结核病(tuberculosis,TB)是由结核分枝杆菌复合群(Mycobacterium tuberculosis complex,MTBC)感染引起的一种经呼吸道传播的传染性疾病;世界上约1/4的人口感染过结核分枝杆菌,TB仍然是全球公共卫生的一个主要威胁[1]。在MTBC感染过程中,巨噬细胞是最早遇到分枝杆菌的免疫细胞,也是分枝杆菌的主要宿主细胞。巨噬细胞可以激活细胞凋亡、自噬、活性氧/一氧化氮(reactive oxygen species/nitric oxide,ROS/NO)等信号通路来抵抗病原体入侵,在分枝杆菌感染和宿主免疫中起关键作用[2-4]。MTBC也进化出多种机制,如干扰吞噬体的成熟等,来逃避巨噬细胞的免疫杀伤作用[5]。深入研究巨噬细胞抗MTBC感染的免疫过程,以及MTBC对巨噬细胞免疫逃逸的具体机制,对于结核病新的治疗靶点的发现以及宿主靶向治疗具有重要意义。
miRNA是一种小的单链非编码RNA,长度约22 nt。miRNA可以被加载到RNA诱导沉默复合体(RNA-induced silencing complex,RISC)中,并通过miRNA介导的mRNA降解或翻译抑制来指导RISC沉默靶mRNA[6]。已有证据[7]表明,在健康和疾病状态下,巨噬细胞的免疫应答等细胞过程都受到各种miRNA的调控。病原体会诱导细胞miRNA水平的变化,表达水平改变的miRNA可以通过调节先天性和适应性免疫应答来影响疾病进展[8]。在我们的前期工作中,通过构建卡介苗即牛结核分枝杆菌减毒活菌株(Mycobacterium bovis Bacillus Calmette-Guérin,M. bovis BCG,BCG)和人结核分枝杆菌减毒株H37Ra菌株感染的巨噬细胞模型,提取感染的巨噬细胞外泌体进行转录组测序、生物信息学分析,并收集结核病患者的血浆标本进行验证,发现miR-185-5p是TB的一种很有潜力的新型生物标志物[9]。但miR-185-5p在分枝杆菌感染中的具体功能尚无研究和报道。
自噬是真核细胞内溶酶体依赖性的细胞降解途径,可以通过形成自噬体将病原体递送到溶酶体中进行降解。自噬作为一种控制细胞内病原体的先天防御机制,参与MTBC与巨噬细胞的相互作用[2]。GUTIERREZ等[10]在2004年最早报道了自噬是一种抑制BCG和人结核分枝杆菌毒株H37Rv菌株在感染的巨噬细胞中存活的防御机制:刺激巨噬细胞的自噬途径导致分枝杆菌吞噬体成熟为吞噬溶酶体,从而抑制分枝杆菌在细胞内存活。MTBC也进化出多种机制来抑制细胞自噬反应,例如上调增强细胞内存活(enhanced intracellular survival,Eis)基因、上调与自噬相关的miRNA、减弱干扰素γ(interferon-γ,IFN-γ)诱导的自噬体生物发生等[11]。总的来说,刺激自噬会促进巨噬细胞对MTBC的杀伤作用,而抑制自噬有利于MTBC的细胞内存活。因此,在本研究中,我们使用BCG感染人单核细胞白血病(THP-1)细胞诱导分化的巨噬细胞,进一步确认BCG感染对miR-185-5p表达的影响,并探究miR-185-5p对巨噬细胞自噬的影响以及在宿主‒病原体相互作用中发挥的功能,以期为研究TB的感染发病机制和治疗方案提供新的思路。
1 材料和方法
1.1 实验材料
1.1.1 细胞和菌株
人单核细胞白血病THP-1细胞购自上海中乔新舟生物科技有限公司。BCG来自本实验室储存传代的菌株库。
1.1.2 试剂和仪器
