
上海交通大学学报(医学版) ›› 2025, Vol. 45 ›› Issue (4): 434-442.doi: 10.3969/j.issn.1674-8115.2025.04.005
王思睿1,2, 孔盖2, 李惠2, 钱禛颖2, 崔慧茹2, 唐莺莹1,2( )
)
收稿日期:2024-12-10
接受日期:2024-12-31
出版日期:2025-04-28
发布日期:2025-04-28
通讯作者:
唐莺莹,研究员,博士;电子信箱:yytang@smhc.org.cn。作者简介:王思睿(1999—),女,硕士生;电子信箱:wangsr0305@163.com。
基金资助:
WANG Sirui1,2, KONG Gai2, LI Hui2, QIAN Zhenying2, CUI Huiru2, TANG Yingying1,2( )
)
Received:2024-12-10
Accepted:2024-12-31
Online:2025-04-28
Published:2025-04-28
Contact:
TANG Yingying, E-mail: yytang@smhc.org.cn.Supported by:摘要:
目的·比较抑郁症患者接受经颅磁刺激(transcranial magnetic stimulation,TMS)治疗前后杏仁核及海马亚区体积的纵向变化,并探讨其与TMS抗抑郁与抗焦虑疗效的相关性。方法·纳入2018年1月—2023年8月于上海交通大学医学院附属精神卫生中心门诊就诊的抑郁症患者58例,使用汉密尔顿抑郁量表(Hamilton Depression Scale,HAMD)、蒙哥马利-阿斯伯格抑郁量表(Montgomery-Asberg Depression Rating Scale,MADRS)和汉密尔顿焦虑量表(Hamilton Anxiety Scale,HAMA)评估患者基线及TMS治疗后的临床抑郁和焦虑症状。所有被试在基线磁共振扫描之后接受干预左侧背外侧前额叶(dorsolateral prefrontal cortex,DLPFC)的TMS治疗,频率10 Hz,共20次。治疗结束当日进行1次磁共振随访。利用FreeSurfer v6.0.0软件对采集到的杏仁核及海马图谱进行亚区分割并计算各亚区体积,使用双因素方差分析对亚区体积进行纵向比较。控制年龄、性别和颅内容积,对抑郁症患者杏仁核和海马亚区体积与基线临床量表(HAMD、MADRS和HAMA)得分进行偏相关分析,并分析体积变化显著的的脑区中体积变化率与TMS干预前后临床量表(HAMD、MADRS和HAMA)减分的相关性。结果·经过TMS治疗后,抑郁症患者右侧杏仁核中央核的体积增大,差异具有统计学意义(t=-2.441,P=0.018);海马亚区除双侧海马伞体积下降外,部分海马亚区及总海马体积均有所增加(P<0.05)。基线时,未发现患者杏仁核及海马亚区体积与抑郁和焦虑症状存在显著相关性;仅在TMS治疗有效的患者中,发现左侧海马尾部的体积变化率与临床焦虑症状减分呈正相关(HAMA:r=0.334,P=0.044)。结论·TMS高频干预左侧DLPFC可能引起右侧杏仁核中央核及双侧部分海马亚区体积增大,并且TMS治疗有效者的左侧海马尾部体积变化率与TMS抗焦虑疗效之间存在联系,提示TMS高频干预左侧DLPFC可能引起右侧杏仁核中央核及双侧部分海马亚区的神经可塑性改变。
中图分类号:
王思睿, 孔盖, 李惠, 钱禛颖, 崔慧茹, 唐莺莹. 经颅磁刺激治疗对抑郁症患者杏仁核及海马亚区体积的影响[J]. 上海交通大学学报(医学版), 2025, 45(4): 434-442.
WANG Sirui, KONG Gai, LI Hui, QIAN Zhenying, CUI Huiru, TANG Yingying. Impact of transcranial magnetic stimulation therapy on the volumes of amygdala and hippocampal subfields in patients with major depressive disorder[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2025, 45(4): 434-442.
