
上海交通大学学报(医学版) ›› 2023, Vol. 43 ›› Issue (1): 8-19.doi: 10.3969/j.issn.1674-8115.2023.01.002
谢林1,2( ), 程烨2(
), 程烨2( ), 郑琦敏1, 张熙2, 付莉莉3, 陈敏2, 王怡2, 梅长林3, 谢静远1, 顾向晨1,2(
), 郑琦敏1, 张熙2, 付莉莉3, 陈敏2, 王怡2, 梅长林3, 谢静远1, 顾向晨1,2( )
)
收稿日期:2022-04-29
接受日期:2022-12-16
出版日期:2023-01-28
发布日期:2023-01-28
通讯作者:
顾向晨,电子信箱:gettygugu@126.com。作者简介:谢林(1994—),女,博士生;电子信箱:18516260652@163.com基金资助:
XIE Lin1,2( ), CHENG Ye2(
), CHENG Ye2( ), ZHENG Qimin1, ZHANG Xi2, FU Lili3, CHEN Min2, WANG Yi2, MEI Changlin3, XIE Jingyuan1, GU Xiangchen1,2(
), ZHENG Qimin1, ZHANG Xi2, FU Lili3, CHEN Min2, WANG Yi2, MEI Changlin3, XIE Jingyuan1, GU Xiangchen1,2( )
)
Received:2022-04-29
Accepted:2022-12-16
Online:2023-01-28
Published:2023-01-28
Contact:
GU Xiangchen, E-mail: gettygugu@126.com.Supported by:摘要:
目的·探究淫羊藿苷在急性肾损伤(acute kidney disease,AKI)向慢性肾脏病(chronic kidney disease,CKD)转化中的作用并探讨其可能的机制。方法·C57BL/6小鼠腹腔注射马兜铃酸2周,制作AKI向CKD转化模型,并于造模前1周或马兜铃酸注射2周结束时持续灌胃给予淫羊藿苷5周或2周。监测小鼠肾功能和肾脏病理损伤,并用透射电子显微镜观察肾小管上皮细胞线粒体结构,采用荧光定量PCR(qPCR)及免疫组织化学染色法检测脂肪酸氧化代谢通路、纤维化及炎症指标等。采用Transwell细胞小室共培养大鼠肾脏成纤维细胞株和大鼠肾小管上皮细胞株,模拟2种细胞之间的相互作用。先提前1 h在肾小管上皮细胞培养液中加入淫羊藿苷,再加入马兜铃酸干预24 h;去除药物干预后,将肾小管上皮细胞与成纤维细胞共培养24 h。qPCR或Western blotting检测肾脏上皮细胞过氧化物酶体增殖物激活受体α(peroxisome proliferator activated receptor α,Ppara)、促纤维化因子、炎症因子、活化胱天蛋白酶(cleaved caspase-3),以及肾脏成纤维细胞细胞外基质相关基因mRNA或蛋白表达的变化。用Ppara-siRNA转染肾小管上皮细胞,观察淫羊藿苷提前于马兜铃酸干预后PPARα下游线粒体脂肪酸氧化代谢相关指标以及cleaved caspase-3的表达变化。结果·马兜铃酸注射2周结束时和注射结束后2周(造模4周),仅注射马兜铃酸的小鼠血清肌酐及尿素氮水平较对照组均显著升高,肾脏炎症细胞大量浸润,肾小管损伤严重;而提前给予淫羊藿苷干预的小鼠较马兜铃酸组肾功能明显改善,肾脏病理损伤减轻。造模4周时马兜铃酸组肾小管上皮细胞线粒体结构损伤严重,脂肪酸氧化代谢通路相关基因mRNA水平较对照组显著降低,伴巨噬细胞浸润及肾纤维化,提前给予淫羊藿苷干预后上述损伤或指标均明显缓解。在马兜铃酸注射2周结束时给予淫羊藿苷的小鼠血清肌酐和尿素氮水平、肾脏病理损伤、纤维化指标均与马兜铃酸组无明显差异。体外实验中,马兜铃酸可抑制肾小管上皮细胞Ppara mRNA表达,促进cleaved caspase-3、炎症因子及促纤维化因子表达升高,并上调共培养的肾脏成纤维细胞细胞外基质相关基因的表达,而淫羊藿苷提前干预对上述变化有明显的纠正作用。用siRNA敲低肾小管上皮细胞的Ppara后,淫羊藿苷对马兜铃酸抑制脂肪酸氧化代谢及促进细胞凋亡的预防保护作用减弱。结论·淫羊藿苷能预防马兜铃酸诱导的小鼠AKI向CKD转化;它可能是通过改善肾小管上皮细胞线粒体脂肪酸氧化代谢通路(尤其是PPARα)发挥作用。
中图分类号:
谢林, 程烨, 郑琦敏, 张熙, 付莉莉, 陈敏, 王怡, 梅长林, 谢静远, 顾向晨. 淫羊藿苷对急性肾损伤向慢性肾脏病转化小鼠模型的预防性保护作用[J]. 上海交通大学学报(医学版), 2023, 43(1): 8-19.
XIE Lin, CHENG Ye, ZHENG Qimin, ZHANG Xi, FU Lili, CHEN Min, WANG Yi, MEI Changlin, XIE Jingyuan, GU Xiangchen. Preventive effect of icariin on transition from acute kidney injury to chronic kidney disease in mouse model[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2023, 43(1): 8-19.
