
上海交通大学学报(医学版) ›› 2025, Vol. 45 ›› Issue (5): 540-548.doi: 10.3969/j.issn.1674-8115.2025.05.002
收稿日期:2024-12-26
接受日期:2025-04-23
出版日期:2025-05-28
发布日期:2025-05-22
通讯作者:
刘 艳,高级工程师,博士;电子信箱:ly30689@rjh.com.cn作者简介:高欣洁(2001—),女,硕士生;电子信箱:gxj0701@sjtu.edu.cn。
基金资助:
GAO Xinjie( ), LIU Yan(
), LIU Yan( ), WANG Dawei(
), WANG Dawei( )
)
Received:2024-12-26
Accepted:2025-04-23
Online:2025-05-28
Published:2025-05-22
Contact:
LIU Yan, E-mail: ly30689@rjh.com.cnSupported by:摘要:
地中海贫血的传统治疗方式为定期输血和异基因造血干细胞移植(allogenic hematopoietic stem cell transplantation,allo-HSCT)。近年兴起的基因修饰自体造血干细胞移植是治愈输血依赖型地中海贫血(transfusion dependent thalassemia,TDT)的新策略,有望替代传统治疗手段,使TDT患者终身受益。地中海贫血基因治疗现有2种技术路线:将外源性β-珠蛋白基因转导造血干细胞(hematopoietic stem cell,HSC)的基因添加;利用CRISPR-Cas9系统重新激活γ-珠蛋白表达的基因编辑。该文结合已获批上市的药物和临床试验的研究进展,具体分析了2种路线各自的优势与局限性,讨论了目前地中海贫血基因治疗药物的有效性和安全性,以及干细胞体外扩增与干性维持、载体递送介导体内基因修饰等关联技术的未来攻克方向。在实现临床转化层面,该文就工艺开发困境、临床试验开展、监管审批流程、商业化及支付体系等临床试验面临的现阶段挑战,进行深入思考并阐述可行性解决方案。
中图分类号:
高欣洁, 刘艳, 王大威. 地中海贫血基因治疗研究进展及思考[J]. 上海交通大学学报(医学版), 2025, 45(5): 540-548.
GAO Xinjie, LIU Yan, WANG Dawei. Research progress and considerations for thalassemia gene therapy[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2025, 45(5): 540-548.
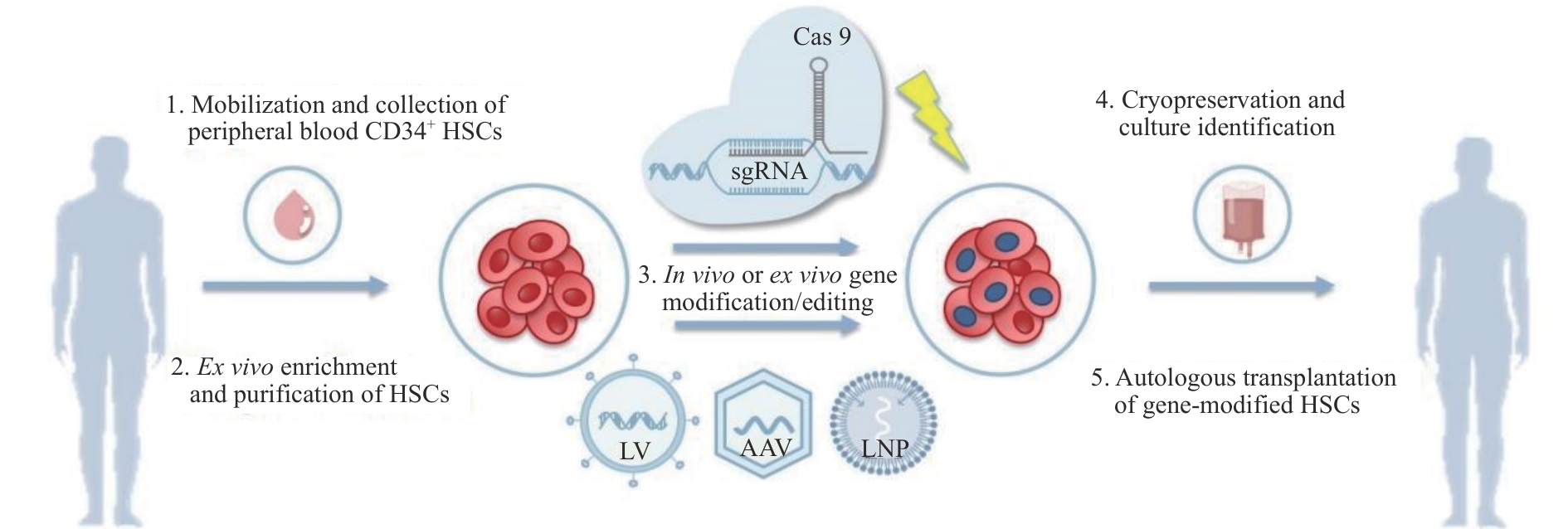
图1 地中海贫血基因治疗策略示意图Note: HSCs—hematopoietic stem cells; Cas9—CRISPR-associated protein 9; sgRNA—single guide RNA; LV—lentiviral; AAV—adeno-associated virus; LNP—lipid nanoparticle.