| Clinical scale | Before TMS | After TMS | T value | P value |
|---|---|---|---|---|
| HAMD-24 | 27 (12, 40) | 10 (0, 27) | 3.0 | <0.001 |
| MADRS | 27 (16, 36) | 7 (0, 26) | 1.5 | <0.001 |
| HAMA | 19 (9, 39) | 10 (0, 27) | 28.0 | <0.001 |
表1 被试TMS治疗前后临床抑郁和焦虑量表得分变化
Tab 1 Clinical assessment scores of depressive and anxiety symptoms before and after TMS treatment
| Clinical scale | Before TMS | After TMS | T value | P value |
|---|---|---|---|---|
| HAMD-24 | 27 (12, 40) | 10 (0, 27) | 3.0 | <0.001 |
| MADRS | 27 (16, 36) | 7 (0, 26) | 1.5 | <0.001 |
| HAMA | 19 (9, 39) | 10 (0, 27) | 28.0 | <0.001 |
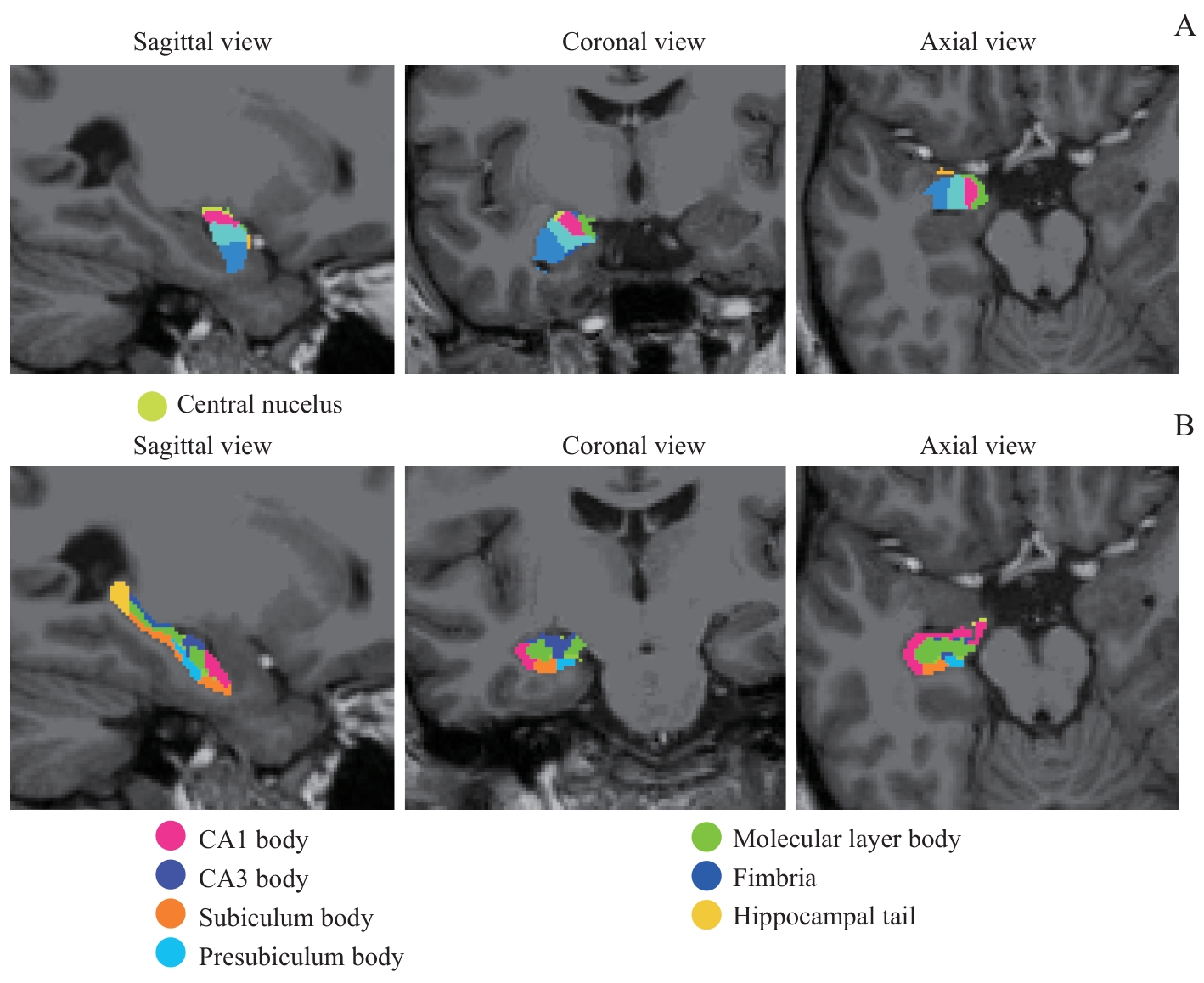
图1 右侧杏仁核、海马亚区分割示意图Note: A. Subfields of the right amygdala. B. Subfields of the right hippocampus.
