根据最新数据显示,在全部恶性肿瘤中,2020年全球肺癌新发病例位居第2位,死亡病例数位居首位。在我国,肺癌死亡率位居第一,发病率仅次于乳腺癌[1]。按照WHO分型标准,肺癌包括肺腺癌(lung adenocarcinoma,LUAD)和肺鳞状细胞癌(lung squamous cell carcinoma,LUSC)等7种类型,其中LUSC占30%~40%[2]。目前,与临床上治疗LUAD时可选择的小分子靶向药物相比,LUSC的治疗办法有限,仍以化疗为主;其次由于其早期进展隐匿,诊断时已为局部晚期且常伴有转移,导致LUSC患者总生存期(overall survival,OS)短[3]。因此,亟需寻找用于LUSC早期诊断及预测患者预后的新型标志物。
研究[6]发现,GGPS1作为甲羟戊酸途径中的一种分支酶,参与蛋白质异戊二烯化,在多种肿瘤中呈过表达,并且与肿瘤发生和发展密切相关,影响着患者预后。然而对于GGPS1在LUSC中的相关研究,目前尚无报道。本研究基于生物信息学和免疫组织化学法分析GGPS1参与LUSC进程的潜在分子机制,以期为LUSC的诊治和预防提供一个新分子靶点。
1 对象与方法
1.1 研究对象
选取2016年7月—2018年7月于内蒙古自治区人民医院住院治疗的LUSC患者作为研究对象,在行肺叶切除术切除离体后1 h内,收集病变及正常组织,将其固定于福尔马林溶液中。纳入标准:① 经病理检验确诊为LUSC。② 首次确诊患者,术前未行放化疗等抗肿瘤治疗。③ 病例资料保留齐全。排除标准:① LUAD及肺小细胞癌患者。② 合并其他部位肿瘤。③ 术前接受放化疗等其他治疗。④ 患者生存信息缺失。
1.2 生物信息学分析
1.2.1 GGPS1泛癌分析
本研究利用TIMER2.0(
1.2.2 数据下载与整理
本研究的数据集下载自UCSC Xena(由TCGA数据库衍生的一个非常直观的在线网站,
1.2.3 GGPS1表达与LUSC患者临床特征与预后的关系
本研究利用UALCAN[8](
运用Kaplan-Meier Plotter数据库(该数据库是基于GEO、TCGA及EGA等多个平台的基因芯片和RNA-seq数据构建而成,
1.2.4 风险模型与预测模型的构建及验证
将UCSC Xena数据库中影响LUSC患者OS的危险因素,通过“glmnet”和“survival”包进行最小绝对收缩和选择算子(least absolute shrinkage and selection operator,LASSO)回归分析。应用“ggplot2”和“rms”包来生成列线图,上部为评分系统,下部为预测系统。列线图通过各因素的总分准确预测LUSC患者的1、2、3、5、10年生存率,绘制校准曲线以显示预测效果。
1.2.5 GGPS1蛋白质相互作用网络分析
1.2.6 GGPS1差异基因的筛选和功能富集分析
为了鉴定GGPS1在LUSC中的生物学功能,选定相关性系数>0.5、P<0.05为标准,筛选与GGPS1存在相关性的差异表达基因。将所选出的相关基因进行基因本体论(Gene Ontology,GO)功能和基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)通路富集分析。运用org.Hs.eg.db、clusterProfiler包进行功能注释和结果可视化。
1.3 免疫组织化学染色
新鲜的LUSC及癌旁组织标本石蜡包埋、连续切片,脱蜡,水化,抗原在热柠檬酸中高压修复,给予GGPS1一抗(φ=1∶100)室温孵育,之后加入二抗试剂孵育30 min,二氨基联苯胺(3,3′-diaminobenzidine,DAB)显色,苏木精复染,中性胶密封。
1.4 免疫组织化学检测与判读
按细胞的染色程度记分:不着色为0分,淡黄色为1分,棕黄色为2分,棕褐色为3分。按阳性染色细胞百分比记分:0为0分,1%~25%为1分,26%~50%为2分,51%~75%为3分,76%~100%为4分。将两项得分相乘即为总评分:≤4分为低表达组,>4分为高表达组。
1.5 随访
随访采用门诊复查、电话回访等方法进行,计算生存时间方法为手术日期到随访截止日期,或非LUSC原因死亡及因复发转移的日期。随访截止时间为2023年6月,中位随访时间56.50个月(0~68个月),无1例失访。
1.6 统计学分析