| Gene | Forward (5′→3′) | Reward (5′→3′) |
|---|---|---|
| Fn (mouse) | ATGGTACAGCTGATCCTGCC | GCCCTGGTTTGTACCTGCTA |
| Acta2 (mouse) | GGAACCCTGAGACGCTGCT | CATTCCAACCATTACTCCC |
| Col1a1 (mouse) | CCCAGCCGCAAAGAGTCTAC | AGCATACCTCGGGTTTCCAC |
| Tgfb1 (mouse) | GGGAAGCAGTGCCCGAACCC | TGGGGGTCAGCAGCCGGTTA |
| Hprt (mouse) | TATGCCGAGGATTTGGAAAA | TCCCATCTCCTTCATGACATC |
| Cpt1a (mouse) | CTTCCCATTTGACACCTTTG | ATACGTGAGGCAGAACTTGC |
| Col3a1 (mouse) | ACAGCTGGTGAACCTGG | ACCAGGAGATCCATCTCGAC |
| Vimentin (mouse) | GGATCAGCTCACCAACGACA | GGTCAAGACGTGCCAGAGAA |
| Cpt2 (mouse) | CCCAGGCTGCCTATCCCT | TCCTTCCCAATGCCGTTC |
| Ppara (mouse) | AGAGCCCCATCTGTCCTCTC | ACTGGTAGTCTGCAAAACCAAA |
| Acox2 (mouse) | GAGGGTGAGAATACGGT | GGTCTTGGTGTGGCGAG |
| Ppara (rat) | AACATCGAGTGTCGAATATGTGG | CCGAATAGTTCGCCGAAAGAA |
| Tnfa (rat) | ATGGGCTCCCTCTCATCAGT | GCTTGGTGGTTTGCTACGAC |
| Fn (rat) | AGAGGGGAGTGGAAGTGTGA | GTTGTAGGTGAACGGGAGAA |
| Tgfb1 (rat) | CTAATGGTGGACCGCAACAAC | CACTGCTTCCCGAATGTCTGA |
| Ctgf (rat) | AACCGTGTGTCATTGTCAT | CACCTTAGTGTGCGTTCTG |
| Cpt1a (rat) | ATGGAGGTTGTCTACGA | CAGGGGTTTACTTTTTA |
| Cpt2 (rat) | CAGCTGACCAAAGAAGCAGC | GTTCAGAGTGCTGGTGGACA |
| Col1a1 (rat) | CTGACGCATGGCCAAGAAGA | TTGGGTCCCTCGACTCCTAT |
| β-actin (rat) | GTAAAGACCTCTATGCCAACA | GGACTCATCGTACTCCTGCT |
表1 实时荧光定量PCR引物序列
Tab 1 Primer sequences for real-time qPCR
| Gene | Forward (5′→3′) | Reward (5′→3′) |
|---|---|---|
| Fn (mouse) | ATGGTACAGCTGATCCTGCC | GCCCTGGTTTGTACCTGCTA |
| Acta2 (mouse) | GGAACCCTGAGACGCTGCT | CATTCCAACCATTACTCCC |
| Col1a1 (mouse) | CCCAGCCGCAAAGAGTCTAC | AGCATACCTCGGGTTTCCAC |
| Tgfb1 (mouse) | GGGAAGCAGTGCCCGAACCC | TGGGGGTCAGCAGCCGGTTA |
| Hprt (mouse) | TATGCCGAGGATTTGGAAAA | TCCCATCTCCTTCATGACATC |
| Cpt1a (mouse) | CTTCCCATTTGACACCTTTG | ATACGTGAGGCAGAACTTGC |
| Col3a1 (mouse) | ACAGCTGGTGAACCTGG | ACCAGGAGATCCATCTCGAC |
| Vimentin (mouse) | GGATCAGCTCACCAACGACA | GGTCAAGACGTGCCAGAGAA |
| Cpt2 (mouse) | CCCAGGCTGCCTATCCCT | TCCTTCCCAATGCCGTTC |
| Ppara (mouse) | AGAGCCCCATCTGTCCTCTC | ACTGGTAGTCTGCAAAACCAAA |
| Acox2 (mouse) | GAGGGTGAGAATACGGT | GGTCTTGGTGTGGCGAG |
| Ppara (rat) | AACATCGAGTGTCGAATATGTGG | CCGAATAGTTCGCCGAAAGAA |
| Tnfa (rat) | ATGGGCTCCCTCTCATCAGT | GCTTGGTGGTTTGCTACGAC |
| Fn (rat) | AGAGGGGAGTGGAAGTGTGA | GTTGTAGGTGAACGGGAGAA |
| Tgfb1 (rat) | CTAATGGTGGACCGCAACAAC | CACTGCTTCCCGAATGTCTGA |
| Ctgf (rat) | AACCGTGTGTCATTGTCAT | CACCTTAGTGTGCGTTCTG |
| Cpt1a (rat) | ATGGAGGTTGTCTACGA | CAGGGGTTTACTTTTTA |
| Cpt2 (rat) | CAGCTGACCAAAGAAGCAGC | GTTCAGAGTGCTGGTGGACA |
| Col1a1 (rat) | CTGACGCATGGCCAAGAAGA | TTGGGTCCCTCGACTCCTAT |
| β-actin (rat) | GTAAAGACCTCTATGCCAACA | GGACTCATCGTACTCCTGCT |
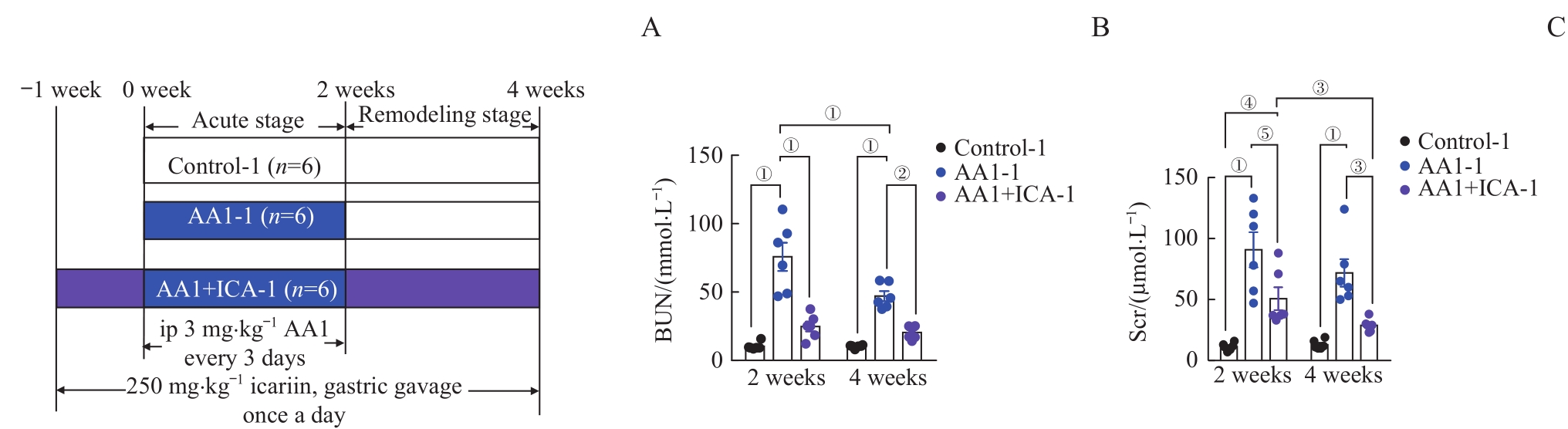
图1 马兜铃酸诱导的AKI-CKD小鼠模型的肾功能损伤以及淫羊藿苷的预防作用Note: A. The grouping diagram of the AKI-CKD mice model induced by AA1 and intervened by ICA. B. Blood urea nitrogen (BUN) after 2 weeks or 4 weeks. C. Serum creatinine (Scr) after 2 weeks or 4 weeks. ip—intraperitoneal injection. ①P=0.000, ②P=0.001, ③P=0.003, ④P=0.007, ⑤P=0.006.