Fig 1 Illustrations of the segmentation of the right amygdala and hippocampal subfields
| Subfield | Time | Laterality | Time × Laterality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F value | P value | η2 | F value | P value | η2 | F value | P value | η2 | |
| La | 0.897 | 0.347 | 0.015 | 18.151 | <0.001 | 0.242 | 5.533 | 0.022 | 0.088 |
| Ba | 0.352 | 0.556 | 0.006 | 59.562 | <0.001 | 0.511 | 3.425 | 0.069 | 0.057 |
| AB | 0.075 | 0.785 | 0.001 | 108.26 | <0.001 | 0.655 | 2.702 | 0.106 | 0.045 |
| AAA | 3.796 | 0.056 | 0.062 | 89.549 | <0.001 | 0.611 | 1.767 | 0.189 | 0.030 |
| Ce | 4.204 | 0.045 | 0.069 | 44.796 | <0.001 | 0.440 | 2.440 | 0.124 | 0.041 |
| Me | 2.307 | 0.134 | 0.390 | 29.001 | <0.001 | 0.337 | 3.143 | 0.082 | 0.052 |
| Co | 0.242 | 0.625 | 0.004 | 102.340 | <0.001 | 0.642 | 0.914 | 0.343 | 0.016 |
| CAT | 0.410 | 0.525 | 0.007 | 59.627 | <0.001 | 0.511 | 0.281 | 0.598 | 0.005 |
| PL | 0.446 | 0.507 | 0.008 | 6.263 | 0.015 | 0.099 | 0.030 | 0.863 | 0.001 |
| Whole amygdala | 0.170 | 0.681 | 0.003 | 83.962 | <0.001 | 0.596 | 4.825 | 0.032 | 0.078 |
表2 TMS干预前后双侧杏仁核亚区体积双因素方差分析
Tab 2 Two-way ANOVA of bilateral amygdala subfield volumes before and after TMS treatment
| Subfield | Time | Laterality | Time × Laterality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F value | P value | η2 | F value | P value | η2 | F value | P value | η2 | |
| La | 0.897 | 0.347 | 0.015 | 18.151 | <0.001 | 0.242 | 5.533 | 0.022 | 0.088 |
| Ba | 0.352 | 0.556 | 0.006 | 59.562 | <0.001 | 0.511 | 3.425 | 0.069 | 0.057 |
| AB | 0.075 | 0.785 | 0.001 | 108.26 | <0.001 | 0.655 | 2.702 | 0.106 | 0.045 |
| AAA | 3.796 | 0.056 | 0.062 | 89.549 | <0.001 | 0.611 | 1.767 | 0.189 | 0.030 |
| Ce | 4.204 | 0.045 | 0.069 | 44.796 | <0.001 | 0.440 | 2.440 | 0.124 | 0.041 |
| Me | 2.307 | 0.134 | 0.390 | 29.001 | <0.001 | 0.337 | 3.143 | 0.082 | 0.052 |
| Co | 0.242 | 0.625 | 0.004 | 102.340 | <0.001 | 0.642 | 0.914 | 0.343 | 0.016 |
| CAT | 0.410 | 0.525 | 0.007 | 59.627 | <0.001 | 0.511 | 0.281 | 0.598 | 0.005 |
| PL | 0.446 | 0.507 | 0.008 | 6.263 | 0.015 | 0.099 | 0.030 | 0.863 | 0.001 |
| Whole amygdala | 0.170 | 0.681 | 0.003 | 83.962 | <0.001 | 0.596 | 4.825 | 0.032 | 0.078 |
| Subfield | Time | Laterality | Time × Laterality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F value | P value | η2 | F value | P value | η2 | F value | P value | η2 | |
| CA1-body | 3.910 | 0.053 | 0.064 | 67.705 | <0.001 | 0.543 | 4.673 | 0.035 | 0.067 |
| CA1-head | 0.148 | 0.702 | 0.003 | 86.886 | <0.001 | 0.604 | 1.217 | 0.275 | 0.021 |