采用SPSS 25.0软件进行统计学分析,运用R软件(4.2.3版本)进行生物信息学分析和绘图。定性资料以频数(百分率)表示,组间比较使用χ2检验。符合正态性分布的定量资料以x±s表示,组间比较采用t检验;不符合正态分布的定量资料以M(Q1,Q3)表示,组间比较采用Mann-Whitney U检验。使用Cox比例风险回归模型,对数据进行单因素和多因素分析。通过Kaplan-Meier法绘制生存曲线,采用Log-rank检验对结果进行验证。P<0.05表示差异有统计学意义。
2 结果
2.1 GGPS1 在LUSC中的生物信息学分析
2.1.1 GGPS1在泛癌中的表达情况
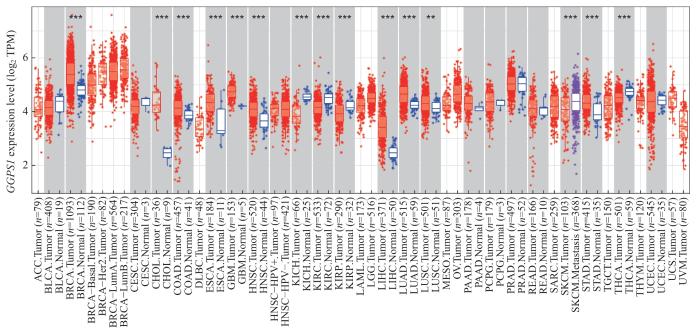
GGPS1在多种癌症类型中表达显著升高(图1),仅在在肾透明细胞癌(kidney renal clear cell carcinoma,KIRC)、肾嫌色细胞癌(kidney chromophobe,KICH)、肾乳头状细胞癌(kidney renal papillary cell carcinoma,KIRP)、皮肤黑色素瘤(skin cutaneous melanoma,SKCM)、甲状腺癌(thyroid carcinoma,THCA)中的表达显著降低。在LUSC中,GGPS1表达水平明显升高。
图1
图1
GGPS1 在泛癌中的表达
Note: ACC—adrenocortical carcinoma; BLCA—bladder urothelial carcinoma; BRCA—breast invasive carcinoma; CESC—cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL—cholangiocarcinoma; COAD—colon adenocarcinoma; DLBC—diffuse large B cell lymphoma; ESCA—esophageal carcinoma; GBM—glioblastoma multiforme; HNSC—head and neck squamous cell carcinoma; KICH—kidney chromophobe; KIRC—kidney renal clear cell carcinoma; KIRP—kidney renal papillary cell carcinoma; LAML—acute myeloid leukemia; LGG—brain lower grade glioma; LIHC—liver hepatocellular carcinoma; LUAD—lung adenocarcinoma; LUSC—lung squamous cell carcinoma; OV—ovarian serous cystadenocarcinoma; PAAD—pancreatic adenocarcinoma; PCPG—pheochromocytoma and paraganglioma; PRAD—prostate adenocarcinoma; READ—rectum adenocarcinoma; SARC—sarcoma; SKCM—skin cutaneous melanoma; STAD—stomach adenocarcinoma; THCA—thyroid carcinoma; THYM—thymoma; UCEC—uterine corpus endometrial carcinoma; UCS—uterine carcinosarcoma. Blue represents normal tissue and red represents tumor tissue. **P<0.01, ***P<0.001.
Fig 1
The expression of GGPS1 in pan-carcinoma
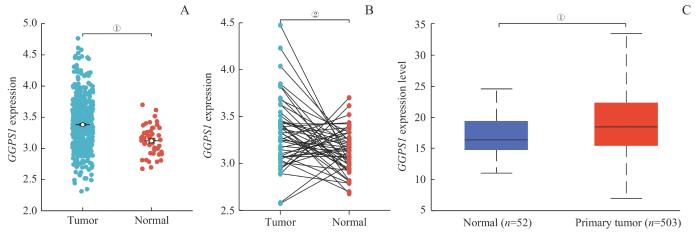
2.1.2 GGPS1在LUSC及癌旁组织中的差异表达
图2
图2
UCSC Xena和UALCAN数据库中 GGPS1 在LUSC和癌旁正常组织的差异表达
Note: A. GGPS1 expression in the tumors and normal tissues. B. GGPS1 expression in the 49 pairs of tumors and normal tissues. C. The expression of GGPS1 in LUSC in UALCAN database. ①P=0.000, ②P=0.003.