Fig 1 Schematic diagram of thalassemia gene therapy strategies
| The average neutrophil and platelet engraftment times were both 14 d. TI occurred in all patients, with the longest duration lasting 18 months[ | ||||
| The median duration of TI among | ||||
表1 β-地中海贫血基因治疗的临床试验
Tab 1 Clinical trials of β-thalassemia gene therapy
| The average neutrophil and platelet engraftment times were both 14 d. TI occurred in all patients, with the longest duration lasting 18 months[ | ||||
| The median duration of TI among | ||||
| 1 | KATTAMIS A, KWIATKOWSKI J L, AYDINOK Y. Thalassaemia[J]. Lancet, 2022, 399(10343): 2310-2324. |
| 2 | 中华医学会血液学分会红细胞疾病贫血学组. 中国输血依赖型β地中海贫血诊断与治疗指南(2022年版)[J]. 中华血液学杂志, 2022, 43(11): 889-896. |
| Red Blood Cell Diseases (Anemia) Group, Chinese Society of Hematology, Chinese Medical Association. Chinese guideline for diagnosis and treatment of transfusion dependent β-thalassemia (2022)[J]. Chinese Journal of Hematology, 2022, 43(11): 889-896. | |
| 3 | LAL A, LOCATELLI F, KWIATKOWSKI J L, et al. Northstar-3: interim results from a phase 3 study evaluating lentiglobin gene therapy in patients with transfusion-dependent β-thalassemia and either a β0 or IVS-I-110 mutation at both alleles of the HBB gene[J]. Blood, 2019, 134: 815. |
| 4 | LOCATELLI F, LANG P, WALL D, et al. Exagamglogene autotemcel for transfusion-dependent β- thalassemia[J]. N Engl J Med, 2024, 390(18): 1663-1676. |
| 5 | PIEL F B, WEATHERALL D J. The α-thalassemias[J]. N Engl J Med, 2014, 371(20): 1908-1916. |
| 6 | KATTAMIS A, FORNI G L, AYDINOK Y, et al. Changing patterns in the epidemiology of β-thalassemia[J]. Eur J Haematol, 2020, 105(6): 692-703. |
| 7 | WANG W D, HU F, ZHOU D H, et al. Thalassaemia in China[J]. Blood Rev, 2023, 60: 101074. |
| 8 | 中华医学会医学遗传学分会遗传病临床实践指南撰写组. β-地中海贫血的临床实践指南[J]. 中华医学遗传学杂志, 2020, 37(3): 243-251. |
| Writing Group for Practice Guidelines for Diagnosis and Treatment of Genetic Diseases, Medical Genetics Branch of Chinese Medical Associatio. Clinical practice guidelines for β-thalassemia [J]. Chinese Journal of Medical Genetics, 2020, 37(3): 243-251. | |
| 9 | ZHEN X M, MING J, ZHANG R Q, et al. Economic burden of adult patients with β-thalassaemia major in mainland China[J]. Orphanet J Rare Dis, 2023, 18(1): 252. |
| 10 | 陈辉, 贾玉艳, 黄粤, 等. 地中海贫血基因治疗进展和现状[J]. 广西医科大学学报, 2024, 41(1): 1-10. |
| CHEN H, JIA Y Y, HUANG Y, et al. Progress and current status of gene therapy for thalassemia[J]. Journal of Guangxi Medical University, 2024, 41(1): 1-10. | |
| 11 | MARKTEL S, SCARAMUZZA S, CICALESE M P, et al. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent β-thalassemia[J]. Nat Med, 2019, 25(2): 234-241. |
| 12 | THOMPSON A A, WALTERS M C, KWIATKOWSKI J, et al. Gene therapy in patients with transfusion-dependent β-thalassemia[J]. N Engl J Med, 2018, 378(16): 1479-1493. |