| CA3-body | 12.769 | <0.001 | 0.183 | 82.281 | <0.001 | 0.591 | 7.449 | 0.008 | 0.166 |
| CA3-head | 0.261 | 0.611 | 0.005 | 21.449 | <0.001 | 0.273 | 1.851 | 0.179 | 0.031 |
| CA4-body | 6.293 | 0.015 | 0.099 | 1.844 | 0.180 | 0.031 | 0.002 | 0.968 | <0.001 |
| CA4-head | 0.026 | 0.872 | 0.0005 | 20.487 | <0.001 | 0.264 | 3.107 | 0.083 | 0.052 |
| Fimbria | 15.254 | <0.001 | 0.211 | 7.824 | 0.007 | 0.121 | 1.837 | 0.181 | 0.031 |
| Fissure | 0.874 | 0.354 | 0.015 | 1.970 | 0.166 | 0.033 | 0.247 | 0.621 | 0.004 |
| GC-DG-body | 6.720 | 0.012 | 0.105 | 1.966 | 0.166 | 0.033 | 0.125 | 0.725 | 0.002 |
| GC-DG-head | 0.001 | 0.973 | <0.001 | 25.200 | <0.001 | 0.307 | 2.654 | 0.109 | 0.044 |
| HATA | 0.370 | 0.546 | 0.006 | 6.398 | 0.014 | 0.101 | 0.684 | 0.412 | 0.012 |
| Hippocampus tail | 26.765 | <0.001 | 0.320 | 65.029 | <0.001 | 0.533 | 0.267 | 0.608 | 0.005 |
| ML-body | 20.469 | <0.001 | 0.264 | 44.278 | <0.001 | 0.437 | 7.873 | 0.007 | 0.121 |
| ML-head | 0.007 | 0.936 | <0.001 | 34.724 | <0.001 | 0.379 | 2.142 | 0.149 | 0.036 |
| Parasubiculum | 0.064 | 0.801 | 0.001 | 13.377 | <0.001 | 0.190 | 0.279 | 0.599 | 0.005 |
| Presubiculum-body | 6.277 | 0.015 | 0.099 | 18.069 | <0.001 | 0.241 | 1.514 | 0.224 | 0.026 |
| Presubiculum-head | 3.017 | 0.083 | 0.052 | 15.834 | <0.001 | 0.217 | 0.637 | 0.428 | 0.011 |
| SUB-body | 17.011 | <0.001 | 0.230 | 0.010 | 0.921 | <0.001 | 1.019 | 0.317 | 0.018 |
| SUB-head | 0.278 | 0.600 | 0.005 | 1.118 | 0.295 | 0.019 | 0.166 | 0.685 | 0.003 |
| Whole hippocampus | 10.728 | 0.002 | 0.158 | 65.042 | <0.001 | 0.533 | 2.941 | 0.092 | 0.049 |
| Whole hippocampus-body | 14.750 | <0.001 | 0.206 | 12.169 | <0.001 | 0.176 | 3.168 | 0.080 | 0.053 |
| Whole hippocampus-head | 0.034 | 0.855 | 0.001 | 33.231 | <0.001 | 0.368 | 1.459 | 0.232 | 0.025 |
表3 TMS干预前后双侧海马亚区体积双因素方差分析
Tab 3 Two-way ANOVA of bilateral hippocampal subfield volumes before and after TMS treatment
| Subfield | Time | Laterality | Time × Laterality | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F value | P value | η2 | F value | P value | η2 | F value | P value | η2 | |
| CA1-body | 3.910 | 0.053 | 0.064 | 67.705 | <0.001 | 0.543 | 4.673 | 0.035 | 0.067 |
| CA1-head | 0.148 | 0.702 | 0.003 | 86.886 | <0.001 | 0.604 | 1.217 | 0.275 | 0.021 |
| CA3-body | 12.769 | <0.001 | 0.183 | 82.281 | <0.001 | 0.591 | 7.449 | 0.008 | 0.166 |
| CA3-head | 0.261 | 0.611 | 0.005 | 21.449 | <0.001 | 0.273 | 1.851 | 0.179 | 0.031 |