Middlebrook 7H9肉汤基础培养基、Middlebrook 7H11琼脂基础培养基、Middlebrook OADC增菌液购自北京索莱宝科技有限公司;罗氏培养管购自珠海贝索生物技术有限公司。RPMI 1640培养基、TRIzol、Lipofectamine 3000购自美国Thermo;胎牛血清(fetal bovine serum,FBS)购自以色列Biological Industries;佛波酯(phorbol 12-myristate 13-acetate,PMA)、PKH26荧光染料购自德国Sigma;miScriptⅡ RT加尾法试剂盒、miScript SYBR Green PCR试剂盒购自德国Qiagen;miR-185-5p模拟物及对照(mimic/mimic NC)、抑制剂及对照(inhibitor/inhibitor NC)购自广州锐博科技有限公司;SensGFP-StubRFP-LC3慢病毒购自上海吉凯基因科技有限公司。Triton X-100、qPCR引物购自生工生物工程(上海)股份有限公司;4%多聚甲醛(paraformaldehyde,PFA)、PBS、RIPA裂解液、苯甲基磺酰氟(phenylmethanesulfonyl fluoride,PMSF)、BCA蛋白浓度测定试剂盒、超敏ECL化学发光试剂盒、辣根过氧化物酶标记山羊抗兔IgG (H+L)购自上海碧云天生物技术有限公司。微管相关蛋白1轻链3(microtubule-associated protein 1 light chain 3,LC3)、白细胞分化抗原9(cluster differentiation 9,CD9)、肿瘤易感基因101(tumor susceptibility gene 101,TSG101)兔单克隆抗体购自英国Abcam(货号分别为ab192890、ab236630、ab236630)。生物安全柜、二氧化碳培养箱、qPCR仪来自美国Thermo,荧光显微镜、Leica TCS Sp8 STED共聚焦显微镜来自德国Leica,透射电子显微镜(transmission electron microscopy,TEM)来自日本JEOL,纳米颗粒跟踪分析(nanoparticle tracking analysis,NTA)仪来自德国Particle Metrix。
1.2 实验方法
1.2.1 外泌体分离鉴定、外泌体摄取
分别收集BCG感染与未感染的THP-1诱导分化的巨噬细胞培养上清液,在4 ℃下,依次按照以下条件离心:300×g、10 min,2 000×g、30 min,12 000 ×g、45 min。去除死细胞和碎片,110 000×g超速离心120 min,PBS重悬沉淀,110 000×g超速离心70 min,所得沉淀即为外泌体颗粒。将其重悬于PBS,直接使用或-80 ℃冷冻备用。首先通过Western blotting检测外泌体标志蛋白CD9和TSG101的表达。再通过NTA技术检测外泌体的粒径大小和浓度范围,样本稀释8 000~10 000倍后上机检测。最后,通过TEM进行外泌体形态鉴定:将外泌体悬液加到固定好的碳网上,室温静置20 min;之后在碳网上滴加2%磷钨酸,静置20 s,在TEM(80 kV)下观察并拍照。
将分离得到的感染BCG的巨噬细胞中的外泌体用PKH26荧光染料进行标记,将标记的外泌体(20 μg/mL)与未感染BCG的巨噬细胞共培养,使用4% PFA固定细胞,在荧光显微镜下观察不同时间点(0、12、24、48 h)细胞内PKH26荧光斑点的分布。
1.2.2 RNA提取和qPCR分析