Fig 2
Differential expression of GGPS1 in the LUSC and paracancerous normal tissues in the UCSC Xena and UALCAN databases
2.1.3 GGPS1的表达与LUSC患者临床病理特征及预后的关系
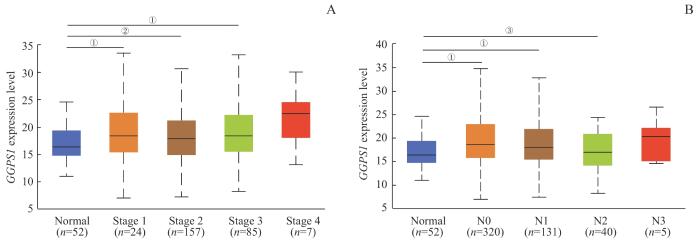
进一步利用UALCAN数据库分析LUSC患者GGPS1的表达与临床分期和淋巴结转移的关系,结果显示:与正常肺组织相比,GGPS1在Stage 1、2和3组和N0、N1和N2组的表达差异有统计学意义(图3A、B,均P<0.05)。
图3
图3
UALCAN数据库中 GGPS1 表达与LUSC患者临床病理特征的关系
Note: A. Comparison of GGPS1 expression between different stages of patients. B. Comparison of GGPS1 expression between different N stages of patients. ①P=0.000, ②P=0.001, ③P=0.027.
Fig 3
Relationship between GGPS1 expression and clinicopathological characteristics of LUSC patients in UALCAN database
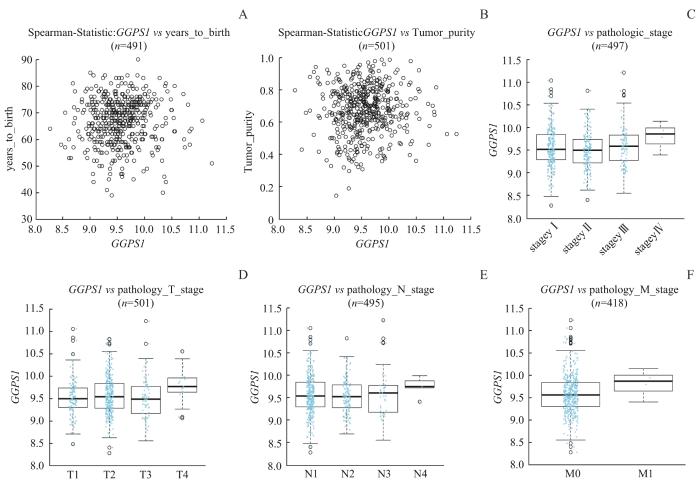
图4
图4
LinkedOmics数据库中 GGPS1 表达与LUSC患者临床病理特征的关系
Note: A. Expression level of GGPS1 of different ages. B. Expression level of GGPS1 in different tumor purity. C. Expression level of GGPS1 in different pathological grades. D. Expression level of GGPS1 in different T stages. E. Expression level of GGPS1 in different N stages. F. Expression level of GGPS1 in different M stages.
Fig 4
Relationship between GGPS1 expression and clinicopathological characteristics of LUSC patients in LinkedOmics database
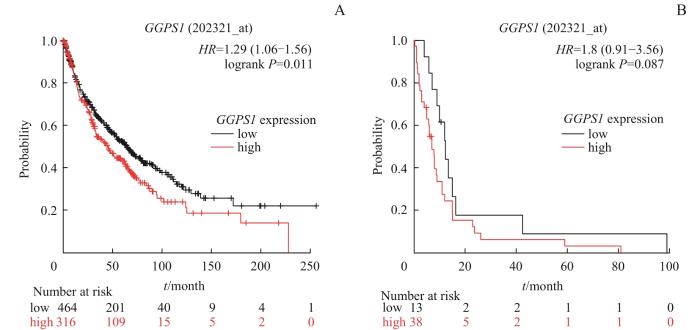
图5
图5
Kaplan-Meier Plotter 数据库中 GGPS1 表达与LUSC患者预后的关系
Note: A. Relationship between GGPS1 expression and overall survival. B. Relationship between GGPS1 expression and post progression survival.