| 13 | LOCATELLI F, THOMPSON A A, KWIATKOWSKI J L, et al. Betibeglogene autotemcel gene therapy for non-β0/β0 genotype β-thalassemia[J]. N Engl J Med, 2022, 386(5): 415-427. |
| 14 | HAN N. Interim results of gene therapy using optimized LentiHBBT87Q vector in five Chinese patients with transfusion dependent β-thalassemia[C]//EHA2024 Hybrid Congress. Madrid, Spain: EHA Library, 2024: 1517. |
| 15 | LI S Q, LING S K, WANG D W, et al. Modified lentiviral globin gene therapy for pediatric β0/β0 transfusion-dependent β-thalassemia: a single-center, single-arm pilot trial[J]. Cell Stem Cell, 2024, 31(7): 961-973.e8. |
| 16 | HUANG J Q, ZHANG Y M, LIANG L, et al. Gene therapy of transfusion-dependent β-thalassemia patients with quick engraftment of reinfused hematopoietic stem cells: an investigator-initiated trial of KL003[J]. Blood, 2023, 142: 4998. |
| 17 | WALTERS M C, SMITH A R, SCHILLER G J, et al. Updated results of a phase 1/2 clinical study of zinc finger nuclease-mediated editing of BCL11A in autologous hematopoietic stem cells for transfusion-dependent β thalassemia[J]. Blood, 2021, 138(Supplement 1): 3974. |
| 18 | FRANGOUL H, HANNA R, WALTERS M C, et al. Reni-cel, the first AsCas12a gene-edited cell therapy, shows promising preliminary results in key clinical outcomes in transfusion-dependent β- thalassemia patients treated in the EdiThaltrial[C]//EHA2024 Hybrid Congress. Madrid, Spain: EHA Library, 2024: 1476. |
| 19 | SHI J, FANG R G, GAO Z, et al. Preliminary safety and efficacy results of EDI001: an investigator initiated trial on CRISPR/Cas9-modified autologous CD34+ hematopoietic stem and progenitor cells for patients with transfusion dependent β-thalassemia[J]. Blood, 2022, 140(Supplement 1): 10652-10653. |
| 20 | ZHENG B, LIU R R, ZHANG X H, et al. Efficacy and safety of brl-101, CRISPR-Cas9-mediated gene editing of the BCL11A enhancer in transfusion-dependent β-thalassemia[J]. Blood, 2023, 142: 4995. |
| 21 | LIU R R, WANG L, XU H, et al. Safety and efficacy of RM-001 (autologous HBG1/2 promoter-modified CD34+ hematopoietic stem and progenitor cells) in patients with transfusion-dependent β-thalassemia[J]. Blood, 2023, 142: 4994. |
| 22 | KUSCU C, ARSLAN S, SINGH R, et al. Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease[J]. Nat Biotechnol, 2014, 32(7): 677-683. |
| 23 | MORGAN R A, UNTI M J, ALESHE B, et al. Improved titer and gene transfer by lentiviral vectors using novel, small β-globin locus control region elements[J]. Mol Ther, 2020, 28(1): 328-340. |
| 24 | GIOMMETTI A, PAPANIKOLAOU E. Advancements in hematopoietic stem cell gene therapy: a journey of progress for viral transduction[J]. Cells, 2024, 13(12): 1039. |