THP-1细胞经75 nmol/L PMA诱导48 h后分化为贴壁的巨噬细胞。加入感染复数(multiplicity of infection,MOI)为10的BCG感染巨噬细胞,感染4 h后更换新鲜RPMI 1640培养基(含10% FBS),继续培养至感染12、24、48 h时收集细胞RNA。通过TRIzol法分别提取细胞RNA和外泌体RNA,使用miScriptⅡ RT加尾法试剂盒将RNA反转录为cDNA,使用miScript SYBR Green PCR试剂盒对miRNA进行qPCR检测。miR-185-5p的qPCR特异性正向引物序列为5′-TCTGGA-GAGAAAGGCAGTTCCTGA-3′,反向引物为试剂盒自带。qPCR反应程序为:95 ℃,15 min;40个循环(95 ℃,15 s;55 ℃,30 s;70 ℃,30 s)。使用U6作为内参,正向引物序列为5′-CTCGCTTCGGCAG-CACA-3′,反向引物序列为5′-AACGCTTCACGAA-TTTGCGT-3′。采用2-ΔΔCT方法计算miRNA相对表达水平。
1.2.3 CFU实验
提前配置7H11琼脂平板,即3.8 g Middlebrook 7H11琼脂基础培养基溶于180 mL蒸馏水,加1 mL甘油,121 ℃高压灭菌10 min,冷却至50~55 ℃后添加Middlebrook OADC增菌液20 mL,倾倒于平板中备用。THP-1细胞诱导贴壁后,按照Lipofectamine 3000使用说明瞬时转染miR-185-5p mimic/mimic NC和inhibitor/inhibitor NC,按照“1.2.2”方法检测转染效率。转染成功后,按照MOI为10加入BCG感染细胞4 h后,使用PBS洗涤3次,去除细胞外BCG,在新鲜培养基中继续培养细胞至BCG感染48 h。用1% Triton X-100裂解细胞,分别收集感染4、48 h的细胞内BCG,PBS连续稀释10、100倍后接种于7H11琼脂平板,37 ℃培养21 d,进行菌落形成单位(colony-forming unit,CFU)计数,分别用于分析巨噬细胞吞噬BCG和BCG在巨噬细胞中存活的情况。
1.2.4 Western blotting
LC3蛋白的Western blotting检测:THP-1细胞诱导贴壁,转染mimic/mimic NC和inhibitor/inhibitor NC 24 h后,加入BCG感染24 h,自噬抑制剂氯喹(chloroquine,CQ)处理组同时加入终浓度为10 μmol/L的CQ处理24 h;使用含1 mmol/L PMSF的RIPA裂解液裂解细胞,收集总蛋白,BCA法进行蛋白定量;使用12% SDS-PAGE凝胶,上样20 μg蛋白,在80 V、50 min及120 V、60 min条件下电泳,在300 mA、70 min条件下转膜(PVDF膜),5%脱脂奶粉室温封闭1 h;4 ℃孵育LC3抗体(1∶1 000稀释)过夜,室温孵育二抗(1∶1 000稀释)1 h后,对蛋白条带进行显影分析。LC3抗体可以同时孵育出LC3Ⅰ和LC3Ⅱ 2条蛋白条带,通常以LC3Ⅱ/内参来评估自噬活性。
CD9、TSG101蛋白的Western blotting检测:收集到的外泌体沉淀同样使用含1 mmol/L PMSF的RIPA裂解液裂解,后续步骤同LC3蛋白的Western blotting检测步骤,CD9、TSG101抗体均1∶1 000稀释。
1.2.5 自噬流检测