Fig 5
Relationship between GGPS1 expression and prognosis of LUSC patients in Kaplan-Meier Plotter database
2.1.4 风险评分模型与预后模型的构建及验证
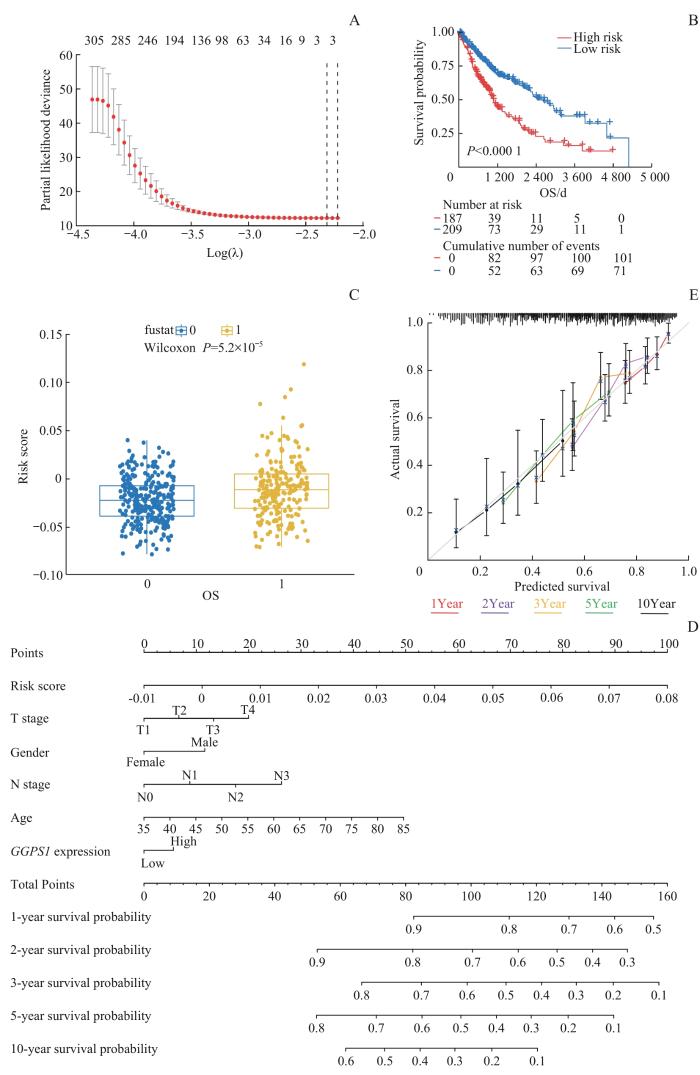
通过LASSO回归分析筛选出细胞周期调定点激酶(cell cycle chekpoint kinase 2,CHEK2)、骨髓分化相关标记(myeloid-associated differentiation marker,MYADM)和三基序蛋白58(tripartite motif protein 58,TRIM58)这3个候选基因以及相应的λ值来计算每个患者的风险评分,将评分中位数0.02786设定为截断值分为高风险组与低风险组(图6A)。之后分析高、低风险组的LUSC患者OS之间的相关性,Kaplan-Meier曲线结果(图6B)表明高风险组LUSC患者OS短于低风险组。为评估LASSO风险预测效能,绘制箱线图结果(图6C)显示:LUSC死亡患者的评分显著高于非死亡患者得分,差异具有统计学意义。以上结果提示基于LASSO回归评估LUSC患者有较好的风险预测效能。
图6
图6
LUSC 患者LASSO回归分析与列线图的建立
Note: A. Screening of candidate genes by LASSO-COX analysis. B. Relationship between risk score and overall survival. C. Effect of risk scores on survival outcomes. D. A nomogram for assessing the survival probability of 1-,2-, 3-,5- and 10-year for LUSC. E. Calibration curve of the prognostic risk model.
Fig 6
LASSO regression analyses and establishment of nomogram for LUSC patients
2.1.5 GGPS1的PPI网络分析
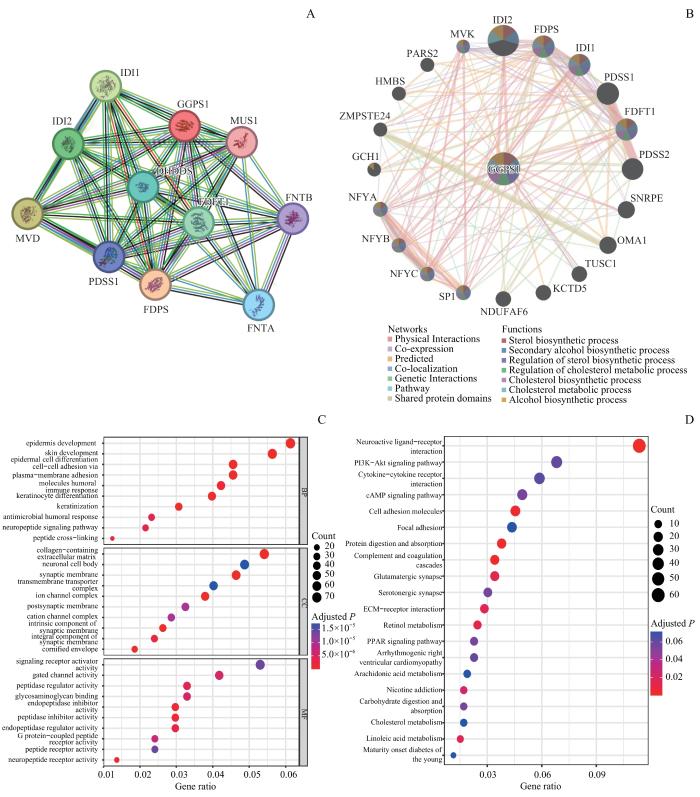
使用STRING数据库探究GGPS1相关蛋白的关系,局部聚类系数为0.931,蛋白网络共有节点数11个,相互作用关系50个,相互作用蛋白网络富集明显(图7A,P=0.000)。筛选出与GGPS1蛋白密切相关的10个基因。使用GeneMANIA数据库发现与GGPS1相关的20个基因。如图7B所示,与GGPS1关系最密切的异戊烯基焦磷酸异构酶2(isopentenyl diphosphate isomerase,IDI2)、IDI1、法尼基焦磷酸合酶(farnesyl diphosphate synthase,FDPS)和法尼基二磷酸法尼基转移酶1(farnesyl-diphosphate farnesyltransferase 1,FDFT1)等基因与胆固醇合成有关。
图7
图7
GGPS1的PPI网络及GO、KEGG分析
Note: A/B. PPI network analysis of STRING database (A) and GeneMANIA database (B). C/D. Bubble plot of GO enrichment analysis (C) and KEGG enrichment analysis (D). MVD—mevalonate diphosphate decarboxylase; DHDDS—dehydrodolichyl diphosphate synthase subunit; PDSS1—decaprenyl diphosphate dynthase subunit 