Fig 1 Renal function injury in the AA1-induced AKI-CKD murine model and preventive effect of ICA
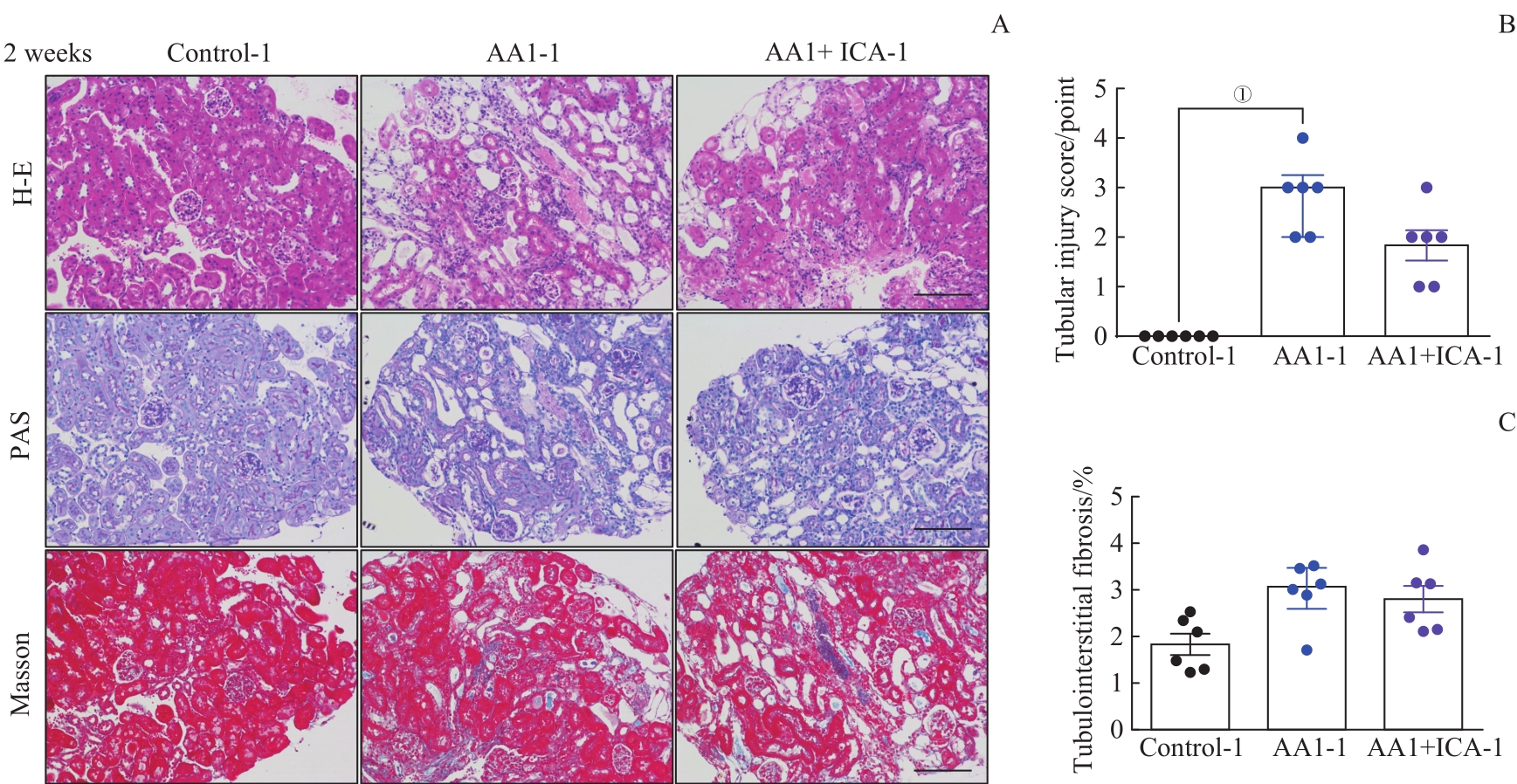
图2 AKI-CKD小鼠模型急性期肾脏的病理损伤以及淫羊藿苷的预防效果Note: A. Representative images of H-E, PAS and Masson staining kidney sections of AKI-CKD model after 2 weeks (×200). B. Renal tubular injury scores of the three groups after 2 weeks. C. Quantification of Masson trichrome staining after 2 weeks. Scale bar=100 μm. ①P=0.000.
Fig 2 Pathological changes of kidney in the AKI-CKD murine model at acute stage and preventive effect of ICA
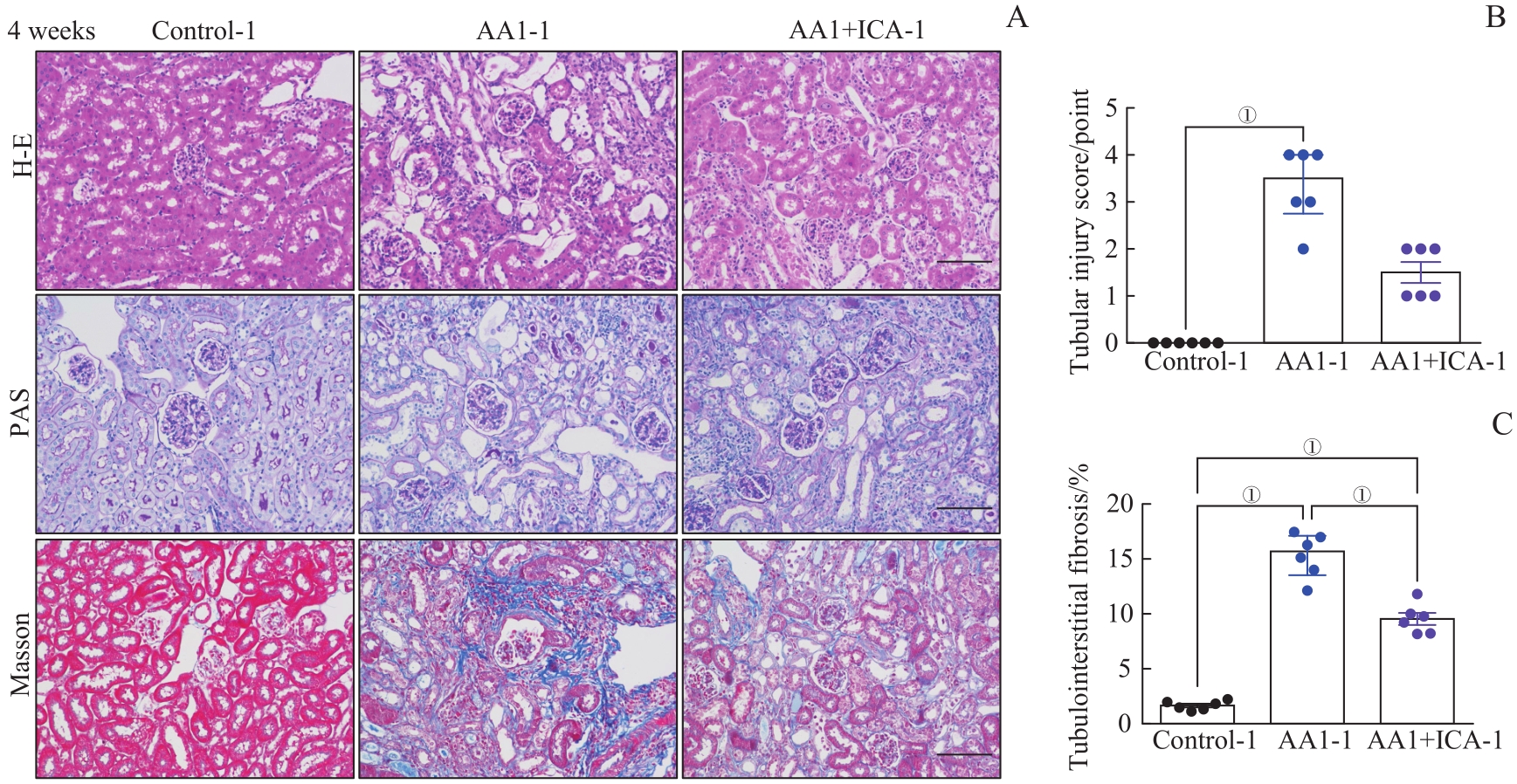
图3 AKI-CKD小鼠模型重塑期肾脏的病理损伤以及淫羊藿苷的预防效果Note: A. Representative images of H-E, PAS and Masson staining kidney sections of AKI-CKD model after 4 weeks (×200). B. Renal tubular injury scores of the three groups after 4 weeks. C. Quantification of Masson trichrome staining after 4 weeks. Scale bar=100 μm. ①P=0.000.