SensGFP-StubRFP-LC3慢病毒以MOI为50感染THP-1细胞,细胞于600 ×g、37 ℃离心90 min后,再在培养箱中感染24 h。加入2 μg/mL嘌呤霉素筛选3 d,清除未感染细胞,传代时加入1 μg/mL嘌呤霉素维持,以获得SensGFP-StubRFP-LC3稳定表达的THP-1细胞。将上述细胞铺板于玻璃底24孔板,PMA诱导细胞贴壁,转染miR-185-5p mimic和inhibitor,并加入BCG感染24 h。4% PFA固定细胞,用Hoechst染料对细胞核染色,通过Leica TCS Sp8 STED共聚焦显微镜观察细胞内荧光斑点的分布。GFP(绿色)和RFP(红色)分别用于指示LC3蛋白在自噬体和自噬溶酶体中的聚集情况;在pH中性的自噬体中,GFP和RFP一起产生黄色信号,而pH敏感的GFP信号在自噬体与酸性溶酶体融合后减弱,导致RFP的红色荧光增强。
1.2.6 生物信息学分析
使用TargetScan (
1.3 统计学分析
使用GraphPad Prism软件进行统计分析并绘图。定量资料用x±s表示。2组间比较采用独立样本t检验,多组间比较采用单因素方差分析(ANOVA)。P<0.05表示差异有统计学意义。
2 结果
2.1 BCG感染巨噬细胞使细胞内和外泌体中的miR-185-5p均表达上调
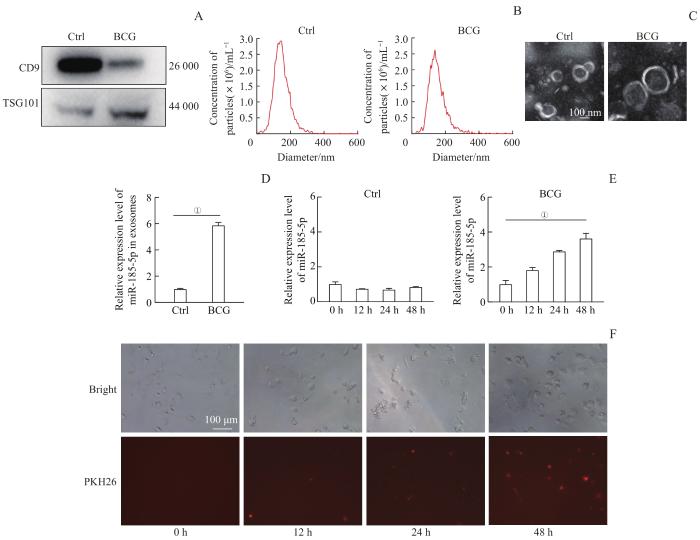
在我们之前的工作中,通过外泌体全转录组测序分析发现BCG和H37Ra感染会使外泌体中miR-185-5p的表达水平显著上调,提示miR-185-5p可能在分枝杆菌感染中有一定的作用[9]。为了进一步研究miR-185-5p在BCG感染中的功能,我们首先对之前的发现进行进一步验证。通过超速离心法分离外泌体,Western blotting检测到外泌体标志蛋白CD9和TSG101的表达(图1A);NTA检测外泌体粒径及浓度大小(图1B),结果显示,外泌体直径约150 nm,浓度约1010个/mL;TEM观察到外泌体的双层膜结构(图1C)。以上结果表明,提取到的外泌体的纯度和质量达标,可用于后续实验。
图1
图1
外泌体鉴定、摄取及BCG感染对巨噬细胞和外泌体中miR-185-5p表达水平的影响
Note:A. Western blotting analysis of the expression levels of exosome marker protein CD9 and TSG101. Ctrl—uninfected macrophages. B. Detection of exosome diameter and concentration by NTA. C. TEM analysis of exosome characteristics. D. The relative expression level of miR-185-5p in exosomes from macrophages with BCG infection for 48 h. E. The relative expression level of miR-185-5p in macrophages with BCG infection for 0, 12, 24 and 48 h. F. Cellular uptake of exosomes extracted from BCG-infected macrophages by uninfected macrophages after 0, 12, 24 and 48 h of co-culture. ①P=0.000.