1; FNTA—farnesyltransferase, CAAX box, α; FNTB—farnesyltransferase, CAAX box, β; MVK—mevalonate kinase; PARS2—prolyl-TRNA synthetase 2, mitochondrial; SNRPE—small nuclear ribonucleoprotein polypeptide E; OMA1—OMA1 zinc metallopeptidase; HMBS—hydroxymethylbilane synthase; ZMPSTE24—zinc metallopeptidase STE24; GCH1—GTP cyclohydrolase 1; NFYA—nuclear transcription factor Y subunit α; NFYB—nuclear transcription factor Y subunit β; NFYC—nuclear transcription factor Y subunit γ; SP1—Sp1 transcription factor; NDUFAF6—NADH:ubiquinone oxidoreductase complex assembly factor 6; KCTD5—potassium channel tetramerization domain containing 5; TUSC1—tumor suppressor candidate 1.
Fig 7
PPI network and GO and KEGG analysis of GGPS1
2.1.6 GO、KEGG分析
为了探索与GGPS1表达相关的潜在生物学功能,进行了GO、KEGG功能富集分析。GO分析(图7C)显示GGPS1相关基因在生物过程(biological process,BP)主要集中在表皮发育、表皮细胞分化细胞黏附、质膜黏附和分子体液免疫应答等过程;在分子功能(molecular function,MF)方面,主要富集于神经元细胞体、突触、跨膜转运复合体和离子通道复合体等功能;在细胞组分(cellular component,CC)中主要集中在G蛋白-偶联肽受体活性、门控通道活性和糖胺聚糖结合等。KEGG分析结果(图7D)显示,GGPS1相关基因主要参与磷脂酰肌醇3激酶(phosphatidylinositol3-kinase,PI3K)/蛋白激酶B(protein kinase B,AKT)/哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin,mTOR)信号通路、蛋白质代谢、过氧化物酶体增殖物激活受体(peroxisome proliferator-activated receptors,PPAR)信号通路、脂代谢调节、胆固醇代谢、尼古丁成瘾等。
2.2 LUSC组织中GGPS1免疫组织化学分析
2.2.1 LUSC患者基线资料
最终纳入90例LUSC患者,其中男84例,女6例;年龄36~78岁[(63.92±8.73)岁],≤64岁者49例,>64岁者41例;有吸烟史41例,无吸烟史49例;病变位于左肺者40例,右肺者50例;肿瘤大小≤3.0 cm者26例,>3.0 cm者64例;淋巴结有转移44例,淋巴结无转移46例;Ⅰ~Ⅱ期47例,Ⅲ~Ⅳ期43例。
2.2.2 LUSC组织中GGPS1的表达情况
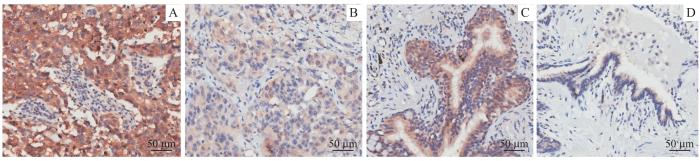
图8
图8
LUSC及癌旁组织中GGPS1免疫组化染色(×200)
Note: A/B. High expression (A) and low expression (B) of GGPS1 in cancer tissues. C/D. High expression (A) and low expression (B) of GGPS1 in adjacent tissues.