Fig 3 Pathological changes of kidney in the AKI-CKD murine model at remodeling stage and preventive effect of ICA
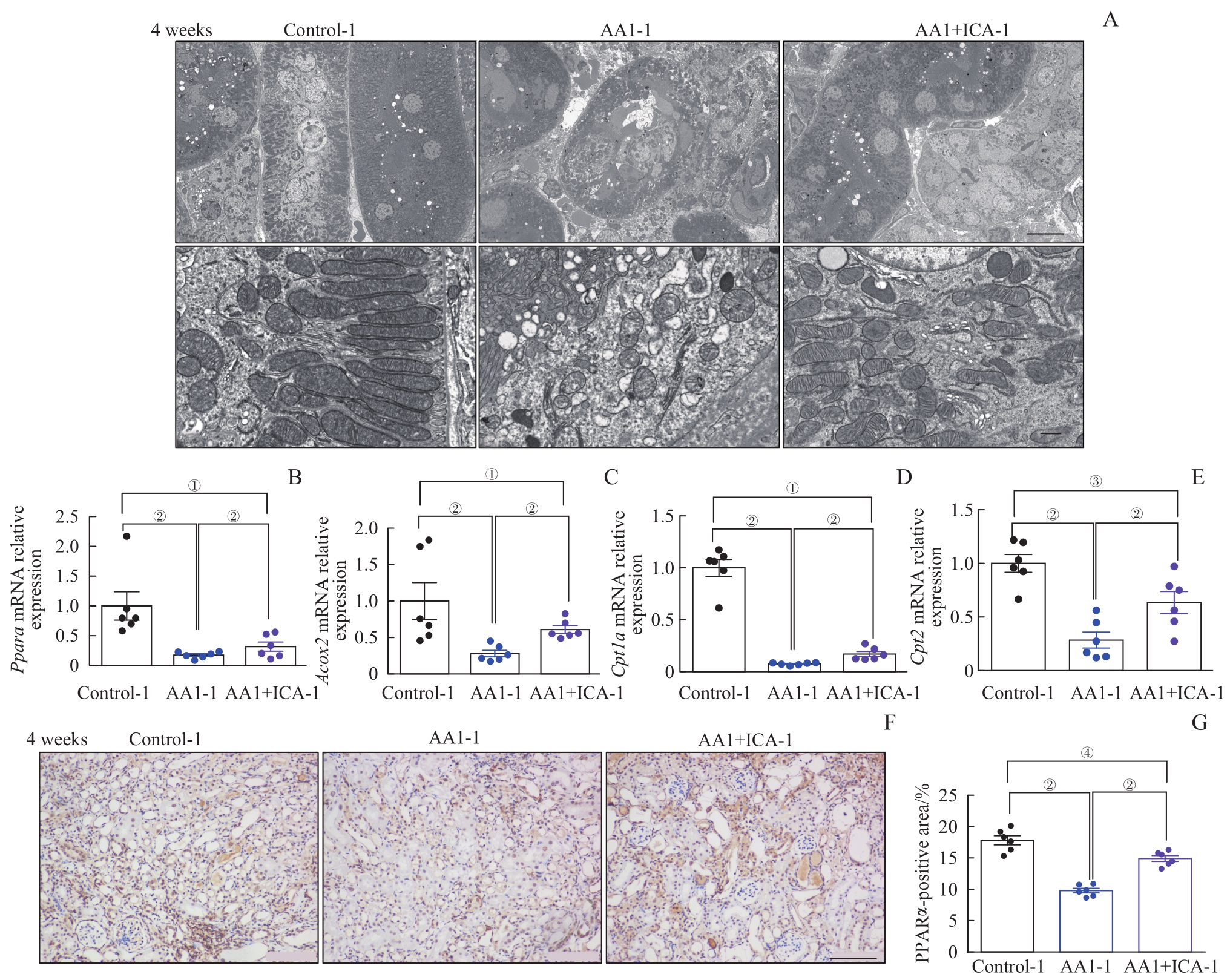
图4 AKI-CKD小鼠模型重塑期肾小管线粒体结构损伤与相关mRNA的表达水平以及淫羊藿苷的预防效果Note: A. Representative electron microscopic images of mitochondria in renal tubular epithelial cells after 4 weeks (above ×2 500, scale bar=10 μm; below ×25 000, scale bar=500 nm). B?E. Relative mRNA expression levels of Ppara (B), Acox2 (C), Cpt1a (D) and Cpt2 (E) in renal tissues after 4 weeks. F. Representative images of kidney sections with immunohistochemical staining PPARα after 4 weeks (×200). G. Quantification of PPARα-positive areas in kidney sections after 4 weeks (scale bar=100 μm). ①P=0.001, ②P=0.000, ③P=0.002, ④P=0.007.
Fig 4 Mitochondrial structural damage and expression levels of related mRNAs in the AKI-CKD murine model at remodeling stage and preventive effect of ICA
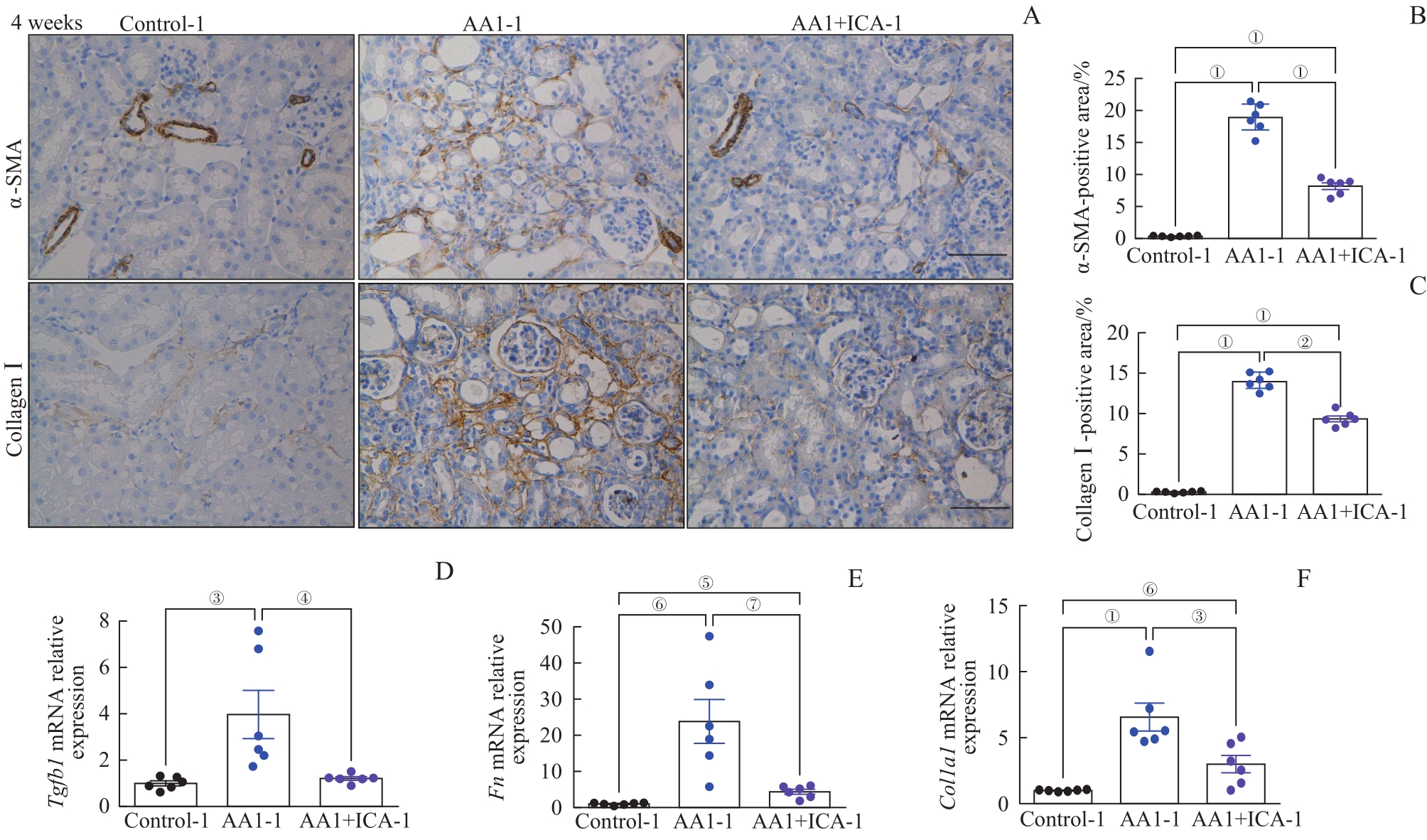
图5 AKI-CKD小鼠模型重塑期的肾纤维化以及淫羊藿苷的预防作用Note: A. Representative images of kidney sections with immunohistochemical staining of α-SMA and collagen Ⅰ after 4 weeks (×200). B. Quantification of α-SMA-positive areas in kidney sections after 4 weeks. C. Quantification of collagen