| 25 | SEGURA E E R, AYOUB P G, HART K L, et al. Gene therapy for β-hemoglobinopathies: from discovery to clinical trials[J]. Viruses, 2023, 15(3): 713. |
| 26 | GAMBARI R. Alternative options for DNA-based experimental therapy of β-thalassemia[J]. Expert Opin Biol Ther, 2012, 12(4): 443-462. |
| 27 | BREDA L, PAPP T E, TRIEBWASSER M P, et al. In vivo hematopoietic stem cell modification by mRNA delivery[J]. Science, 2023, 381(6656): 436-443. |
| 28 | ESRICK E B, LEHMANN L E, BIFFI A, et al. Post-transcriptional genetic silencing of BCL11A to treat sickle cell disease[J]. N Engl J Med, 2021, 384(3): 205-215. |
| 29 | LIU B Y, BRENDEL C, VINJAMUR D S, et al. Development of a double shmiR lentivirus effectively targeting both BCL11A and ZNF410 for enhanced induction of fetal hemoglobin to treat β-hemoglobinopathies[J]. Mol Ther, 2022, 30(8): 2693-2708. |
| 30 | CAVAZZANA-CALVO M, PAYEN E, NEGRE O, et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia[J]. Nature, 2010, 467(7313): 318-322. |
| 31 | DUNCAN C N, BLEDSOE J R, GRZYWACZ B, et al. Hematologic cancer after gene therapy for cerebral adrenoleukodystrophy[J]. N Engl J Med, 2024, 391(14): 1287-1301. |
| 32 | POESCHLA E M. Integrase, LEDGF/p75 and HIV replication[J]. Cell Mol Life Sci, 2008, 65(9): 1403-1424. |
| 33 | DORMIANI K, MIR MOHAMMAD SADEGHI H, SADEGHI-ALIABADI H, et al. Long-term and efficient expression of human β-globin gene in a hematopoietic cell line using a new site-specific integrating non-viral system[J]. Gene Ther, 2015, 22(8): 663-674. |
| 34 | PFEIFER A, BRANDON E P, KOOTSTRA N, et al. Delivery of the Cre recombinase by a self-deleting lentiviral vector: efficient gene targeting in vivo[J]. Proc Natl Acad Sci USA, 2001, 98(20): 11450-11455. |
| 35 | BAHAL R, ALI MCNEER N, QUIJANO E, et al. In vivo correction of anaemia in β-thalassemic mice by γPNA-mediated gene editing with nanoparticle delivery[J]. Nat Commun, 2016, 7: 13304. |
| 36 | LAZARIS V M, SIMANTIRAKIS E, STAVROU E F, et al. Non-viral episomal vector mediates efficient gene transfer of the β-globin gene into K562 and human haematopoietic progenitor cells[J]. Genes (Basel), 2023, 14(9): 1774. |
| 37 | STAVROU E F, SIMANTIRAKIS E, VERRAS M, et al. Episomal vectors based on S/MAR and the β-globin replicator, encoding a synthetic transcriptional activator, mediate efficient γ-globin activation in haematopoietic cells[J]. Sci Rep, 2019, 9(1): 19765. |
| 38 | REES H A, MINELLA A C, BURNETT C A, et al. CRISPR-derived genome editing therapies: progress from bench to bedside[J]. Mol Ther, 2021, 29(11): 3125-3139. |
| 39 | ENACHE O M, RENDO V, ABDUSAMAD M, et al. Cas9 activates the p53 pathway and selects for p53-inactivating mutations[J]. Nat Genet, 2020, 52(7): 662-668. |
