磷酸核糖焦磷酸合成酶(phosphoribosyl pyrophosphate synthetase,PRPS)是核苷酸合成途径中的限速酶,可催化核糖-5-磷酸合成磷酸核糖焦磷酸(phosphoribosyl pyrophosphate,PRPP),而PRPP是核苷酸合成的前体[5]。PRPS1是PRPS发挥功能的最主要的亚型,且在人体绝大部分组织中均有表达[6]。生理状态下,PRPS1是一个由3个同源二聚体构成的六聚体,呈高度对称的螺旋状结构[7];其中,每个同源二聚体都含有1个活性位点和2个高度保守的变构调节位点[8]。迄今为止,在PRPS1基因中发现的突变均为错义突变,且PRPS1基因突变会导致其编码的蛋白结构发生改变,进而影响PRPS1活性。相关研究[9-11]显示,升高或降低PRPS1的活性会导致个体的嘌呤、嘧啶核苷酸代谢紊乱,进而诱发一系列疾病,如进行性神经性腓骨肌萎缩症、X染色体连锁非综合征性感音神经性耳聋2型、Arts综合征等。本课题组的前期研究[12]显示,ALL患儿存在多位点的复发特异性PRPS1基因突变,这也是儿童ALL研究领域中的首次发现。
6-巯基嘌呤(6-mercaptopurine,6-MP)、6-硫代鸟嘌呤(6-thioguanine,6-TG)是最常用的巯嘌呤类化学治疗(化疗)药物,也是ALL联合化疗法的主要药物;作为前体性药物,其二者均可通过嘌呤补救途径转化为细胞毒性的硫鸟嘌呤核苷酸掺入正在合成的DNA中,引起DNA的错配修复,最终导致DNA双链断裂及细胞凋亡[13]。研究[12]发现,突变的PRPS1基因所编码的PRPS1突变体蛋白可通过解除ADP/GDP对PRPS1的负反馈抑制使PRPS1活性上升,最终导致ALL细胞对6-MP、6-TG耐药;其中,PRPS1 A190T突变是ALL中PRPS1的热点突变。目前,临床上已新发现了诸多PRPS1突变位点,但其对药物敏感性的影响尚不明确。
表1 ALL患者中未验证功能变化的 PRPS1 突变的信息
Tab 1
| Sample ID | Gender | Immuno-phenotype | Allele change | Amino acid change | Relapse time/d | Time of relapse① | Subtype |
|---|---|---|---|---|---|---|---|
| CCCG2018 | M | B-ALL | A>T | I72F | 638 | early relapse | BCR_ABL1 |
| ALL208 | M | B-ALL | A>C | R177S | 341 | early relapse | PAX5_MPRIP |
| ALL086 | M | B-ALL | G>C | V316L | 427 | early relapse | ETV6_RUNX1 |
表2 已报道的ALL细胞中未验证功能变化的 PRPS1 突变的信息
Tab 2
| ALL cell line | Lineage | Gene | Mutation | Amino acid change |
|---|---|---|---|---|
| KOPN72bi | BCP-ALL | PRPS1 | c.623T>C | V208A |
| RS4;11 | BCP-ALL | PRPS1 | c.866T>C | V289A |
表3 PRPS1 I72位点的随机突变
Tab3
| Amino acid change | Allele change |
|---|---|
| I72M | C>G |
| I72V | A>G |
| I72F | A>T |
| I72L | A>C |
| I72N | T>A |