Ⅰ-positive areas in kidney sections after 4 weeks. D?F. Relative mRNA expression levels of Tgfb1 (D), Fn (E) and Col1a1 (F) in renal tissues after 4 weeks. Scale bar=50 μm. ①P=0.000, ②P=0.044, ③P=0.008, ④P=0.014, ⑤P=0.007, ⑥P=0.001, ⑦P=0.003.
Fig 5 Renal fibrosis in the AKI-CKD murine model at remodeling stage and preventive effect of ICA
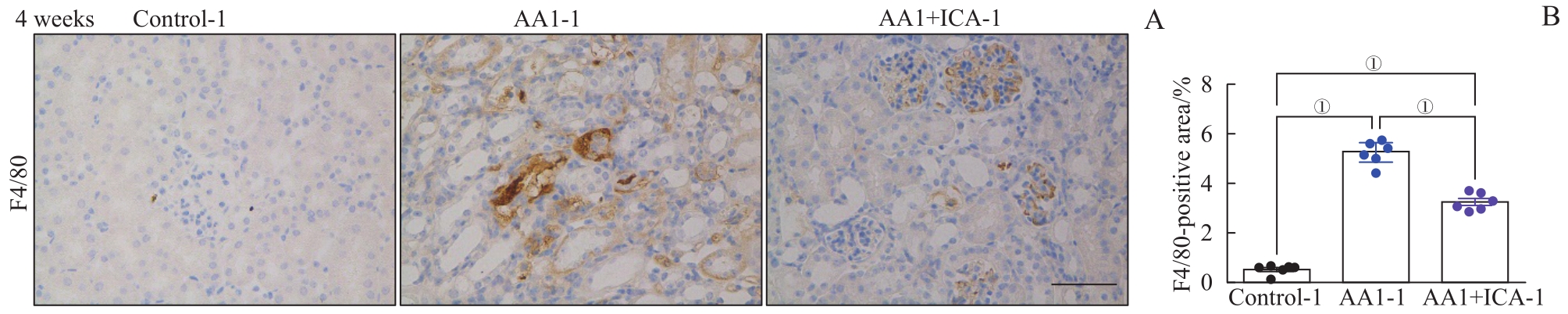
图6 AKI-CKD小鼠模型重塑期肾脏巨噬细胞浸润及淫羊藿苷的预防作用Note: A. Representative images of kidney sections with immunohistochemical staining of F4/80 after 4 weeks. B. Quantification of F4/80 positive areas in kidney sections after 4 weeks. Scale bar=50 μm. ①P=0.000.
Fig 6 Renal macrophage infiltration in the AKI-CKD murine model at remodeling stage and preventive effect of ICA
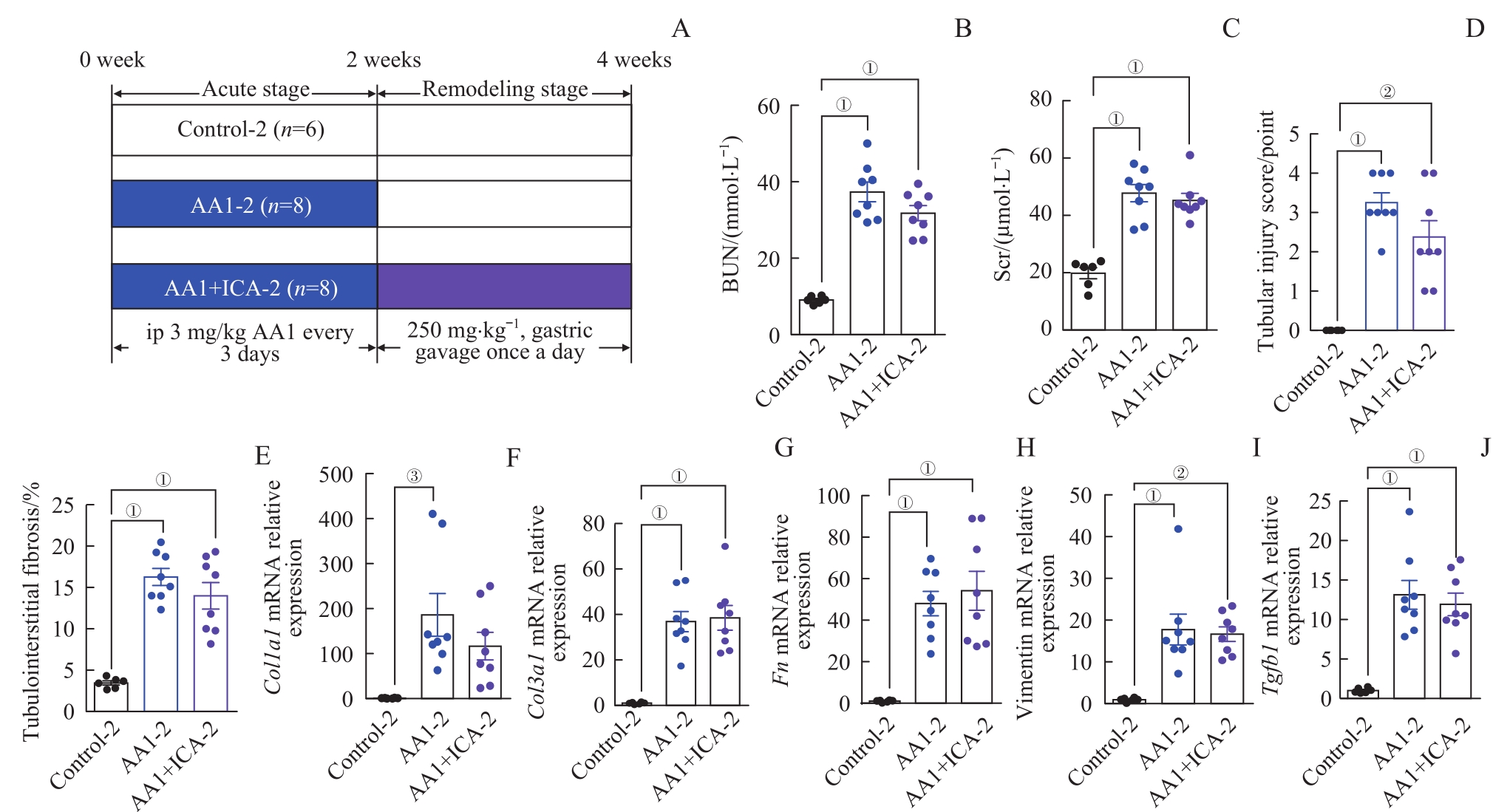
图7 AKI-CKD小鼠模型急性期结束给予淫羊藿苷对小鼠肾脏的影响Note: A. The grouping diagram of the AKI-CKD mice model induced by AA1 and intervened by ICA. B. BUN after 4 weeks. C. Scr after 4 weeks. D. Renal tubular injury scores of the three groups after 4 weeks. E. Quantification of Masson trichrome staining after 4 weeks. F?J. Relative mRNA expression levels of Col1a1 (F), Col3a1 (G), Fn (H), vimentin (I) and Tgfb1 (J)in renal tissues after 4 weeks.