Fig 1
Identification and uptake of exosomes and the effect of BCG infection on the expression of miR-185-5p
我们还对提取到的BCG感染的巨噬细胞外泌体进行荧光染色,将其与未感染的巨噬细胞进行共培养,发现外泌体可以转移到未感染的巨噬细胞内,且在共培养48 h时巨噬细胞对外泌体的摄取量最高(图1F),提示外泌体可能作为介质进行胞间传递,且其被细胞的摄取具有时间依赖性。
2.2 miR-185-5p促进BCG在THP-1诱导分化的巨噬细胞中的存活
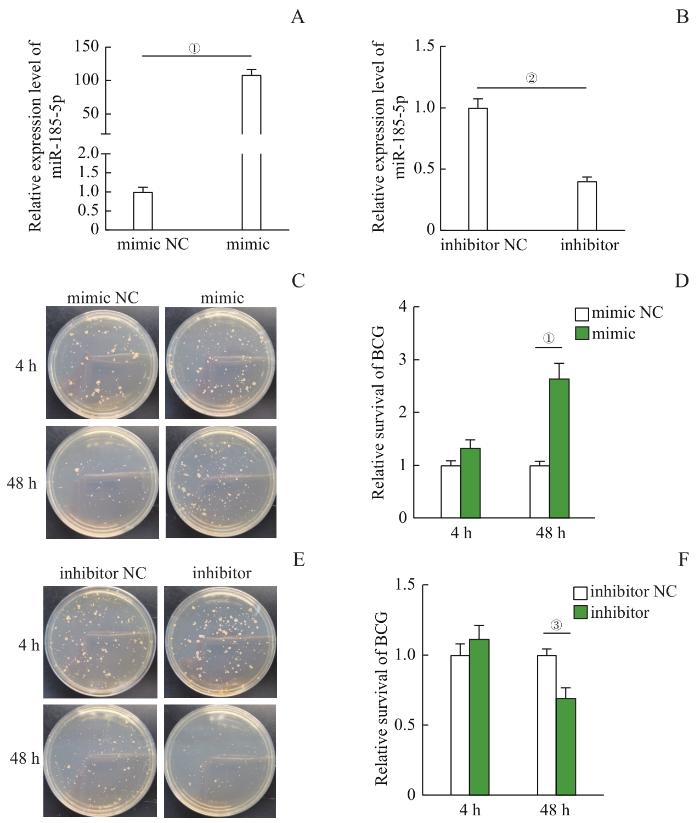
为了探索miR-185-5p在分枝杆菌感染中的作用,我们首先验证了miR-185-5p对胞内BCG的生长存活是否有影响。首先设计合成miR-185-5p mimic/mimic NC和inhibitor/inhibitor NC,转染THP-1诱导分化的巨噬细胞,结果显示:相比于mimic NC,mimic显著上调miR-185-5p的表达水平(图2A,P=0.000);相比于inhibitor NC,inhibitor显著抑制miR-185-5p的表达水平(图2B,P=0.002)。转染了mimic和inhibitor的巨噬细胞经BCG感染4 h和48 h后,分别收取巨噬细胞内的BCG进行CFU计数。结果显示,感染4 h时,mimic NC组与mimic组BCG的数量差异无统计学意义(P>0.05),同样inhibitor NC组与inhibitor组BCG数量差异也无统计学意义(P>0.05),表明BCG感染4 h时miR-185-5p未明显影响巨噬细胞对BCG的吞噬作用。而在BCG感染48 h时,相比于mimic NC组,mimic组BCG的CFU数量显著增加(图2C、D,P=0.000);相比于inhibitor NC组,inhibitor组BCG的CFU数量显著减少(图2E、F,P=0.041)。这些结果表明,miR-185-5p在BCG感染过程中发挥着有利于BCG生长存活、不利于巨噬细胞清除杀灭BCG的作用。
图2
图2
miR-185-5p对BCG胞内存活的影响
Note:A. The relative expression level of miR-185-5p in THP-1-derived macrophages transfected with miR-185-5p mimic and mimic NC. B. The relative expression level of miR-185-5p in THP-1-derived macrophages transfected with miR-185-5p inhibitor and inhibitor NC. C. The effect of miR-185-5p up-regulation on the survival of BCG in macrophages after 4 h and 48 h of BCG infection. D. Relative quantification of BCG survival in macrophages with miR-185-5p up-regulation. E. The effect of miR-185-5p down-regulation on the survival of BCG in macrophages after 4 h and 48 h of BCG infection. F. Relative quantification of BCG survival in macrophages with miR-185-5p down-regulation. ①P=0.000, ②P=0.002, ③P=0.041.