| I72S | T>G |
| I72T | T>C |
1 材料与方法
1.1 实验材料
1.1.1 细胞与质粒
人胚肾细胞系HEK-293T(简称293T)、人急性B淋巴细胞白血病细胞系REH均购自中国科学院典型培养物保藏委员会细胞库。
慢病毒包装质粒pMD2.G和psPAX2均购自美国Addgene公司,携带有绿色荧光蛋白(green fluorescent protein,GFP)标签的pGV303质粒购自上海吉凯基因医学科技股份有限公司。重组质粒pGV303 PRPS1 WT(携带野生型PRPS1基因编码区序列)、pGV303 PRPS1 A190T(携带具有A190T突变位点的PRPS1基因编码区序列)由本实验室前期构建。重组质粒pGV303 PRPS1 I72V、I72F、R177S、V316L、V208A、V289A、I72M、I72L、I72N、I72S、I72T(分别携带具有I72V、I72F、R177S、V316L、V208A、V289A、I72M、I72L、I72N、I72S、I72T突变位点的PRPS1基因编码区序列)均由本实验室通过KOD高保真酶以pGV303 PRPS1 WT质粒为模板进行环状PCR构建而成(所有质粒均进行了DNA测序,以确保质粒构建正确)。
1.1.2 主要试剂与仪器
DMEM培养基、1×磷酸盐缓冲液(1×PBS)、1×胰蛋白酶-EDTA溶液(1×TE)、青霉素-链霉素、RPMI 1640培养基(Gibco,美国),胎牛血清(fetal bovine serum,FBS)(苏州依科赛生物科技股份有限公司),IRDye® 800CW荧光标记的山羊抗兔IgG(LI-COR,美国),Cell Titer-Glo试剂盒(Promega,美国),6-MP、6-TG(Sigma,美国),多功能成像系统(Bio-rad,美国),Annexin V/DAPI凋亡检测试剂盒(BD,美国),jetPRIME®转染试剂及缓冲液(Polyplus-transfection,法国),MoFlo XDP超速流式细胞分选仪(Beckman Coulter,德国),多功能酶标仪(Biotek,美国)。
1.2 实验方法
1.2.1 细胞培养
293T细胞(贴壁细胞)采用含10% FBS、100 U/mL青霉素和100 μg/mL链霉素的DMEM培养基进行培养。REH细胞(悬浮细胞)采用含10% FBS、100 U/mL青霉素和100 μg/mL链霉素的RPMI 1640培养基进行培养。所有细胞的培养条件均为37 ℃、5% CO2,取对数生长期的细胞用于后续实验。
1.2.2 瞬时转染及蛋白表达检测
根据jetPRIME®转染试剂说明书配制jetPRIME®转染混合液,分别向其中添加重组质粒(包括pGV303 PRPS1 WT、A190T、I72V、I72F、R177S、V316L、V208A、V289A、I72M、I72L、I72N、I72S、I72T)以及空载体pGV303,制备含上述14种质粒的转染混合液。将293T细胞以1×106个/孔接种于6孔板中,培养24 h后向其中分别添加上述14种转染混合液,继续培养24 h后使用荧光显微镜检测各转染后细胞的GFP阳性率。待GFP阳性率≥70%时,使用蛋白质印迹法(Western blotting)检测293T细胞中各外源性PRPS1的表达,以转染pGV303空载体的细胞为阴性对照;待外源性PRPS1均有良好表达后,收集细胞并用于后续实验。
1.2.3 药物敏感性实验检测经6-MP或6-TG处理的各293T细胞的IC50