Fig 7 Effect of ICA on kidney of the AKI-CKD murine model at the end of acute phase
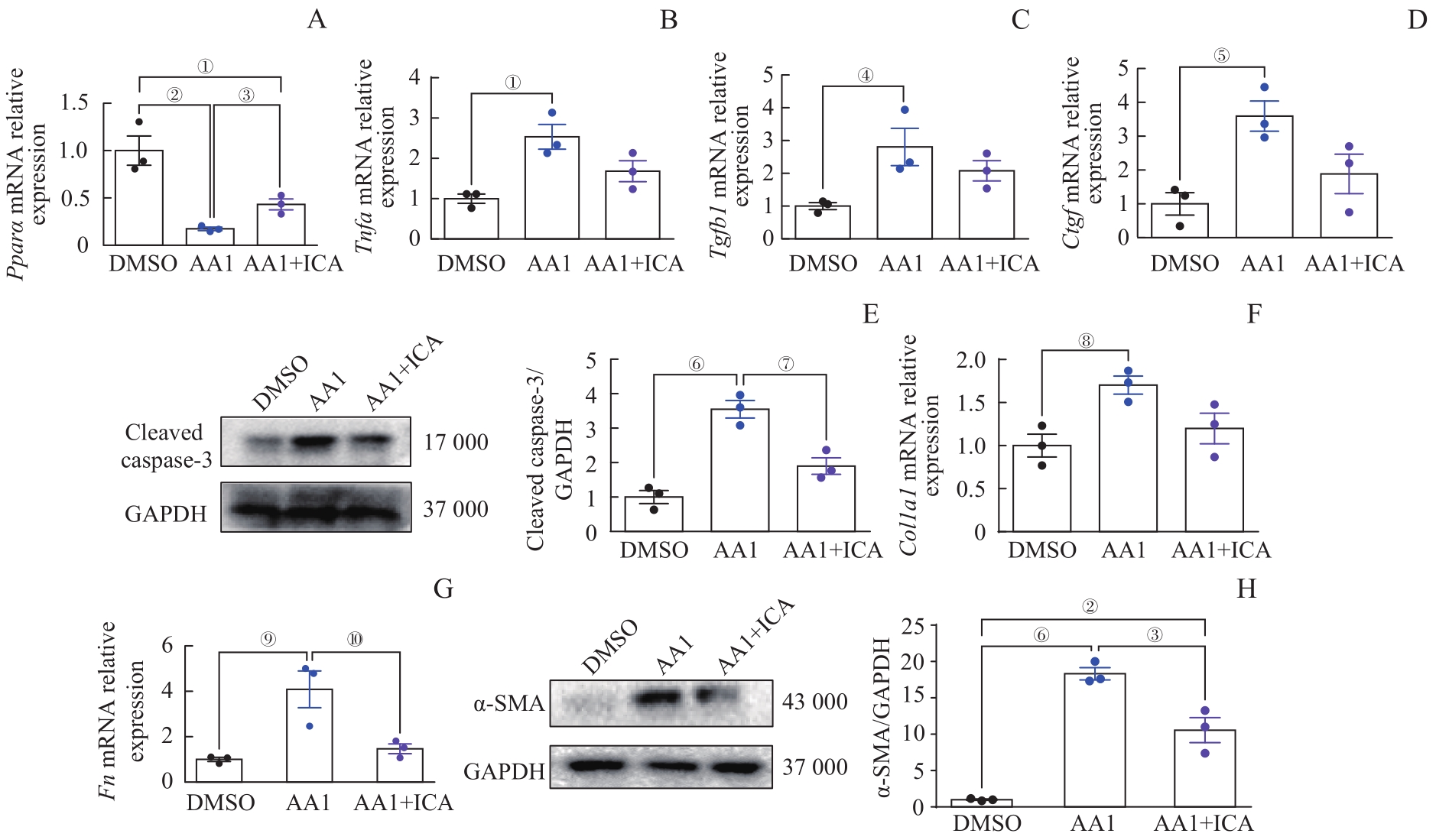
图8 淫羊藿苷通过保护肾小管上皮细胞抑制肾成纤维细胞活化Note: A?D. Relative mRNA expression levels of Ppara (A), Tnfa (B), Tgfb1 (C) and Ctgf (D) in renal tubular epithelial cells. E.Western blotting analysis of cleaved caspase-3 in renal tubular epithelial cells. F/G. Relative mRNA expression levels of Fn (F) and Col1a1 (G) in renal fibroblasts. H. Western blotting analysis of α-SMA in renal fibroblasts. Each experiment was repeated three times. n=3 in each group. ①P=0.009, ②P=0.002, ③P=0.006, ④P=0.034, ⑤P=0.017, ⑥P=0.000, ⑦P=0.005, ⑧P=0.029, ⑨P=0.010, ⑩P=0.020.