| 40 | ANTONIOU P, MICCIO A, BRUSSON M. Base and prime editing technologies for blood disorders[J]. Front Genome Ed, 2021, 3: 618406. |
| 41 | HRYHOROWICZ M, LIPIŃSKI D, ZEYLAND J. Evolution of CRISPR/cas systems for precise genome editing[J]. Int J Mol Sci, 2023, 24(18): 14233. |
| 42 | WU Z W, ZHANG Y F, YU H P, et al. Programmed genome editing by a miniature CRISPR-Cas12f nuclease[J]. Nat Chem Biol, 2021, 17(11): 1132-1138. |
| 43 | FERRARI S, JACOB A, CESANA D, et al. Choice of template delivery mitigates the genotoxic risk and adverse impact of editing in human hematopoietic stem cells[J]. Cell Stem Cell, 2022, 29(10): 1428-1444.e9. |
| 44 | LAMSFUS-CALLE A, DANIEL-MORENO A, UREÑA-BAILÉN G, et al. Universal gene correction approaches for β-hemoglobinopathies using CRISPR-Cas9 and adeno-associated virus serotype 6 donor templates[J]. CRISPR J, 2021, 4(2): 207-222. |
| 45 | NUALKAEW T, JEARAWIRIYAPAISARN N, HONGENG S, et al. Restoration of correct βIVS2-654-globin mRNA splicing and HbA production by engineered U7 snRNA in β-thalassaemia/HbE erythroid cells[J]. Sci Rep, 2019, 9(1): 7672. |
| 46 | KYLE CROMER M, CAMARENA J, MARTIN R M, et al. Gene replacement of α-globin with β-globin restores hemoglobin balance in β-thalassemia-derived hematopoietic stem and progenitor cells[J]. Nat Med, 2021, 27(4): 677-687. |
| 47 | PAVANI G, FABIANO A, LAURENT M, et al. Correction of β-thalassemia by CRISPR/Cas9 editing of the α-globin locus in human hematopoietic stem cells[J]. Blood Adv, 2021, 5(5): 1137-1153. |
| 48 | LU D, XU Z L, PENG Z Y, et al. Induction of fetal hemoglobin by introducing natural hereditary persistence of fetal hemoglobin mutations in the γ-globin gene promoters for genome editing therapies for β-thalassemia[J]. Front Genet, 2022, 13: 881937. |
| 49 | SHYR D C, LOWSKY R, MILLER W, et al. One year follow-up on the first patient treated with nula-cel: an autologous CRISPR/Cas9 gene corrected CD34+ cell product to treat sickle cell disease[J]. Blood, 2023, 142: 5000. |
| 50 | ZHANG H K, SUN R L, FEI J, et al. Correction of β-thalassemia IVS-II-654 mutation in a mouse model using prime editing[J]. Int J Mol Sci, 2022, 23(11): 5948. |
| 51 | UCHIDA N, TISDALE J F, DONAHUE R E, et al. A single dose of CD117 antibody drug conjugate enables hematopoietic stem cell based gene therapy in nonhuman Primates[J]. Biol Blood Marrow Transplant, 2020, 26(3): S6. |
| 52 | NGUYEN G N, EVERETT J K, KAFLE S, et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells[J]. Nat Biotechnol, 2021, 39(1): 47-55. |
| 53 | WANG H J, GEORGAKOPOULOU A, PSATHA N, et al. In vivo hematopoietic stem cell gene therapy ameliorates murine thalassemia intermedia[J]. J Clin Invest, 2019, 129(2): 598-615. |