Fig 8
Immunohistochemical staining of GGPS1 in LUSC and adjacent tissues (×200)
表1 GGPS1在中LUSC组织与癌旁正常肺组织表达差异
Tab 1
| Expression | LUSC (n=90) | Normal (n=90) | χ2 | P value |
|---|---|---|---|---|
| GGPS1/n(%) | 8.193 | 0.004 | ||
| Low | 42 (46.7) | 61 (67.8) | ||
| High | 48 (53.3) | 29 (32.2) |
2.2.3 GGPS1表达与LUSC临床病理特征的关系
GGPS1表达与LUSC的肿瘤大小、淋巴结转移及TNM分期(均P<0.05)相关,而与性别、年龄、吸烟史、肿瘤位置无关(均P>0.05),提示GGPS1可能参与LUSC的发生、发展过程(表2)。
表2 GGPS1表达水平与LUSC临床病理特征的相关性
Tab 2
| Characteristic | Total/n | GGPS1/n(%) | χ2 value | P value | |
|---|---|---|---|---|---|
| Low expression | High expression | ||||
| Gender | 1.213 | 0.271 | |||
| Male | 84 | 41 (48.8) | 43 (51.2) | ||
| Female | 6 | 1 (16.7) | 5 (83.3) | ||
| Age | 0.003 | 0.955 | |||
| ≤64 years old | 49 | 23 (46.9) | 26 (53.1) | ||
| >64 years old | 41 | 19 (46.3) | 22 (53.7) | ||
| History of smoking | 2.691 | 0.101 | |||
| No | 41 | 23 (56.1) | 18 (43.9) | ||
| Yes | 49 | 19 (38.8) | 30 (61.2) | ||
| Tumor location | 0.984 | 0.321 | |||
| Left | 40 | 21 (52.5) | 19 (47.5) | ||
| Right | 50 | 21 (42.0) | 29 (58.0) | ||
| Tumor size | 10.247 | 0.001 | |||
| ≤3 cm | 26 | 19 (73.1) | 7 (26.9) | ||
| >3 cm | 64 | 23 (35.9) | 41 (64.1) | ||
| Lymph node metastasis | 7.626 | 0.006 | |||
| No | 46 | 28 (60.9) | 18 (39.1) | ||
| Yes | 44 | 14 (31.8) | 30 (68.2) | ||
| TNM stage | 8.935 | 0.003 | |||
| Ⅰ~Ⅱ | 47 | 29 (61.7) | 18 (38.3) | ||
| Ⅲ~Ⅳ | 43 | 13 (30.2) | 30 (69.8) | ||
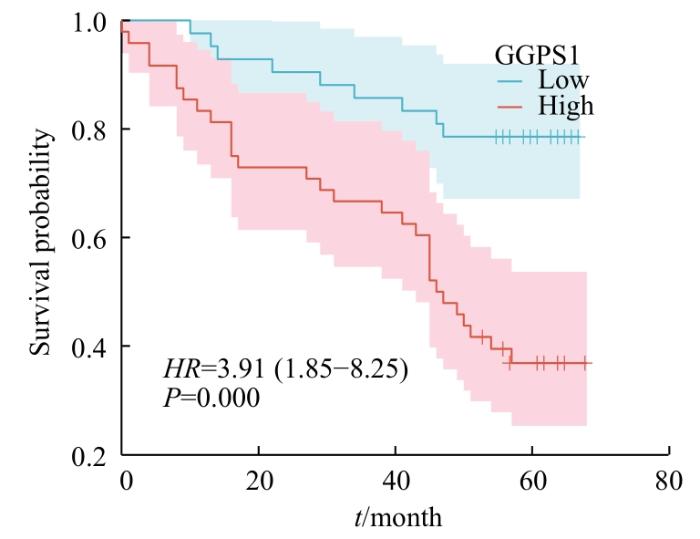
2.2.4 GGPS1表达与LUSC患者预后的关系
随访5年,90例LUSC患者中39例死亡,分析LUSC患者
GGPS1表达水平与预后的相关性,Kaplan-Meier分析结果如图9所示,GGPS1蛋白阳性表达LUSC患者5年生存期(37.5%,18/48)低于GGPS1蛋白阴性表达患者(78.6%,33/42),且差异具有统计学意义(χ2 =14.779,P=0.000)。
图9
图9
GGPS1不同表达水平LUSC患者OS的生存曲线
Fig 9
Survival curves of OS in LUSC patients with different GGPS1 expression levels
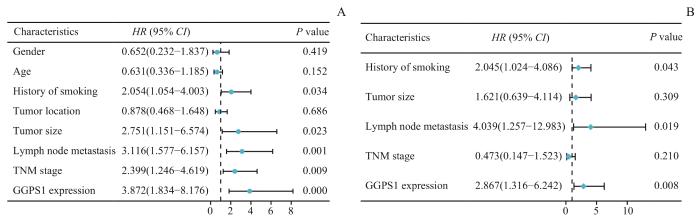
2.2.5 LUSC患者总生存期的影响因素分析