Fig 8 ICA inhibiting renal fibroblast activation through protecting renal tubular epithelial cells
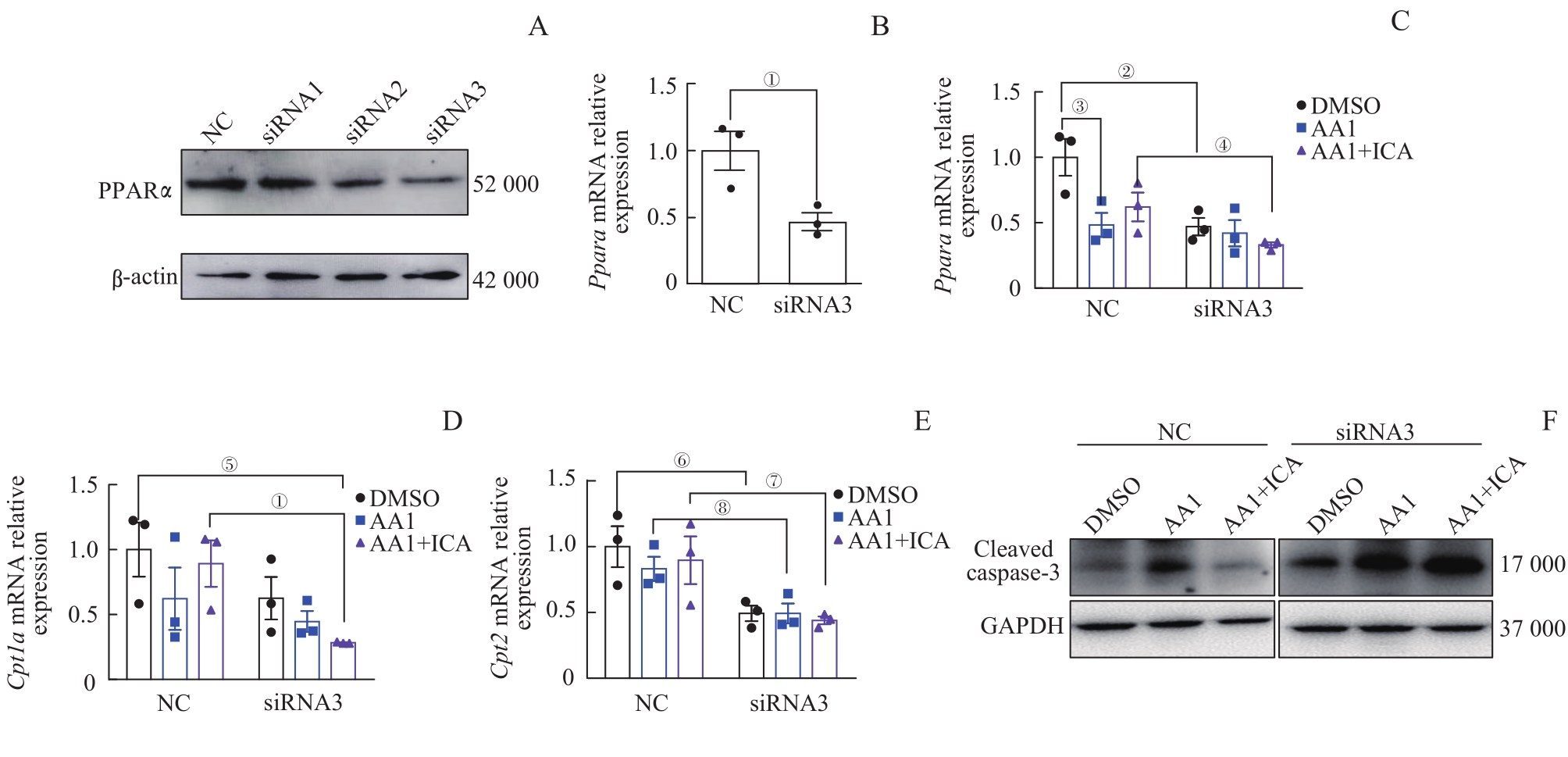
图9 敲低 Ppara 后淫羊藿苷对肾小管上皮细胞脂肪酸氧化代谢相关基因及凋亡蛋白表达水平的影响Note: A. Western blotting analysis of PPARα protein expression in epithelial cells after siRNAs intervention. B. Relative mRNA expression level of Ppara in epithelial cells after siRNA3 intervention. C?E. Relative mRNA expression levels of Ppara (C), Cpt1a (D)and Cpt2 (E) in epithelial cells with NC-siRNA or siRNA3 intervention. F. Western blotting analysis of cleaved caspase-3 protein expression in epithelial cells with NC-siRNA or siRNA3 intervention. Each experiment was repeated three times. n=3. ①P=0.027, ②P=0.020, ③P=0.037, ④P=0.060, ⑤P=0.026, ⑥P=0.038, ⑦P=0.046, ⑧P=0.045.
Fig 9 Effect of ICA on expression levels of fatty acid oxidative metabolism-related mRNAs and the apoptotic protein in renal tubular epithelial cells after knocking down Ppara
| 1 | KURZHAGEN J T, DELLEPIANE S, CANTALUPPI V, et al. AKI: an increasingly recognized risk factor for CKD development and progression[J]. J Nephrol, 2020, 33(6): 1171-1187. |
| 2 | BUCALOIU I D, KIRCHNER H L, NORFOLK E R, et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury[J]. Kidney Int, 2012, 81(5): 477-485. |
| 3 | HE L Y, WEI Q Q, LIU J, et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms[J]. Kidney Int, 2017, 92(5): 1071-1083. |
| 4 | VENKATACHALAM M A, WEINBERG J M, KRIZ W, et al. Failed tubule recovery, AKI-CKD transition, and kidney disease progression[J]. J Am Soc Nephrol, 2015, 26(8): 1765-1776. |
| 5 | 顾向晨, 张红利, 程烨, 等. 淫羊藿苷对血管紧张素Ⅱ诱导肾纤维化的保护作用研究[J]. 上海医学, 2020, 43(10): 611-617. |
| GU X C, ZHANG H L, CHENG Y, et al. Protective effect of icariin on angiotensin Ⅱ induced renal fibrosis[J]. Shanghai Medical Journal, 2020, 43(10): 611-617. | |
| 6 | XIE L, FU L L, MEI C L, et al. Icariin attenuates renal interstitial fibrosis through G protein-coupled estrogen receptor in a UUO murine model[J]. Am J Transl Res, 2022, 14(3): 1567-1577. |
| 7 | MA P, ZHANG S, SU X L, et al. Protective effects of icariin on cisplatin-induced acute renal injury in mice[J]. Am J Transl Res, 2015, 7(10): 2105-2114. |
| 8 | JADOT I, COLOMBARO V, MARTIN B, et al. Restored nitric oxide bioavailability reduces the severity of acute-to-chronic transition in a mouse model of aristolochic acid nephropathy[J]. PLoS One, 2017, 12(8): e0183604. |
| 9 | HUANG L H, SCARPELLINI A, FUNCK M, et al. Development of a chronic kidney disease model in C57BL/6 mice with relevance to human pathology[J]. Nephron Extra, 2013, 3(1): 12-29. |
| 10 | FU Y, TANG C Y, CAI J, et al. Rodent models of AKI-CKD transition[J]. Am J Physiol Renal Physiol, 2018, 315(4): F1098-F1106. |
| 11 | SHIMIZU A, MASUDA Y, MORI T, et al. Vascular endothelial growth factor165 resolves glomerular inflammation and accelerates glomerular capillary repair in rat anti-glomerular basement membrane glomerulonephritis[J]. J Am Soc Nephrol, 2004, 15(10): 2655-2665. |
| 12 | TAKAORI K, NAKAMURA J, YAMAMOTO S, et al. Severity and frequency of proximal tubule injury determines renal prognosis[J]. J Am Soc Nephrol, 2016, 27(8): 2393-2406. |
| 13 | GU X C, YANG H L, SHENG X, et al. Kidney disease genetic risk variants alter lysosomal β-mannosidase (MANBA) expression and disease severity[J]. Sci Transl Med, 2021, 13(576): eaaz1458. |
| 14 | CHAWLA L S, EGGERS P W, STAR R A, et al. Acute kidney injury and chronic kidney disease as interconnected syndromes[J]. N Engl J Med, 2014, 371(1): 58-66. |
| 15 | HOSTE E A J, KELLUM J A, SELBY N M, et al. Global epidemiology and outcomes of acute kidney injury[J]. Nat Rev Nephrol, 2018, 14(10): 607-625. |