Fig 2
Effect of miR-185-5p on intracellular BCG survival
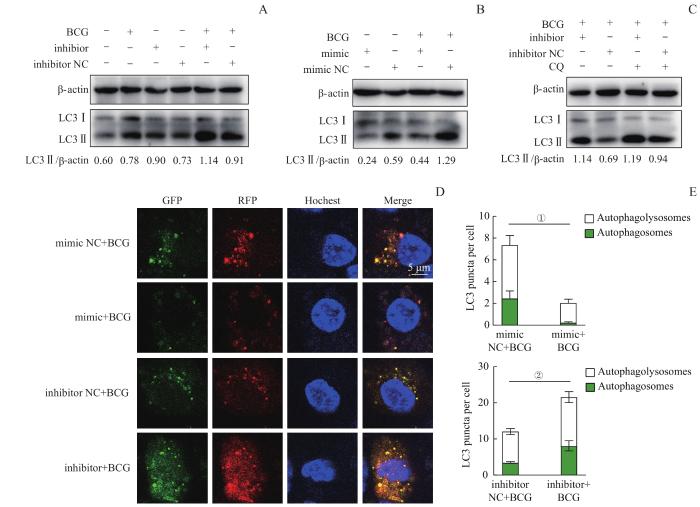
2.3 miR-185-5p抑制BCG感染诱导的细胞自噬
图3
图3
miR-185-5p对巨噬细胞自噬流的影响
Note:A–C. Western blotting analysis of autophagy protein LC3 Ⅱ in macrophages. A. THP-1-derived macrophages were treated with inhibitor/inhibitor NC with or without BCG infection. B. THP-1-derived macrophages were treated with mimic/mimic NC with or without BCG infection. C. THP-1-derived macrophages were transfected with miR-185-5p inhibitor followed by BCG infection and CQ treatment (10 μmol/L). D. Confocal images of LC3 protein. GFP (green) and RFP (red) indicate the aggregation of LC3 protein in autophagosomes and autophagolysosomes, respectively. E. Quantification of RFP and GFP fluorescent puncta. ①P=0.034, ②P=0.042.
Fig 3
Effect of miR-185-5p on macrophage autophagic flux
使用CQ进一步验证miR-185-5p对BCG感染的巨噬细胞中自噬流的影响。CQ可以通过破坏溶酶体的结构和功能来抑制自噬,导致LC3Ⅱ聚集。结果显示,CQ处理后,使用miR-185-5p inhibitor仍然使BCG感染的巨噬细胞中LC3Ⅱ的表达增加(图3C),表明miR-185-5p可以抑制BCG感染的巨噬细胞自噬。
为了进一步揭示miR-185-5p对自噬流的影响,我们使用SensGFP-StubRFP-LC3来跟踪LC3在自噬体和自噬溶酶体中的聚集情况。发现在BCG感染时,相比于mimic NC组,miR-185-5p mimic组GFP、RFP荧光斑点均明显减少(图3D),即早期自噬体的生成减少(图E,P=0.034);而相比于inhibitor NC组,使用inhibitor抑制miR-185-5p后则会使早期自噬体的生成显著增多(图3D、E,P=0.042)。以上结果表明,BCG感染时,通过上调miR-185-5p的表达可以抑制早期自噬体的生成,抑制细胞自噬过程,从而有利于BCG在巨噬细胞内的生长存活。
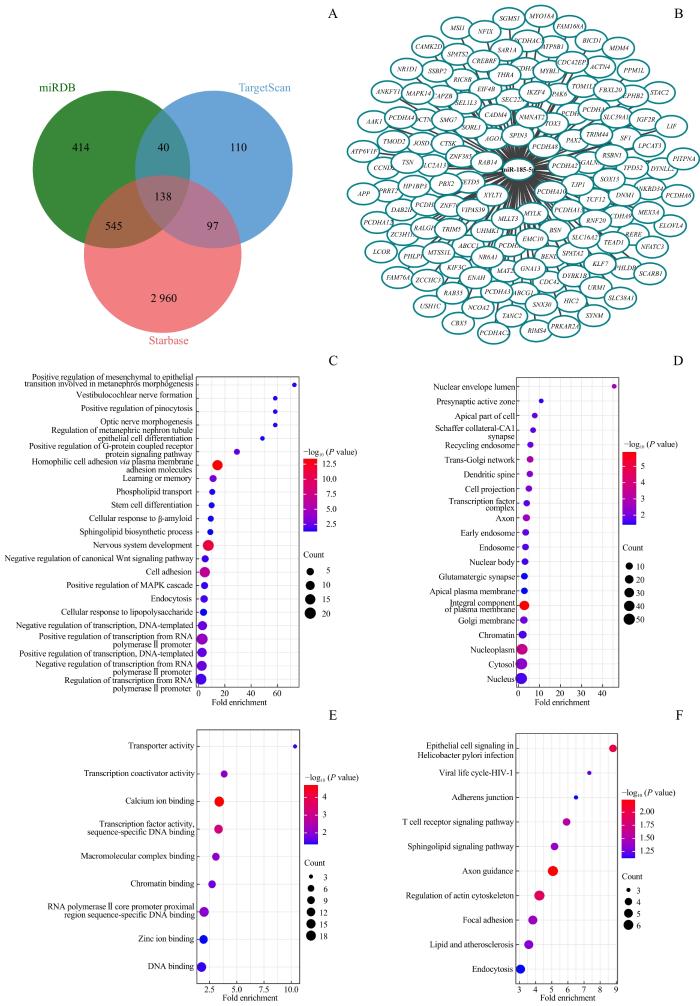
2.4 miR-185-5p潜在靶基因预测及富集分析
图4
图4
miR-185-5p潜在靶基因预测及富集分析