| CA4-body | 6.293 | 0.015 | 0.099 | 1.844 | 0.180 | 0.031 | 0.002 | 0.968 | <0.001 |
| CA4-head | 0.026 | 0.872 | 0.0005 | 20.487 | <0.001 | 0.264 | 3.107 | 0.083 | 0.052 |
| Fimbria | 15.254 | <0.001 | 0.211 | 7.824 | 0.007 | 0.121 | 1.837 | 0.181 | 0.031 |
| Fissure | 0.874 | 0.354 | 0.015 | 1.970 | 0.166 | 0.033 | 0.247 | 0.621 | 0.004 |
| GC-DG-body | 6.720 | 0.012 | 0.105 | 1.966 | 0.166 | 0.033 | 0.125 | 0.725 | 0.002 |
| GC-DG-head | 0.001 | 0.973 | <0.001 | 25.200 | <0.001 | 0.307 | 2.654 | 0.109 | 0.044 |
| HATA | 0.370 | 0.546 | 0.006 | 6.398 | 0.014 | 0.101 | 0.684 | 0.412 | 0.012 |
| Hippocampus tail | 26.765 | <0.001 | 0.320 | 65.029 | <0.001 | 0.533 | 0.267 | 0.608 | 0.005 |
| ML-body | 20.469 | <0.001 | 0.264 | 44.278 | <0.001 | 0.437 | 7.873 | 0.007 | 0.121 |
| ML-head | 0.007 | 0.936 | <0.001 | 34.724 | <0.001 | 0.379 | 2.142 | 0.149 | 0.036 |
| Parasubiculum | 0.064 | 0.801 | 0.001 | 13.377 | <0.001 | 0.190 | 0.279 | 0.599 | 0.005 |
| Presubiculum-body | 6.277 | 0.015 | 0.099 | 18.069 | <0.001 | 0.241 | 1.514 | 0.224 | 0.026 |
| Presubiculum-head | 3.017 | 0.083 | 0.052 | 15.834 | <0.001 | 0.217 | 0.637 | 0.428 | 0.011 |
| SUB-body | 17.011 | <0.001 | 0.230 | 0.010 | 0.921 | <0.001 | 1.019 | 0.317 | 0.018 |
| SUB-head | 0.278 | 0.600 | 0.005 | 1.118 | 0.295 | 0.019 | 0.166 | 0.685 | 0.003 |
| Whole hippocampus | 10.728 | 0.002 | 0.158 | 65.042 | <0.001 | 0.533 | 2.941 | 0.092 | 0.049 |
| Whole hippocampus-body | 14.750 | <0.001 | 0.206 | 12.169 | <0.001 | 0.176 | 3.168 | 0.080 | 0.053 |
| Whole hippocampus-head | 0.034 | 0.855 | 0.001 | 33.231 | <0.001 | 0.368 | 1.459 | 0.232 | 0.025 |
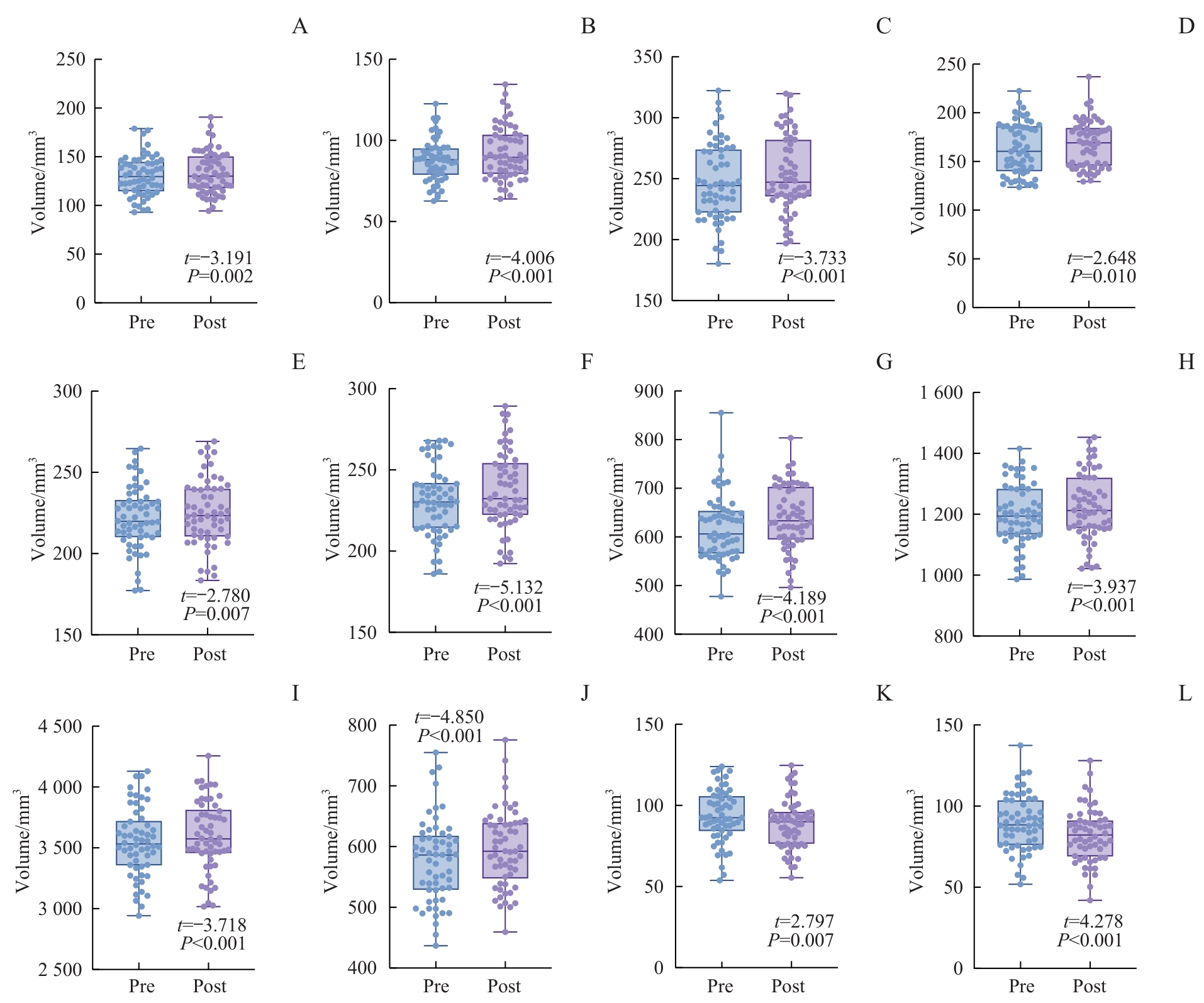
图3 TMS干预前后双侧海马亚区体积比较Note: A. Right CA1 body. B. Right CA3 body. C. Right subiculum body. D. Right presubiculum body. E. Left molecular layer body. F. Right molecular layer body. G. Right hippocampal tail. H. Right hippocampal body. I. Right total hippocampus. J. Left hippocampal tail. K/L. Bilateral hippocampal fimbria (K. Left. L. Right).
Fig 3 Comparison of volumes of bilateral hippocampal subfields before and after TMS treatment
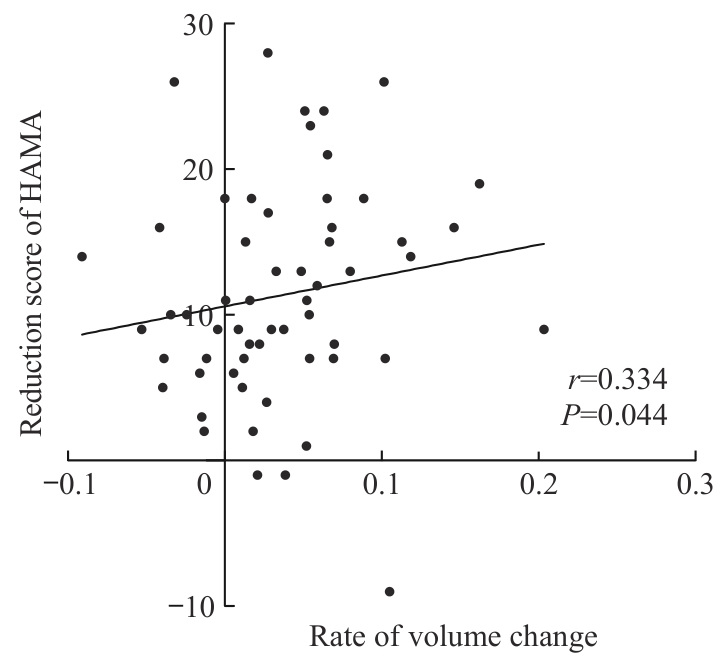
图4 TMS干预前后左侧海马尾体积变化率与焦虑症状减分在TMS治疗有效者中的相关性
Fig 4 Correlation between the rate of left hippocampal tail volume change and reduction scores in anxiety symptoms in TMS responders
| 1 | LU J, XU X F, HUANG Y Q, et al. Prevalence of depressive disorders and treatment in China: a cross-sectional epidemiological study[J]. Lancet Psychiatry, 2021, 8(11): 981-990. |