分别将“1.2.2”部分获得的能够表达pGV303蛋白以及pGV303 PRPS1 WT、A190T、I72V、I72F、R177S、V316L、V208A、V289A、I72M、I72L、I72N、I72S和I72T蛋白的293T细胞重悬为单细胞并计数,以4×103个/孔接种于96孔板中。培养24 h后,分别向其中添加待测药物6-MP或6-TG,6-MP或6-TG的质量终浓度均依次为0、0.02、0.05、0.14、0.41、1.23、3.70、11.11、33.33、100 μg/mL;每个浓度、每种细胞均设置3个复孔。药物处理72 h后,使用Cell Titer-Glo试剂盒测定其细胞活力,使用多功能酶标仪读取相对发光强度(relative light unit,RLU)。以各组中药物质量终浓度为0的细胞的RLU作为空白对照,计算各浓度药物下的细胞存活率(cell viability,CV)及半数抑制浓度(half maximal inhibitory concentration,IC50),实验重复3次。选取表达pGV303 PRPS1 A190T蛋白的293T细胞为对6-MP或6-TG耐药的阳性对照,表达pGV303蛋白、pGV303 PRPS1 WT和I72V蛋白的293T细胞为对6-MP或6-TG不耐药的阴性对照。当待检测细胞IC50>阴性对照IC50且P<0.05时,我们认为该细胞对该药物具有耐药性。
1.2.4 慢病毒包装、细胞感染及蛋白表达检测
将重组质粒pGV303 PRPS1 WT、A190T、I72F、I72V及空载体pGV303分别与包装慢病毒质粒pMD2.G和psPAX2混合后,添加至配制好的jetPRIME®转染混合液中,以制备含上述5种质粒的慢病毒转染混合液。将293T细胞以4×106个/皿接种于培养皿中,培养24 h后向其中分别添加上述5种慢病毒转染混合液,培养48、72 h后收集上清液并进行浓缩。
向REH细胞(2×106个)中分别添加上述5种病毒原液(100 μL)进行感染,培养24 h后换液。待细胞生长至合适密度时,用MoFlo XDP超速流式细胞分选仪分选出GFP阳性表达的细胞并继续进行培养,使用Western blotting检测REH细胞中各外源性PRPS1的表达,以转染pGV303空载体的细胞为阴性对照。待外源性PRPS1均有良好表达后,取生长状态良好并处于对数生长期的REH细胞用于后续研究。
1.2.5 药物敏感性实验检测经6-MP或6-TG处理的各REH细胞的IC50
分别将“1.2.4”部分获得的能够表达pGV303蛋白以及pGV303 PRPS1 WT、A190T、I72F和I72V蛋白的REH细胞重悬为单细胞并计数,以1.2×104个/孔接种于96孔板中进行培养,而后分别向其中加入待测药物6-MP或6-TG,继续培养72 h后使用Cell Titer-Glo试剂盒测定其细胞活力,并计算CV及IC50。其中,药物浓度、实验处理等均与“1.2.3”部分相一致。选取表达pGV303 PRPS1 A190T蛋白的REH细胞为对6-MP或6-TG耐药的阳性对照,表达pGV303蛋白、pGV303 PRPS1 WT和I72V蛋白的REH细胞为对6-MP或6-TG不耐药的阴性对照。细胞耐药性评判原则亦同前。
1.2.6 Annexin Ⅴ/DAPI双染法检测细胞凋亡
分别将“1.2.4”部分获得的5种REH细胞以2×106个/孔接种于12孔板中;其中,用10 μg/mL的6-MP处理48 h的细胞为实验组,不做处理的为对照组。经计数后收集各组细胞,采用Annexin V/DAPI凋亡检测试剂盒对上述细胞进行染色,并于流式细胞仪上检测其凋亡率。实验组和对照组均设置3个复孔。
1.2.7 Western blotting检测REH细胞中DNA损伤和细胞凋亡相关蛋白的表达
