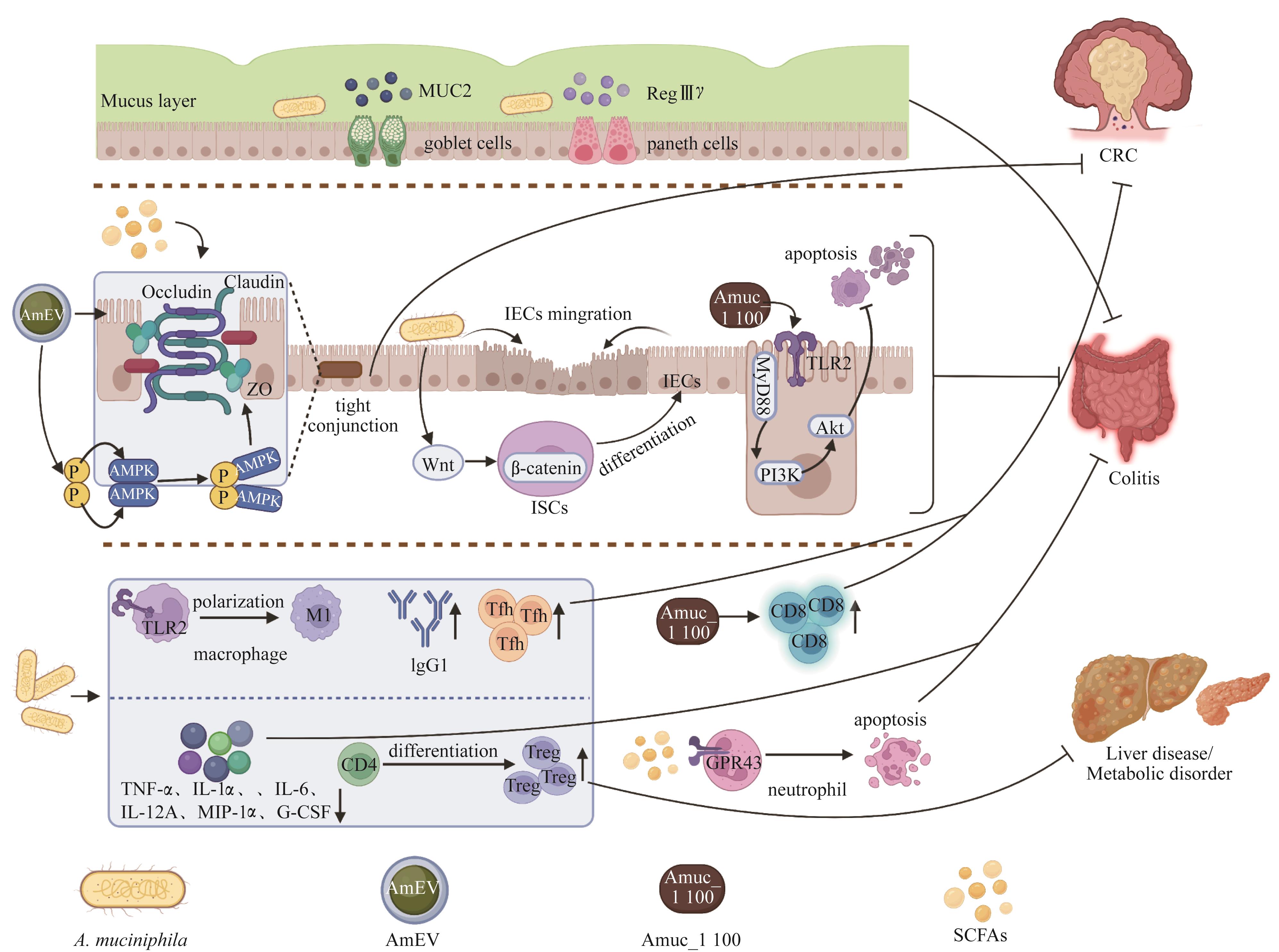
图 2 A. muciniphila 在肠道相关疾病中的作用机制Note:IEC—intestinal epithelial cell; ISC—intestinal stem cell; G-CSF—granulocyte colony stimulating factor; GPR43—G protein-coupled receptor 43.