Note: A. Venn diagram of potential target genes of miR-185-5p. B. 138 target genes of miR-185-5p. C. GO analysis of target genes in biological process. D. GO analysis of target genes in cell component. E. GO analysis of target genes in molecular function. F. KEGG pathway analysis of target genes of miR-185-5p.
Fig 4
Prediction and enrichment analysis of potential target genes of miR-185-5p
值得特别注意的是,在这些基因中,被报道与自噬相关的基因有核受体亚家族1组D成员1(nuclear receptor subfamily 1 group D member 1,NR1D1)、Ras相关蛋白Rab-35(Ras-related protein Rab-35,RAB35)、细胞分裂控制蛋白42同源物(cell division control protein 42 homolog,CDC42)等[12-14],提示miR-185-5p很可能通过直接靶向这些基因来调控细胞自噬,参与抗感染免疫过程。除了自噬相关基因,在预测到的miR-185-5p靶基因中,我们还发现了参与脂代谢途径的ATP 结合盒亚家族G成员1(ATP-binding cassette sub-family G member 1,ABCG1)、清道夫受体B类成员1(scavenger receptor class B member 1,SCARB1)基因,以及具有重要转录调控作用的丝裂原活化蛋白激酶14(mitogen-activated protein kinase 14,MAPK14)等,提示miR-185-5p还可能通过调节脂代谢和细胞因子信号通路等来参与抗分枝杆菌感染的免疫调节过程。
3 讨论
巨噬细胞是MTBC感染过程中的主要宿主细胞。巨噬细胞不仅可以为MTBC的生存提供场所,还可以启动一系列宿主反应来限制MTBC的细胞内生长。自噬在宿主防御中起着重要作用,它可以通过形成成熟的自噬溶酶体降解细菌,限制MTBC的细胞内存活[10]。本研究使用BCG感染THP-1诱导分化的巨噬细胞,发现BCG感染会导致细胞内和外泌体中均高表达miR-185-5p。上调miR-185-5p可以促进BCG在巨噬细胞内的生长和存活,而抑制miR-185-5p则会使巨噬细胞内BCG存活量明显降低。更有意义的是,本研究发现miR-185-5p能够调控巨噬细胞的自噬过程。BCG感染能诱导巨噬细胞自噬;上调miR-185-5p可以通过抑制早期自噬体的生成抑制细胞自噬的发生,而抑制miR-185-5p则会促进自噬体的生成,使细胞自噬活性进一步增强。这些结果表明,miR-185-5p可以通过调控巨噬细胞自噬途径来参与分枝杆菌感染的免疫过程,抑制miR-185-5p会促进巨噬细胞自噬,抑制BCG的细胞内生存,提示其可能成为结核病一种潜在的药物作用靶点。
通过miRNA mimic恢复对宿主友好的miRNA表达或通过miRNA inhibitor阻断异常的miRNA表达,实现miRNA的正向调控或负向调控,可用于疾病治疗[15-16]。我们发现miR-185-5p的上调有利于巨噬细胞内BCG的生存,而抑制miR-185-5p后BCG的存活量减少,提示可以使用miR-185-5p inhibitor来阻断miR-185-5p的表达,以抑制宿主细胞内的细菌生长,起到辅助治疗结核病的作用。