采用Western blotting对“1.2.6”部分中实验组细胞的DNA损伤和细胞凋亡相关蛋白进行检测,具体操作参照说明书进行。其中,使用的一抗(兔抗人抗体)为β-actin、细胞周期检验点激酶2(check point kinase 2,CHK2)抗体、磷酸化的CHK2(phosphorylated CHK2,pCHK2)抗体、H2AX抗体、S139位点磷酸化的组蛋白H2AX(phosphorylated H2AX-S139,γ-H2AX)抗体、聚(腺苷二磷酸核糖)聚合酶剪切体 [cleaved poly(ADP-ribose)polymerase,cleaved PARP]抗体、total PARP(总PARP)抗体,工作浓度均为1∶1 000;使用的二抗为IRDye® 800CW荧光标记的山羊抗兔IgG,工作浓度为1∶100 000。
1.2.8 蛋白质结构分析
采用蛋白质三维结构建模技术对PRPS1 I72位点、PRPS1 I72F和I72V的氨基酸残基及空间构象进行分析,具体步骤如下:①使用PDB数据库查找PRPS1 I72位点的蛋白质结构图,采用PDB数据库中编号为2HCR(PDB code 2HCR)的PRPS1蛋白结构图为本文PRPS1 I72位点的蛋白质结构图。②基于PDB code 2HCR的晶体结构,使用三维成像技术模拟PRPS1 I72F、PRPS1 I72V的氨基酸残基及空间构象,并使用PyMOL软件绘制相应氨基酸残基及空间构象图。
1.3 统计学方法
采用GraphPad Prism 8.0软件进行各项数据分析。定量资料以x±s的形式表示,2组间比较采用独立样本t检验,多组间比较采用单因素方差分析。P<0.05表示差异具有统计学意义。
2 结果
2.1 PRPS1 位点突变对293T细胞巯嘌呤类药物敏感性的影响
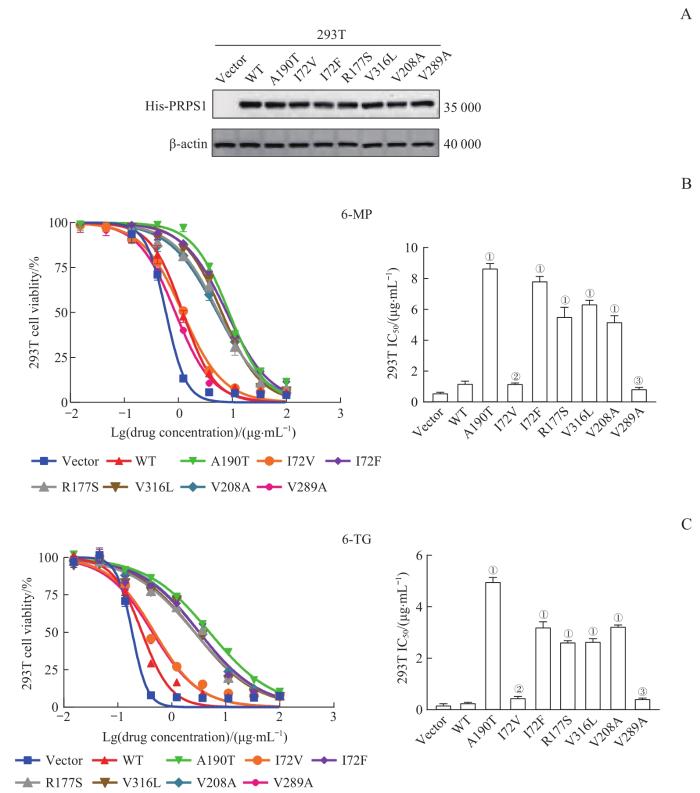
采用Western blotting检测PRPS1各突变体在293T细胞中的蛋白表达情况,结果(图1A)显示,与转染pGV303空载体相比,转染pGV303 PRPS1 WT、A190T、I72V、I72F、R177S、V316L、V208A、V289A重组质粒的293T细胞均可高表达各外源性PRPS1,且各细胞间的表达量相似;继而表明,各外源性PRPS1在293T细胞中均有良好且相一致的蛋白表达。
图1
图1
PRPS1 位点突变对293T细胞巯嘌呤类药物敏感性的影响
Note: A. Protein expression of PRPS1 mutants in 293T cells by Western blotting. His-PRPS1 represents the PRPS1 fusion protein with His label, that is, the exogenous PRPS1 protein. B. Detection of the drug sensitivities of PRPS1 mutations to 6-MP in 293T cells by drug sensitivity experiment. ①P=0.000, ②P=1.000, ③P=0.632, compared with 293T cells transfected with pGV303 PRPS1 WT plasmid. C. Detection of the drug sensitivities of PRPS1 mutations to 6-TG in 293T cells by drug sensitivity experiment. ①P=0.000, ②P=0.135, ③P=0.291, compared with 293T cells transfected with pGV303 PRPS1 WT plasmid.