| 2 | JACOB Y, MORRIS L S, VERMA G, et al. Altered hippocampus and amygdala subregion connectome hierarchy in major depressive disorder[J]. Transl Psychiatry, 2022, 12(1): 209. |
| 3 | PLOSKI J E, VAIDYA V A. The neurocircuitry of posttraumatic stress disorder and major depression: insights into overlapping and distinct circuit dysfunction-a tribute to ron duman[J]. Biol Psychiatry, 2021, 90(2): 109-117. |
| 4 | MCKINNON M C, YUCEL K, NAZAROV A, et al. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder[J]. J Psychiatry Neurosci, 2009, 34(1): 41-54. |
| 5 | SCHMAAL L, POZZI E, HO T C, et al. ENIGMA MDD: seven years of global neuroimaging studies of major depression through worldwide data sharing[J]. Transl Psychiatry, 2020, 10(1): 172. |
| 6 | FRODL T, MEISENZAHL E, ZETZSCHE T, et al. Enlargement of the amygdala in patients with a first episode of major depression[J]. Biol Psychiatry, 2002, 51(9): 708-714. |
| 7 | KRONENBERG G, TEBARTZ VAN ELST L, REGEN F, et al. Reduced amygdala volume in newly admitted psychiatric in-patients with unipolar major depression[J]. J Psychiatr Res, 2009, 43(13): 1112-1117. |
| 8 | KIM H, HAN K M, CHOI K W, et al. Volumetric alterations in subregions of the amygdala in adults with major depressive disorder[J]. J Affect Disord, 2021, 295: 108-115. |
| 9 | CONG E Z, LI Q F, CHEN H Y, et al. Association between the volume of subregions of the amygdala and major depression with suicidal thoughts and anxiety in a Chinese cohort[J]. J Affect Disord, 2022, 312: 39-45. |
| 10 | 储召松. 抑郁症患者杏仁核亚区结构磁共振成像研究[D]. 昆明: 昆明医科大学, 2021. |
| CHU Z S. Structural magnetic resonance imaging study of amygdala subregions in patients with major depressive disorder[D]. Kunming: Kunming Medical University, 2021. | |
| 11 | RODDY D, KELLY J R, FARRELL C, et al. Amygdala substructure volumes in major depressive disorder[J]. Neuroimage Clin, 2021, 31: 102781. |
| 12 | ZAVALIANGOS-PETROPULU A, MCCLINTOCK S M, JOSHI S H, et al. Hippocampal subfield volumes in treatment resistant depression and serial ketamine treatment[J]. Front Psychiatry, 2023, 14: 1227879. |
| 13 | WU C C, JIA L L, MU Q L, et al. Altered hippocampal subfield volumes in major depressive disorder with and without anhedonia[J]. BMC Psychiatry, 2023, 23(1): 540. |
| 14 | HAN K M, KIM A, KANG W, et al. Hippocampal subfield volumes in major depressive disorder and bipolar disorder[J]. Eur Psychiatry, 2019, 57: 70-77. |
| 15 | RODDY D W, FARRELL C, DOOLIN K, et al. The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression[J]. Biol Psychiatry, 2019, 85(6): 487-497. |