但目前本研究仅限于体外实验,想要确定miR-185-5p作为治疗靶点的应用价值,还需要进一步使用动物模型进行体内实验,整体评估其在体内对BCG生长存活的影响,以及使用miR-185-5p inhibitor后宿主抗菌的效果。此外,目前miRNA药物的治疗也具有一定的局限性,主要问题包括miRNA稳定性差、脱靶作用、递送方式不完善等。目前,外泌体作为药物递送载体,因具有保护其内容物免于降解、体积小、免疫原性低、毒性低、药物靶向等优点,而具有广阔的应用前景[17-18]。本研究发现BCG感染不仅诱导巨噬细胞内高表达miR-185-5p,在巨噬细胞分泌的外泌体中也含有高水平的miR-185-5p,且外泌体可以被未感染的巨噬细胞捕获。这提示外泌体在miR-185-5p的应用中可能发挥以下作用:一方面,外泌体作为天然载体,可保护miR-185-5p免受核糖核酸酶(RNase)的降解,为其递送方式提供了思路;另一方面,外泌体还可能将miR-185-5p传递到未感染巨噬细胞或其他免疫细胞中发挥功能。这些都是值得后续深入研究的内容。
寻找新的结核病标志物,并探索其在感染免疫以及宿主-病原相互作用中的机制,对开发新的结核病治疗靶点、完善结核病防控策略具有重要意义。在之前的研究中,我们发现了外泌体miR-185-5p作为结核病临床诊断标志物的应用价值[9]。在本研究中,我们发现了miR-185-5p作为结核病治疗靶点的潜力。miR-185-5p能够通过调节自噬在BCG感染中发挥重要作用,并通过预测发现NR1D1、CDC42可能是其直接靶基因。研究表明,NR1D1可以调节自噬和溶酶体的生物发生[12],抑制白细胞介素10(interleukin,IL-10)基因的表达[19],改善细胞对分枝杆菌的清除作用,提供对分枝杆菌感染的保护作用;还有研究[20]显示,miR-185-5p可以通过CDC42/JNK通路调控巨噬细胞的炎症和吞噬作用。这些研究为我们的结果提供了支持,并为后续研究miR-185-5p调控自噬的具体机制提供了思路。另外,我们还发现miR-185-5p可能直接靶向脂代谢相关基因如ABCG1[21],以及转录调控相关基因如MAPK14[22]。这些基因均被报道与分枝杆菌感染相关,提示miR-185-5p还可能通过调节脂代谢过程或细胞因子转录调控来发挥相应的作用。miR-185-5p在自噬调控之外的作用也值得我们进一步深入研究。
作者贡献声明
吴淇琦、汪豪、林砺、晏博和张舒林参与了实验设计,吴淇琦负责实验操作,吴淇琦和汪豪负责数据分析,吴淇琦、汪豪、晏博和张舒林参与了论文的写作和修改。所有作者均阅读并同意了最终稿件的提交。
AUTHOR's CONTRIBUTIONS
The study was designed by WU Qiqi, WANG Hao, LIN Li, YAN Bo and ZHANG Shulin. The experiments were performed by WU Qiqi. The results were analyzed by WU Qiqi and WANG Hao. The manuscript was drafted and revised by WU Qiqi, WANG Hao, YAN Bo and ZHANG Shulin. All the authors have read the last version of paper and consented for submission.
利益冲突声明
所有作者声明不存在利益冲突。
COMPETING INTERESTS
All authors disclose no relevant conflict of interests.
参考文献