Fig 1
Effect of mutations at PRPS1 locus on the sensitivity of thiopurine chemotherapy drugs in 293T cells
药物敏感性实验的结果(图1B、C)显示,转染pGV303 PRPS1 I72F、R177S、V316L和V208A重组质粒的293T细胞对6-MP或6-TG的IC50远高于各药物的阴性对照(即转染Vector、pGV303 PRPS1 WT、I72V重组质粒的293T细胞)(均P=0.000),而转染pGV303 PRPS1 V289A重组质粒的293T细胞对6-MP或6-TG的IC50与各药物的阴性对照之间的差异均无统计学意义。
2.2 PRPS1I72位点突变对293T细胞巯嘌呤类药物敏感性的影响
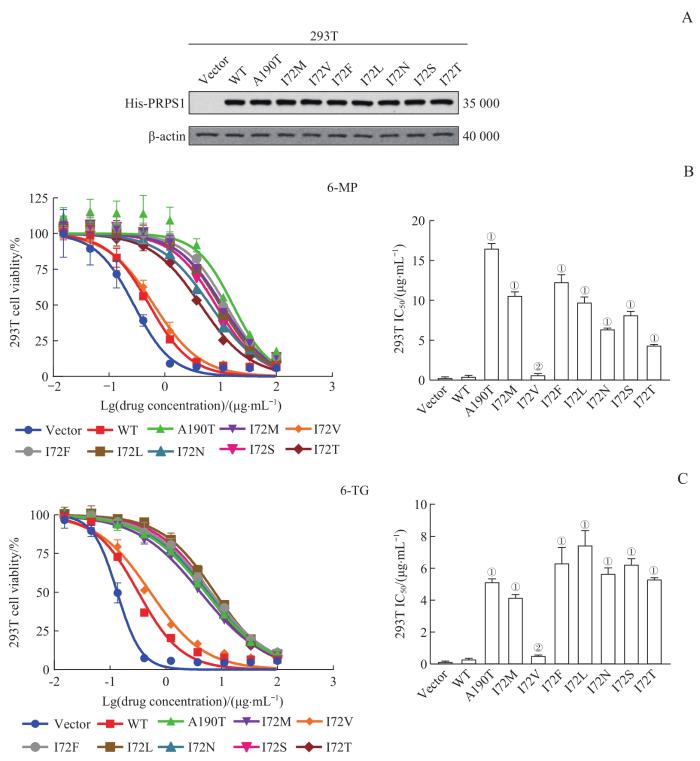
采用Western blotting检测外源性PRPS1 I72位点不同突变体在293T细胞中的蛋白表达情况,结果(图2A)显示,与转染pGV303空载体相比,转染pGV303 PRPS1 WT、A190T、I72M、I72V、I72F、I72L、I72N、I72S、I72T重组质粒的293T细胞均高表达PRPS1不同突变体,且各细胞间的表达量相似;继而证明,外源性PRPS1 I72位点不同突变体在293T细胞中均有良好且相一致的蛋白表达。
图2
图2
PRPS1 I72位点突变对293T细胞巯嘌呤类药物敏感性的影响
Note: A. Protein expression of PRPS1 mutants in 293T cells by Western blotting. His-PRPS1 represents the PRPS1 fusion protein with His label, that is, the exogenous PRPS1 protein. B. Detection of the drug sensitivities of PRPS1 I72 mutations to 6-MP in 293T cells by drug sensitivity experiment. ①P=0.000, ②P=1.000, compared with 293T cells transfected with pGV303 PRPS1 WT plasmid. C. Detection of the drug sensitivities of PRPS1 I72 mutations to 6-TG in 293T cells by drug sensitivity experiment. ①P=0.000, ②P=0.995, compared with 293T cells transfected with pGV303 PRPS1 WT plasmid.