Fig 2 Mechanisms of A. muciniphila in gut-related diseases
| 1 | LYNCH S V, PEDERSEN O. The human intestinal microbiome in health and disease[J]. N Engl J Med, 2016, 375(24): 2369-2379. |
| 2 | JAKOBSSON H E, ABRAHAMSSON T R, JENMALM M C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section[J]. Gut, 2014, 63(4): 559-566. |
| 3 | DERRIEN M, COLLADO M C, BEN-AMOR K, et al. The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract[J]. Appl Environ Microbiol, 2008, 74(5): 1646-1648. |
| 4 | DERRIEN M, VAUGHAN E E, PLUGGE C M, et al. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium[J]. Int J Syst Evol Microbiol, 2004, 54(Pt 5): 1469-1476. |
| 5 | HOLD G L, PRYDE S E, RUSSELL V J, et al. Assessment of microbial diversity in human colonic samples by 16S rDNA sequence analysis[J]. FEMS Microbiol Ecol, 2002, 39(1): 33-39. |
| 6 | SALZMAN N H, DE JONG H, PATERSON Y, et al. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria[J]. Microbiology (Reading), 2002, 148(Pt 11): 3651-3660. |
| 7 | DERRIEN M, VAN PASSEL M W, VAN DE BOVENKAMP J H, et al. Mucin-bacterial interactions in the human oral cavity and digestive tract[J]. Gut Microbes, 2010, 1(4): 254-268. |
| 8 | COLLADO M C, DERRIEN M, ISOLAURI E, et al. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly[J]. Appl Environ Microbiol, 2007, 73(23): 7767-7770. |
| 9 | COLLADO M C, ISOLAURI E, LAITINEN K, et al. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women[J]. Am J Clin Nutr, 2008, 88(4): 894-899. |
| 10 | SONOYAMA K, FUJIWARA R, TAKEMURA N, et al. Response of gut microbiota to fasting and hibernation in Syrian hamsters[J]. Appl Environ Microbiol, 2009, 75(20): 6451-6456. |
| 11 | BELZER C, DE VOS W M. Microbes inside: from diversity to function: the case of Akkermansia[J]. ISME J, 2012, 6(8): 1449-1458. |
| 12 | VAN PASSEL M W J, KANT R, ZOETENDAL E G, et al. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes[J]. PLoS One, 2011, 6(3): e16876. |
| 13 | BAJER L, KVERKA M, KOSTOVCIK M, et al. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis[J]. World J Gastroenterol, 2017, 23(25): 4548-4558. |
| 14 | EARLEY H, LENNON G, BALFE Á, et al. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis[J]. Sci Rep, 2019, 9(1): 15683. |
| 15 | KUMP P, WURM P, GRÖCHENIG H P, et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis[J]. Aliment Pharmacol Ther, 2018, 47(1): 67-77. |
| 16 | PNG C W, LINDÉN S K, GILSHENAN K S, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria[J]. Am J Gastroenterol, 2010, 105(11): 2420-2428. |
| 17 | BIAN X Y, WU W R, YANG L Y, et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice[J]. Front Microbiol, 2019, 10: 2259. |
| 18 | KANG C S, BAN M, CHOI E J, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis[J]. PLoS One, 2013, 8(10): e76520. |
| 19 | WANG L J, TANG L, FENG Y M, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice[J]. Gut, 2020, 69(11): 1988-1997. |
| 20 | HÅKANSSON Å, TORMO-BADIA N, BARIDI A, et al. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice[J]. Clin Exp Med, 2015, 15(1): 107-120. |
| 21 | CASTRO-MEJÍA J, JAKESEVIC M, KRYCH Ł, et al. Treatment with a monoclonal anti-IL-12p40 antibody induces substantial gut microbiota changes in an experimental colitis model[J]. Gastroenterol Res Pract, 2016, 2016: 4953120. |