表3 影响LUSC患者OS的Cox回归模型
Tab 3
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Gender | 0.652 | 0.232‒1.837 | 0.419 | |||
| Age | 0.631 | 0.336‒1.185 | 0.152 | |||
| History of smoking | 2.054 | 1.054‒4.003 | 0.034 | 2.045 | 1.024‒4.086 | 0.043 |
| Tumor location | 0.878 | 0.468‒1.648 | 0.686 | |||
| Tumor size | 2.751 | 1.151‒6.574 | 0.023 | 1.621 | 0.639‒4.114 | 0.309 |
| Lymph node metastasis | 3.116 | 1.577‒6.157 | 0.001 | 4.039 | 1.257‒12.983 | 0.019 |
| TNM stage | 2.399 | 1.246‒4.619 | 0.009 | 0.473 | 0.147‒1.523 | 0.210 |
| GGPS1 expression | 3.872 | 1.834‒8.176 | 0.000 | 2.867 | 1.316‒6.242 | 0.008 |
图10
图10
Cox回归分析森林图
Fig 10
Forest plot of Cox regression analysis
3 讨论
肺癌是目前最主要的癌症死亡原因,每年导致的死亡人数超过胰腺癌、前列腺癌、乳腺癌和结直肠癌的总和[12],因此,急需寻找新的生物标志物。GGPS1作为甲羟戊酸途径中的重要的分支酶,催化法尼基焦磷酸(farnesyl diphosphate,FPP)合成香叶基香叶基焦磷酸(geranylgeranyl diphosphate,GGPP)。通过FTase或GGTaseⅠ/Ⅱ,FPP、GGPP可以转移到含有CaaX基序的蛋白质上,发生蛋白质异戊二烯化——法尼基化和香叶基香叶基化[13];它是脂质修饰的一种,是小GTP酶(如Ras和Rho)的膜定位和激活所必需的。GGPS1的异常表达和活性改变将影响FPP和GGPP的相对含量,从而破坏蛋白质法尼基化和香叶基香叶基化的平衡,进而参与多种疾病的调控。
最近KAIYRZHANO等[14]发现GGPS1双等位基因变异具致病作用,GGPS1基因突变与先天性听力损失和原发性卵巢功能不全相关的肌营养不良症有关。有研究[15]表明,GGPS1在肝硬化诱导的肝细胞癌的发生发展中起重要作用,对预测肝细胞癌的生物学特征具有临床意义。GGPS1可以作为肾脏血管平滑肌脂肪瘤[16]、前列腺癌[17]及口腔鳞状细胞癌[18]的治疗靶点。此外,对于GGPS1抑制剂研究显示:抑制GGPS1能够阻断蛋白的运输,诱导未折叠蛋白反应和细胞凋亡,从而导致胰腺导管腺癌[19]、多发性骨髓瘤[20]、骨肉瘤和尤文肉瘤[21]等肿瘤细胞死亡;抑制GGPS1还可以减缓前列腺癌及乳腺癌的转移能力,导致全身肿瘤负荷减少[6]。但LUSC中GGPS1的表达水平及临床意义未见报道。
本研究运用TIMER2.0、UCSC Xena和UALCAN数据库分析LUSC中GGPS1表达情况,发现GGPS1在多种肿瘤中呈高表达,在LUSC中表达也高于正常肺组织(P<0.05)。通过UALCAN数据库分析发现GGPS1的表达与肿瘤分期和N分期有关(P<0.05),LinkedOmics数据库研究发现GGPS1的表达与LUSC患者T分期相关(P=0.029)。运用Kaplan-Meier Plotter数据库发现LUSC患者GGPS1高表达与预后不良有关。
进一步采用免疫组织化学法检测GGPS1在LUSC及正常肺组织中的表达情况,结果发现,GGPS1蛋白在LUSC组织中的表达要显著高于癌旁组织(P<0.05),这一结果与数据库结论相符。同时,先前发现GGPS1在肺、肝、肾、前列腺及口腔肿瘤中表达显著升高。这些数据表明GGPS1的表达水平可能与肿瘤的发展有关。此外,我们研究结果显示GGPS1的高表达与肿瘤大小、淋巴结转移、TNM分期相关,尤其在肿瘤体积较大、淋巴结转移阳性及晚期LUSC患者中表达水平升高更明显。多因素COX回归显示吸烟史、淋巴结转移和GGPS1表达可以作为LUSC的独立预后因素。这与WANG等[22]在LUAD中研究相吻合,其结果显示,GGPS1在LUAD中呈高表达,且GGPS1的过表达有助于肿瘤转移并与LUAD的不良预后相关,提示GGPS1可能作为LUAD诊断标志物与治疗靶点。以上提示GGPS1表达水平升高与LUSC的发生、发展密切相关,GGPS1高表达可能促进LUSC的侵袭和转移,并影响着患者的预后。