| 16 | LEFAUCHEUR J P, ALEMAN A, BAEKEN C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014‒2018)[J]. Clin Neurophysiol, 2020, 131(2): 474-528. |
| 17 | CASH R F H, ZALESKY A. Personalized and circuit-based transcranial magnetic stimulation: evidence, controversies, and opportunities[J]. Biol Psychiatry, 2024, 95(6): 510-522. |
| 18 | SYDNOR V J, CIESLAK M, DUPRAT R, et al. Cortical-subcortical structural connections support transcranial magnetic stimulation engagement of the amygdala[J]. Sci Adv, 2022, 8(25): eabn5803. |
| 19 | DALHUISEN I, ACKERMANS E, MARTENS L, et al. Longitudinal effects of rTMS on neuroplasticity in chronic treatment-resistant depression[J]. Eur Arch Psychiatry Clin Neurosci, 2021, 271(1): 39-47. |
| 20 | HAYASAKA S, NAKAMURA M, NODA Y, et al. Lateralized hippocampal volume increase following high-frequency left prefrontal repetitive transcranial magnetic stimulation in patients with major depression[J]. Psychiatry Clin Neurosci, 2017, 71(11): 747-758. |
| 21 | SEEWOO B J, RODGER J, DEMITRACK M A, et al. Neurostructural differences in adolescents with treatment-resistant depression and treatment effects of transcranial magnetic stimulation[J]. Int J Neuropsychopharmacol, 2022, 25(8): 619-630. |
| 22 | SAYGIN Z M, KLIEMANN D, IGLESIAS J E, et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas[J]. Neuroimage, 2017, 155: 370-382. |
| 23 | IGLESIAS J E, AUGUSTINACK J C, NGUYEN K, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI[J]. Neuroimage, 2015, 115: 117-137. |
| 24 | KALIN N H, SHELTON S E, DAVIDSON R J. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate[J]. J Neurosci, 2004, 24(24): 5506-5515. |
| 25 | AMARAL D G, PRICE J L, PITKANEN A, et al. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction[M]. New York: Wiley-Liss, 1992: 1-66. |
| 26 | GILPIN N W, HERMAN M A, ROBERTO M. The central amygdala as an integrative hub for anxiety and alcohol use disorders[J]. Biol Psychiatry, 2015, 77(10): 859-869. |
| 27 | TAKAMIYA A, CHUNG J K, LIANG K C, et al. Effect of electroconvulsive therapy on hippocampal and amygdala volumes: systematic review and meta-analysis[J]. Br J Psychiatry, 2018, 212(1): 19-26. |
| 28 | ENNEKING V, LEEHR E J, DANNLOWSKI U, et al. Brain structural effects of treatments for depression and biomarkers of response: a systematic review of neuroimaging studies[J]. Psychol Med, 2020, 50(2): 187-209. |
| 29 | TATU L, VUILLIER F. Structure and vascularization of the human hippocampus[J]. Front Neurol Neurosci, 2014, 34: 18-25. |