Fig 2
Effect of mutations at PRPS1 I72 locus on the sensitivity of thiopurine chemotherapy drugs in 293T cells
药物敏感性实验的结果(图2B、C)显示,6-MP或6-TG对转染pGV303 PRPS1 A190T、I72M、I72F、I72L、I72N、I72S、I72T重组质粒的293T细胞的IC50远高于各药物的阴性对照(即转染Vector、pGV303 PRPS1 WT、I72V重组质粒的293T细胞)(均P=0.000)。
2.3 PRPS1I72位点突变对REH细胞巯嘌呤类药物敏感性的影响
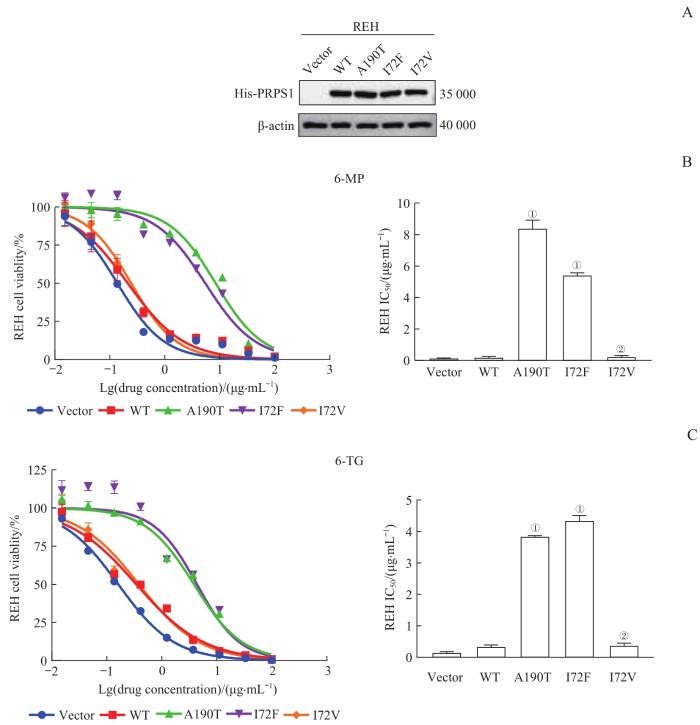
采用Western blotting检测各REH稳定细胞系中PRPS1不同突变体的蛋白表达情况,结果(图3A)显示,与转染pGV303空载体相比,稳定转染pGV303 PRPS1 WT、A190T、I72F、I72V重组质粒的REH细胞均高表达PRPS1,且各细胞间的表达量相似;继而证明,REH稳定细胞系构建成功。
图3
图3
PRPS1 I72位点突变对REH细胞巯嘌呤类药物敏感性的影响
Note:A. Protein expression of PRPS1 mutants in REH cells by Western blotting. His-PRPS1 represents the PRPS1 fusion protein with His label, that is, the exogenous PRPS1 protein. B. Detection of the drug sensitivities of PRPS1 I72 mutations to 6-MP in REH cells by drug sensitivity experiment. ①P=0.000, ②P=0.998, compared with REH cells transfected with pGV303 PRPS1 WT plasmid. C. Detection of the drug sensitivities of PRPS1 I72 mutations to 6-TG in REH cells by drug sensitivity experiment. ①P=0.000, ②P=0.903, compared with REH cells transfected with pGV303 PRPS1 WT plasmid.