此外,采用LASSO-COX回归分析筛选最具代表性的基因标记,从而确定了LUSC的3个基因标记。然后,构建了基于LUSC患者OS的列线图,用于预测LUSC患者生存概率。纳入风险评分、年龄、性别、T分期、N分期和GGPS1表达的列线图成功识别了预后不良的高风险患者。进一步通过构建GGPS1相关基因的PPI网络,结果显示与胆固醇合成有关的基因如IDI2、IDI1、FDPS和FDFT1与GGPS1关系最为密切。GO分析表明,GGPS1与分子体液免疫应答、细胞电生理活动、G蛋白-偶联肽受体活性和门控通道活性等细胞生物过程有关。KEGG分析显示GGPS1具有参与蛋白质代谢,调节脂质、胆固醇代谢过程,尼古丁成瘾等功能,且与PI3K/AKT/mTOR、PPAR信号通路相关。脂质代谢作为癌症中最显著的代谢改变,在促进癌症进展的代谢需求之外发挥着独特的作用[23-24]。肺癌伴随着血管结构异常,限制了肺癌细胞的能量供应,并创造了一个低氧环境,诱导脂质代谢的改变,以支持肺肿瘤细胞的生长[25]。ERSHOV等[26]研究发现胆固醇合成途径中的酶会导致病理性胆固醇累积,这可能是癌症的危险因素。吸烟是目前公认的引起肺癌的主要危险因素之一,香烟中的尼古丁暴露会导致内分泌抵抗途径进一步加重,从而诱发内分泌失调导致LUSC的进展[27]。SHEN等[28]先前研究发现早期生长反应基因-1(earlygrowthresponsegene-1,Egr-1)/GGPS1/Ras通路与吸烟诱导的肺部疾病有关,在小鼠正常肺组织和NSCLC细胞给予烟雾刺激后激活GGPS1转录,从而影响NSCLC的发生与发展。此外,有研究发现mTOR的激活可诱导GGPS1的积累,通过抑制GGPS1可导致PI3K/AKT/mTOR通路被抑制,从而通过自噬诱导TSC2-null细胞凋亡[15]。因此,推测GGPS1在LUSC中可能通过PI3K/AKT/mTOR信号通路发挥其癌基因作用。PPAR能够调节脂质代谢,PPAR信号通路在癌症中发挥多效性功能[29]。因此,GGPS1作为作为甲羟戊酸途径中的反式异戊二烯转移酶,可能通过影响多种代谢途径而影响肿瘤的发生与发展,通过与其关联的蛋白组成信号网络而发挥促癌的作用。
综上所述,本研究通过生信分析和免疫组织化学实验验证发现,在LUSC中GGPS1表达上调,其高表达促进LUSC的转移和增殖,是LUSC患者的独立危险因素,提示GGPS1有望成为LUSC的生物标志物和潜在的治疗靶点。功能富集分析显示GGPS1可能通过多种功能及信号通路参与LUSC的发生与发展,为后续的研究方向提供了参考。
作者贡献声明
王鑫、王晓霞、许天祥参与了实验设计;王鑫负责撰写论文;王鑫、李彦庆、郑永鑫、乌杰、任猛参与了论文修改;王晓霞、贾向东、许天祥参与了论文审阅。所有作者均阅读并同意了最终稿件的提交。
AUTHOR's CONTRIBUTIONS
The study was designed by WANG Xin, WANG Xiaoxia and XU Tianxiang. The manuscript was drafted by WANG Xin and was revised by WANG Xin, LI Yanqing, ZHENG Yongxin, WU Jie and REN Meng. The manuscript was reviewed by WANG Xiaoxia, JIA Xiangdong and XU Tianxiang. All the authors have read the last version of paper and consented for submission.
利益冲突声明
所有作者声明不存在利益冲突。
COMPETING INTERESTS
All authors disclose no relevant conflict of interests.
参考文献