| 54 | LI C, WANG H J, GEORGAKOPOULOU A, et al. In vivo HSC gene therapy using a bi-modular HDAd5/35++ vector cures sickle cell disease in a mouse model[J]. Mol Ther, 2021, 29(2): 822-837. |
| 55 | PASCHOUDI K, YANNAKI E, PSATHA N. Precision editing as a therapeutic approach for β-hemoglobinopathies[J]. Int J Mol Sci, 2023, 24(11): 9527. |
| 56 | LI C, GEORGAKOPOULOU A, MISHRA A, et al. In vivo HSPC gene therapy with base editors allows for efficient reactivation of fetal γ-globin in β-YAC mice[J]. Blood Adv, 2021, 5(4): 1122-1135. |
| 57 | MEAKER G A, WILKINSON A C. Ex vivo hematopoietic stem cell expansion technologies: recent progress, applications, and open questions[J]. Exp Hematol, 2024, 130: 104136. |
| 58 | LI Y H, HE M, ZHANG W S, et al. Expansion of human megakaryocyte-biased hematopoietic stem cells by biomimetic Microniche[J]. Nat Commun, 2023, 14(1): 2207. |
| 59 | WANG H J, GEORGAKOPOULOU A, NIZAMIS E, et al. Auto-expansion of in vivo HDAd-transduced hematopoietic stem cells by constitutive expression of tHMGA2[J]. Mol Ther Methods Clin Dev, 2024, 32(3): 101319. |
| 60 | CORBACIOGLU S, FRANGOUL H, LOCATELLI F, et al. Defining curative endpoints for transfusion-dependent β-thalassemia in the era of gene therapy and gene editing[J]. Am J Hematol, 2024, 99(3): 422-429. |
| [1] | 何苏荟, 赵银龙, 张家毓. 端粒酶基因治疗对压力超负荷心力衰竭小鼠的影响[J]. 上海交通大学学报(医学版), 2025, 45(8): 949-956. |
| [2] | 江凌勇. 牙颌面骨畸形机制研究的现状与发展[J]. 上海交通大学学报(医学版), 2024, 44(6): 663-675. |
| [3] | 陈瑾, 傅瑶. 人角膜内皮细胞自体再生的研究进展[J]. 上海交通大学学报(医学版), 2023, 43(6): 775-780. |
| [4] | 赵洁, 姜言, 郝思国. 弥漫大B细胞淋巴瘤患者临床特征及预后分析[J]. 上海交通大学学报(医学版), 2023, 43(10): 1282-1288. |
| [5] | 周月, 程晨, 郑恩霖, 孟卓, 王鉴, 王青洁, 何勇宁, 孙锟. 利用Crispr/Cas9基因编辑系统在人胚胎干细胞中探索ELABELA的潜在新受体[J]. 上海交通大学学报(医学版), 2022, 42(9): 1258-1264. |
| [6] | 成桢哲, 金晨曦, 冯宝怡, 郑晓飞, 刘祎晴, 吴皓, 陶永. 基于腺相关病毒血清型8型介导的Gjb2基因c.109G>A纯合突变耳聋小鼠的基因治疗[J]. 上海交通大学学报(医学版), 2022, 42(6): 735-741. |
| [7] | 韩夏夏, 顾霜霜, 戴黛, 沈南. 应用CRISPR/Cas9介导的基因编辑系统研究B细胞中转录因子T-bet的调控作用[J]. 上海交通大学学报(医学版), 2022, 42(4): 433-442. |
| [8] | 赵艳娜, 邱荣, 沈南, 唐元家. 构建诱导型CRISPR/Cas9系统用于小鼠免疫细胞基因功能研究[J]. 上海交通大学学报(医学版), 2021, 41(3): 297-301. |
| [9] | 李冬凉,赖昱臣,张薇薇,王 戬,李春红,王甦平,施琼芸,张 行,陶晔璇. 我国转化医学国家重大科技基础设施建设初探[J]. 上海交通大学学报(医学版), 2020, 40(6): 701-706. |
| [10] | 张志愿. 口腔颌面表型组计划——口腔医学可持续发展的新起点[J]. 上海交通大学学报(医学版), 2020, 40(10): 1321-1323. |
| [11] | 李送 1,钟昌明 1,王小文 1, 2,张诚 1, 3,冯波 2,谢晓 4,黄春 1,向小勇 1. Pluronic F-127-胰蛋白酶混合凝胶提高静脉桥血管腺相关病毒感染效率的研究[J]. 上海交通大学学报(医学版), 2019, 39(7): 730-. |
| [12] | 丁文婧1,吴慧1,樊嵘1,徐敏2,仇晓春1. 肿瘤领域的最新转化医学研究[J]. 上海交通大学学报(医学版), 2018, 38(9): 1133-. |
| [13] | 吴慧 1,樊嵘 1,丁文婧 1,章馨曼 2,仇晓春 1. 转化医学领域近期前沿进展[J]. 上海交通大学学报(医学版), 2018, 38(6): 712-. |
| [14] | 吴慧 1,章馨曼 2,丁文婧 1,仇晓春 1. 转化医学领域近期研究进展[J]. 上海交通大学学报(医学版), 2018, 38(4): 481-. |
| [15] | 翁震 1,钮晓音 2,周俊松 1. CRISPR-Cas9技术在非肿瘤性血液病中的应用[J]. 上海交通大学学报(医学版), 2018, 38(11): 1396-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||