Fig 3
Effect of mutations at PRPS1 I72 locus on the sensitivity of thiopurine chemotherapy drugs in REH cells
药物敏感性实验的结果(图3B、C)显示,6-MP或6-TG对稳定转染pGV303 PRPS1 A190T、I72F重组质粒的REH细胞的IC50远高于各药物的阴性对照(即稳定转染Vector、pGV303 PRPS1 WT、I72V重组质粒的REH细胞)(均P=0.000)。
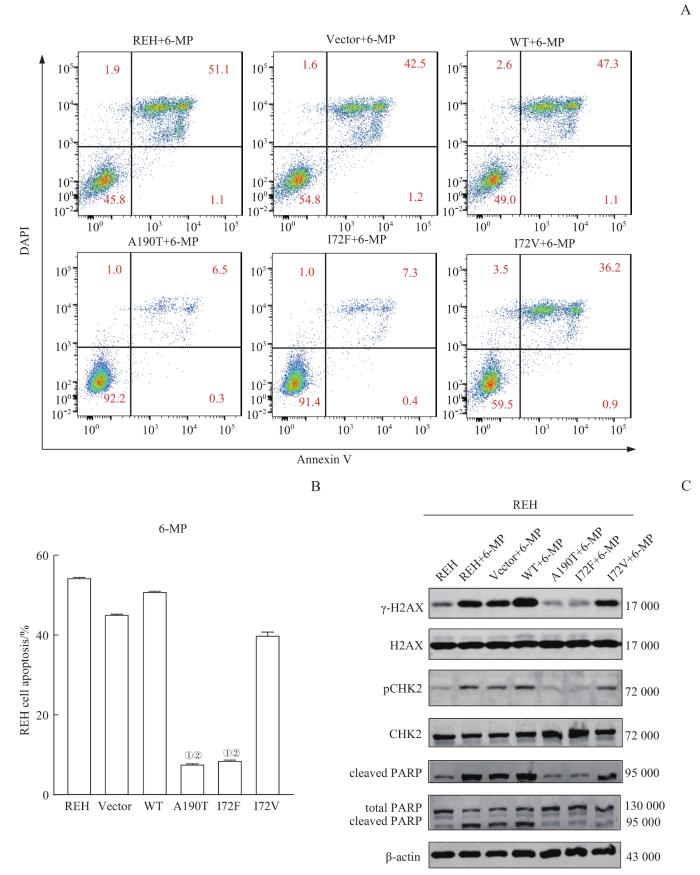
而后,我们采用10 μg/mL 6-MP处理实验组细胞,48 h后经Annexin Ⅴ/DAPI双染并通过流式细胞术检测细胞的凋亡情况,结果(图4A、B)显示,稳定转染pGV303 PRPS1 A190T、I72F重组质粒的REH细胞的凋亡率明显低于阴性对照(稳定转染Vector、pGV303 PRPS1 WT、I72V重组质粒的REH细胞)(均P=0.000)。
图4
图4
PRPS1 I72位点突变对经6-MP诱导的REH细胞的DNA损伤和细胞凋亡的影响
Note: A/B. Flow scatter plots (A) and statistical analysis (B) of the detection of apoptosis rates for each REH cell line by Annexin Ⅴ/DAPI double staining. ①P=0.000, compared with REH cells transfected with pGV303 PRPS1 WT plasmid; ②P=0.000, compared with REH cells transfected with pGV303 PRPS1 I72V plasmid. C. Detection of the protein expression of DNA damage biomarkers and apoptosis-related proteins by Western blotting.
Fig 4
Effect of mutations at PRPS1 I72 locus on the apoptosis and DNA damage in REH cells induced by 6-MP
采用Western blotting检测实验组细胞中DNA损伤、细胞凋亡相关蛋白的表达情况,结果(图4C)显示与转染Vector、pGV303 PRPS1 WT、I72V重组质粒的REH细胞相比,经6-MP处理的稳定转染pGV303 PRPS1 A190T、I72F重组质粒的REH细胞中的γ-H2AX、pCHK2、cleaved PARP的表达水平有明显下调。继而提示,PRPS1 I72F突变可以更加有效地减少由6-MP诱导的细胞凋亡和DNA损伤反应。
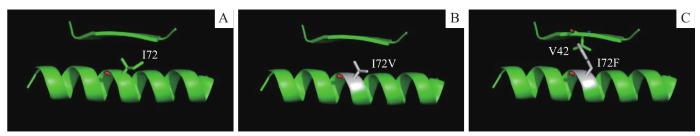
2.4 PRPS1I72位点突变对蛋白质结构的影响
图5
图5
PRPS1 I72位点(A)、PRPS1 I72V(B)和PRPS1 I72F(C)的蛋白质结构示意图
Fig 5
Schematic diagrams of protein structures of PRPS1 I72 locus (A), PRPS1 I72V (B) and PRPS1 I72F(C)
3 讨论