| 22 | GANESH B P, KLOPFLEISCH R, LOH G, et al. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice[J]. PLoS One, 2013, 8(9): e74963. |
| 23 | SEREGIN S S, GOLOVCHENKO N, SCHAF B, et al. NLRP6 protects Il10 -/- mice from colitis by limiting colonization of Akkermansia muciniphila[J]. Cell Rep, 2017, 19(4): 733-745. |
| 24 | BAE M, CASSILLY C D, LIU X, et al. Akkermansia muciniphila phospholipid induces homeostatic immune responses[J]. Nature, 2022, 608(7921): 168-173. |
| 25 | FAN L N, XU C C, GE Q W, et al. A. muciniphila suppresses colorectal tumorigenesis by inducing TLR2/NLRP3-mediated M1-like TAMs[J]. Cancer Immunol Res, 2021, 9(10): 1111-1124. |
| 26 | COLLINS D, HOGAN A M, WINTER D C. Microbial and viral pathogens in colorectal cancer[J]. Lancet Oncol, 2011, 12(5): 504-512. |
| 27 | ROUTY B, LE CHATELIER E, DEROSA L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors[J]. Science, 2018, 359(6371): 91-97. |
| 28 | HOU X Y, ZHANG P, DU H Z, et al. Akkermansia muciniphila potentiates the antitumor efficacy of FOLFOX in colon cancer[J]. Front Pharmacol, 2021, 12: 725583. |
| 29 | MILOSEVIC I, VUJOVIC A, BARAC A, et al. Gut-liver axis, gut microbiota, and its modulation in the management of liver diseases: a review of the literature[J]. Int J Mol Sci, 2019, 20(2): 395. |
| 30 | GRANDER C, ADOLPH T E, WIESER V, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease[J]. Gut, 2018, 67(5): 891-901. |
| 31 | WU W R, LV L X, SHI D, et al. Protective effect of Akkermansia muciniphila against immune-mediated liver injury in a mouse model[J]. Front Microbiol, 2017, 8: 1804. |
| 32 | FAN Y, PEDERSEN O. Gut microbiota in human metabolic health and disease[J]. Nat Rev Microbiol, 2021, 19(1): 55-71. |
| 33 | EVERARD A, BELZER C, GEURTS L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity[J]. Proc Natl Acad Sci USA, 2013, 110(22): 9066-9071. |
| 34 | SANTACRUZ A, COLLADO M C, GARCÍA-VALDÉS L, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women[J]. Br J Nutr, 2010, 104(1): 83-92. |
| 35 | KARLSSON C L J, ONNERFÄLT J, XU J, et al. The microbiota of the gut in preschool children with normal and excessive body weight[J]. Obesity (Silver Spring), 2012, 20(11): 2257-2261. |
| 36 | ZHANG X Y, SHEN D Q, FANG Z W, et al. Human gut microbiota changes reveal the progression of glucose intolerance[J]. PLoS One, 2013, 8(8): e71108. |
| 37 | CHELAKKOT C, CHOI Y, KIM D K, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions[J]. Exp Mol Med, 2018, 50(2): e450. |
| 38 | DAO M C, EVERARD A, ARON-WISNEWSKY J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology[J]. Gut, 2016, 65(3): 426-436. |
| 39 | PLOVIER H, EVERARD A, DRUART C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice[J]. Nat Med, 2017, 23(1): 107-113. |
| 40 | LEE H, KO G. Effect of metformin on metabolic improvement and gut microbiota[J]. Appl Environ Microbiol, 2014, 80(19): 5935-5943. |
| 41 | SHIN N R, LEE J C, LEE H Y, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice[J]. Gut, 2014, 63(5): 727-735. |
| 42 | LI J, LIN S Q, VANHOUTTE P M, et al. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe -/- mice[J]. Circulation, 2016, 133(24): 2434-2446. |