| 16 | HORNE K L, PACKINGTON R, MONAGHAN J, et al. Three-year outcomes after acute kidney injury: results of a prospective parallel group cohort study[J]. BMJ Open, 2017, 7(3): e015316. |
| 17 | YU S M W, BONVENTRE J V. Acute kidney injury and maladaptive tubular repair leading to renal fibrosis[J]. Curr Opin Nephrol Hypertens, 2020, 29(3): 310-318. |
| 18 | KANG H M, AHN S H, CHOI P, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development[J]. Nat Med, 2015, 21(1): 37-46. |
| 19 | WANG M, GAO H Y, LI W H, et al. Icariin and its metabolites regulate lipid metabolism: from effects to molecular mechanisms[J]. Biomed Pharmacother, 2020, 131: 110675. |
| 20 | WU B, FENG J Y, YU L M, et al. Icariin protects cardiomyocytes against ischaemia/reperfusion injury by attenuating sirtuin 1-dependent mitochondrial oxidative damage[J]. Br J Pharmacol, 2018, 175(21): 4137-4153. |
| 21 | PU L Y, MENG Q Y, LI S, et al. Icariin arrests cell cycle progression and induces cell apoptosis through the mitochondrial pathway in human fibroblast-like synoviocytes[J]. Eur J Pharmacol, 2021, 912: 174585. |
| 22 | SONG Y H, CAI H, ZHAO Z M, et al. Icariin attenuated oxidative stress induced-cardiac apoptosis by mitochondria protection and ERK activation[J]. Biomed Pharmacother, 2016, 83: 1089-1094. |
| 23 | LI Y C, DING X S, LI H M, et al. Icariin attenuates high glucose-induced type Ⅳ collagen and fibronectin accumulation in glomerular mesangial cells by inhibiting transforming growth factor-β production and signalling through G protein-coupled oestrogen receptor 1[J]. Clin Exp Pharmacol Physiol, 2013, 40(9): 635-643. |
| 24 | PANT A, RONDINI E A, KOCAREK T A. Farnesol induces fatty acid oxidation and decreases triglyceride accumulation in steatotic HepaRG cells[J]. Toxicol Appl Pharmacol, 2019, 365: 61-70. |
| 25 | CHUNG K W, LEE E K, LEE M K, et al. Impairment of PPARα and the fatty acid oxidation pathway aggravates renal fibrosis during aging[J]. J Am Soc Nephrol, 2018, 29(4): 1223-1237. |
| 26 | LU Y F, XU Y Y, JIN F, et al. Icariin is a PPARα activator inducing lipid metabolic gene expression in mice[J]. Molecules, 2014, 19(11): 18179-18191. |
| [1] | 李玉, 张羽. 慢性肾脏病患者不良妊娠结局的危险因素分析[J]. 上海交通大学学报(医学版), 2024, 44(5): 560-566. |
| [2] | 安俊伊, 陈必颖, 陈循睿, 尹姗姗, 边洲亮, 刘峰. SFXN3在头颈部鳞状细胞癌中的表达及其对细胞增殖的影响[J]. 上海交通大学学报(医学版), 2024, 44(4): 427-434. |
| [3] | 孔汝心, 周亚群, 魏婷宜, 雷鸣. 癌-睾丸抗原CT63在慢性髓系白血病中的作用及其机制[J]. 上海交通大学学报(医学版), 2024, 44(11): 1347-1358. |
| [4] | 克德尔亚·艾山江, 傅怡, 赖冬林, 邬海龙, 龚伟. 肝细胞癌相关的核编码线粒体基因及临床信息的综合预后模型[J]. 上海交通大学学报(医学版), 2024, 44(1): 1-12. |
| [5] | 冯奕源, 徐忠匀, 尹雅芙, 王辉, 程维维. 二甲双胍改善由C9ORF72肌萎缩侧索硬化/额颞叶痴呆相关多聚甘氨酸-精氨酸诱导的线粒体损伤[J]. 上海交通大学学报(医学版), 2023, 43(7): 839-847. |
| [6] | 金芳全, 樊成虎, 唐晓栋, 陈彦同, 齐兵献. 线粒体功能障碍与骨质疏松症相关性研究进展[J]. 上海交通大学学报(医学版), 2023, 43(6): 761-767. |
| [7] | 洪晗馨, 王龙昊, 刘辉辉, 彭浒, 吴皓, 杨涛. 线粒体内膜转位酶8A基因敲除小鼠的构建及其内耳功能研究[J]. 上海交通大学学报(医学版), 2023, 43(3): 261-268. |
| [8] | 刘铁鑫, 林俊卿, 郑宪友. 靶向亚细胞结构治疗脊髓损伤的研究进展[J]. 上海交通大学学报(医学版), 2023, 43(2): 230-236. |
| [9] | 沙旭栋, 王晨飞, 鲁佳, 虞志华. 瞬时受体电位香草素1型对高脂饮食诱导的小胶质细胞代谢的调控[J]. 上海交通大学学报(医学版), 2023, 43(12): 1493-1506. |
| [10] | 冯宝怡, 董庭婷, 郑晓飞, 陶永, 吴皓. 不同月龄C57BL/6J小鼠耳蜗线粒体和NAD+水平比较[J]. 上海交通大学学报(医学版), 2022, 42(8): 980-986. |
| [11] | 赖秀秀, 朱青燕, 谈佳奇, 杨令, 朱琰, 周公民. 小剂量非布司他改善无症状高尿酸血症高龄患者肾功能的临床研究[J]. 上海交通大学学报(医学版), 2022, 42(7): 885-892. |
| [12] | 王亚琨, 许佳瑞, 吴茜茜, 张晓华, 朱迎春, 白寿军. 医养结合综合干预对上海郊区老年慢性肾脏病患者生活质量和精神状态的影响[J]. 上海交通大学学报(医学版), 2022, 42(7): 904-910. |
| [13] | 林祎嘉, 程丽珍, 苗雅. 线粒体自噬异常在阿尔茨海默病中的作用及机制研究综述[J]. 上海交通大学学报(医学版), 2022, 42(3): 387-392. |
| [14] | 魏珊, 纪鸥洋, 陈志豪, 黄泽慧, 李璞, 方均燕, 刘英莉. 慢性肾脏病患者安全用药知信行量表的编制及信度、效度分析[J]. 上海交通大学学报(医学版), 2022, 42(12): 1729-1738. |
| [15] | 熊雷, 易茜, 许明芳, 陈健. MRPL12在肺腺癌中的表达和预后分析[J]. 上海交通大学学报(医学版), 2021, 41(8): 1033-1040. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||