PRPS1作为核苷酸合成途径中的限速酶,在ALL耐药、复发中发挥着重要作用。本研究首先在293T细胞中过表达ALL临床样本及2个ALL细胞系中已发现的多个未知功能的PRPS1突变体,并行巯嘌呤类药物敏感性检测,结果发现表达PRPS1 I72F、R177S、V316L、V208A的突变可使293T细胞对6-MP或6-TG产生耐药,而突变体I72V则不会影响细胞的药物敏感性。因此我们推测,PRPS1 I72位点突变成不同氨基酸后,其对巯嘌呤类药物敏感性的影响可能不尽相同。为此,我们构建了PRPS1 I72的不同突变体(包括I72M、I72L、I72N、I72S、I72T),在293T细胞中就I72不同突变体对巯嘌呤类药物敏感性的影响进行检测,结果发现表达PRPS1 I72M、I72F、I72L、I72N、I72S、I72T的突变可使293T细胞对6-MP或6-TG产生耐药。后续,通过构建REH稳定表达PRPS1各突变体的细胞系行巯嘌呤类药物敏感性检测,并通过Annexin Ⅴ/DAPI双染实验及细胞凋亡、DNA损伤蛋白检测明确了PRPS1 I72位点的不同突变对该类药物敏感性的影响亦不同,与293T细胞获得的结果一致。同时,我们尝试从蛋白质结构变化的角度就I72位点不同突变对巯嘌呤敏感性影响不同的现象做出解释。
本研究通过在293T细胞中瞬时表达和REH细胞稳定表达PRPS1各突变体进行巯嘌呤类药物敏感性检测,发现PRPS1 I72F、R177S、V316L、V208A 属于耐药突变,而I72V、V289A对巯嘌呤类药物敏感性无明显影响,继而证明PRPS1 I72位点不同突变对功能影响存在差别。从PRPS1 I72位点的三维蛋白质结构解析图上来看,PRPS1 I72位点的I突变成F可能对PRPS1蛋白结构有潜在影响,而突变成M时并没有明确影响(该结果在正文中未显示)。且PRPS1 I72位点既不在PRPS1底物结合的口袋和变构位点附近,也不在二聚体界面上;而在理论上,涉及酶活性位点、变构调节位点的突变才能够改变酶的活性。另外,本文所使用的通过PDB数据库预测产生的蛋白质三维结构模型可能与真正结构存在一定出入,因此要想进一步明确PRPS1 I72位点突变产生的耐药机制,尚需要突变体的蛋白结构才能确定。
综上,本研究表明PRPS1的不同位点突变可使细胞对巯嘌呤类化疗药物敏感性产生不同的影响,当我们在临床上发现ALL患儿存在PRPS1 I72F、R177S、V316L、V208A、I72M、I72L、I72N、I72S和I72T突变时,应警惕患儿出现的针对巯嘌呤类化疗药物的耐药性。在此类患儿的治疗中,我们应减少巯嘌呤类化疗药物的使用,而采用其他化疗药物进行个体化治疗,进一步探索针对此类患儿的最佳治疗方案。同时,我们的研究还证实了293T细胞可作为检测PRPS1突变对巯嘌呤类化疗药耐药的快速研究模型,继而提示可通过293T细胞对PRPS1突变是否会引起巯嘌呤类药物耐受进行快速评估,从而为后续临床用药提供指导。
作者贡献声明
李慧和沈树红参与了实验设计,崔芷嫣开展了实验操作,崔芷嫣和陈尧参与了数据分析和整理,李慧、崔芷嫣、陈尧、陶悦参与了论文的写作和修改。所有作者均阅读并同意了最终稿件的提交。
AUTHOR's CONTRIBUTIONS
The study was designed by LI Hui and SHEN Shuhong. The experiments were carried out by CUI Zhiyan. The data was collected and analyzed by CUI Zhiyan and CHEN Yao. The manuscript was drafted and revised by LI Hui, CUI Zhiyan, CHEN Yao and TAO Yue. All the authors have read the last version of paper and consented for submission.
利益冲突声明
所有作者声明不存在利益冲突。
COMPETING INTERESTS
All authors disclose no relevant conflict of interests.
参考文献