| 43 | ZHU L D, LU X X, LIU L, et al. Akkermansia muciniphila protects intestinal mucosa from damage caused by S. pullorum by initiating proliferation of intestinal epithelium[J]. Vet Res, 2020, 51(1): 34. |
| 44 | REUNANEN J, KAINULAINEN V, HUUSKONEN L, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer[J]. Appl Environ Microbiol, 2015, 81(11): 3655-3662. |
| 45 | ALAM A, LEONI G, QUIROS M, et al. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota[J]. Nat Microbiol, 2016, 1: 15021. |
| 46 | GREGORIEFF A, PINTO D, BEGTHEL H, et al. Expression pattern of Wnt signaling components in the adult intestine[J]. Gastroenterology, 2005, 129(2): 626-638. |
| 47 | DERRIEN M, VAN BAARLEN P, HOOIVELD G, et al. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila[J]. Front Microbiol, 2011, 2: 166. |
| 48 | ANSALDO E, SLAYDEN L C, CHING K L, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis[J]. Science, 2019, 364(6446): 1179-1184. |
| 49 | MASLOWSKI K M, VIEIRA A T, NG A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43[J]. Nature, 2009, 461(7268): 1282-1286. |
| 50 | KIM M, FRIESEN L, PARK J, et al. Microbial metabolites, short-chain fatty acids, restrain tissue bacterial load, chronic inflammation, and associated cancer in the colon of mice[J]. Eur J Immunol, 2018, 48(7): 1235-1247. |
| 51 | MENG X, ZHANG J R, WU H, et al. Akkermansia muciniphila aspartic protease Amuc_1434* inhibits human colorectal cancer LS174T cell viability via TRAIL-mediated apoptosis pathway[J]. Int J Mol Sci, 2020, 21(9): 3385. |
| 52 | CARIO E, GERKEN G, PODOLSKY D K. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function[J]. Gastroenterology, 2007, 132(4): 1359-1374. |
| [1] | 王治琪, 王莹. 儿童炎症性肠病相关贫血的诊治研究进展[J]. 上海交通大学学报(医学版), 2025, 45(9): 1232-1238. |
| [2] | 木尔扎特·艾麦提, 张业骞, 刘涛, 白龙, 张浩宇, 倪博, 关玉静, 王书昌, 顾佳毅, 朱纯超, 夏翔, 张子臻. 机器人与腹腔镜辅助近端胃切除联合双肌瓣吻合治疗早期胃上部癌的近期效果对比[J]. 上海交通大学学报(医学版), 2025, 45(7): 874-882. |
| [3] | 赛提尔古丽·克然木, 钱蕾, 丁思怡, 哈娜提·马合力木汗, 杨雪儿, 贾浩. 精氨酸代谢调控间充质干细胞功能的研究进展[J]. 上海交通大学学报(医学版), 2025, 45(7): 910-915. |
| [4] | 赵心雨, 张文超, 陈旭卓, 宋佳琪, 黄慧, 张善勇. 亚精胺对脂多糖诱导的小鼠颅骨炎症性骨溶解的作用研究[J]. 上海交通大学学报(医学版), 2025, 45(6): 673-683. |
| [5] | 杨乐, 周怡, 王钶韵, 赖娅莉. 大黄素改善阿尔茨海默病认知障碍、内质网应激和神经炎症的研究[J]. 上海交通大学学报(医学版), 2025, 45(6): 727-734. |
| [6] | 汤开然, 冯成领, 韩邦旻. 基于单细胞测序与转录组测序构建M2巨噬细胞基因相关的前列腺癌预后模型[J]. 上海交通大学学报(医学版), 2025, 45(5): 549-561. |
| [7] | 张钲佳, 李小敏, 周鑫, 马海荣, 艾松涛. 高阶磁共振功能成像评估骨与软组织肿瘤价值初探[J]. 上海交通大学学报(医学版), 2025, 45(5): 585-596. |
| [8] | 禹恺, 帅哲玮, 黄洪军, 罗艳. 小胶质细胞在中枢神经系统炎症性疾病中的作用和机制研究进展[J]. 上海交通大学学报(医学版), 2025, 45(5): 630-638. |
| [9] | 万宏劲, 胡逸斌, 王昕, 张凯, 秦安, 马培翔, 马辉, 赵杰. 甲基莲心碱通过KEAP1/NRF2/GPX4和NF-κB信号通路减轻椎间盘退行性变[J]. 上海交通大学学报(医学版), 2025, 45(3): 261-270. |
| [10] | 张博源, 姚志荣. 紫外线诱导的DNA损伤促进皮肤恶性肿瘤发生的研究现状[J]. 上海交通大学学报(医学版), 2025, 45(2): 228-232. |
| [11] | PANDIT Roshan, 卢君瑶, 何立珩, 包玉洁, 季萍, 陈颖盈, 许洁, 王颖. 肿瘤坏死因子-α在新型冠状病毒感染合并肾损伤中的作用[J]. 上海交通大学学报(医学版), 2025, 45(1): 1-10. |
| [12] | 王晓红, 方贻儒. 双相障碍神经炎症机制的研究进展[J]. 上海交通大学学报(医学版), 2025, 45(1): 107-112. |
| [13] | 木司塔巴·木台力甫, 王俊杰, 钱云臻, 陈溯源, 邵达, 张志刚, 李冬雪. 预免疫策略结合mVenus-p27K-系统构建休眠肿瘤小鼠模型[J]. 上海交通大学学报(医学版), 2024, 44(9): 1104-1114. |
| [14] | 何蕊, 颜克鹏, 王静. 靶向髓源性抑制细胞的叶酸循环增强肿瘤免疫治疗效果研究[J]. 上海交通大学学报(医学版), 2024, 44(8): 1011-1022. |
| [15] | 陈深册, 陈依明, 王凡, 张梦珂, 杨惟杰, 吕洞宾, 洪武. 饮食干预治疗抑郁相关症状的研究进展[J]. 上海交通大学学报(医学版), 2024, 44(8): 1050-1055. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||