| 30 | TESEN H, WATANABE K, OKAMOTO N, et al. Volume of amygdala subregions and clinical manifestations in patients with first-episode, drug-naïve major depression[J]. Front Hum Neurosci, 2022, 15: 780884. |
| 31 | BROWN S G, RUTLAND J W, VERMA G, et al. Structural MRI at 7T reveals amygdala nuclei and hippocampal subfield volumetric association with Major Depressive Disorder symptom severity[J]. Sci Rep, 2019, 9(1): 10166. |
| 32 | CAO B, PASSOS I C, MWANGI B, et al. Hippocampal subfield volumes in mood disorders[J]. Mol Psychiatry, 2017, 22(9): 1352-1358. |
| 33 | XU Y W, CUI D, ZHAO Y, et al. Volumetric alterations of the hippocampal subfields in major depressive disorder with and without suicidal ideation[J]. Behav Brain Res, 2023: 114733. |
| 34 | FRODL T, SCHAUB A, BANAC S, et al. Reduced hippocampal volume correlates with executive dysfunctioning in major depression[J]. J Psychiatry Neurosci, 2006, 31(5): 316-323. |
| 35 | ZHANG Y X, LIU X, YANG Y, et al. Revealing complexity: segmentation of hippocampal subfields in adolescents with major depressive disorder reveals specific links to cognitive dysfunctions[J]. Eur Psychiatry, 2024, 68(1): e5. |
| 36 | GBYL K, VIDEBECH P. Electroconvulsive therapy increases brain volume in major depression: a systematic review and meta-analysis[J]. Acta Psychiatr Scand, 2018, 138(3): 180-195. |
| 37 | MALLER J J, JEFF DASKALAKIS Z, FITZGERALD P B. Hippocampal volumetrics in depression: the importance of the posterior tail[J]. Hippocampus, 2007, 17(11): 1023-1027. |
| 38 | CHU Z S, YUAN L J, LIAN K, et al. Reduced gray matter volume of the hippocampal tail in melancholic depression: evidence from an MRI study[J]. BMC Psychiatry, 2024, 24(1): 183. |
| 39 | MALLER J J, BROADHOUSE K, RUSH A J, et al. Increased hippocampal tail volume predicts depression status and remission to anti-depressant medications in major depression[J]. Mol Psychiatry, 2018, 23(8): 1737-1744. |
| 40 | NOGOVITSYN N, MULLER M, SOUZA R, et al. Hippocampal tail volume as a predictive biomarker of antidepressant treatment outcomes in patients with major depressive disorder: a CAN-BIND report[J]. Neuropsychopharmacology, 2020, 45(2): 283-291. |
| [1] | 王海红, 袁辰馨, 甘鸿, 江海峰, 赵燕, 杜江, 张毅. 间歇性θ短阵快速脉冲刺激对戒断期酒精依赖患者渴求、情绪和认知功能影响的随机对照研究[J]. 上海交通大学学报(医学版), 2025, 45(3): 349-356. |
| [2] | 陈深册, 陈依明, 王凡, 张梦珂, 杨惟杰, 吕洞宾, 洪武. 饮食干预治疗抑郁相关症状的研究进展[J]. 上海交通大学学报(医学版), 2024, 44(8): 1050-1055. |
| [3] | 孙晨寅, 吴百川, 张慧凤, 方贻儒, 彭代辉. 体动记录仪评估抑郁症昼夜节律:一项系统综述和meta分析[J]. 上海交通大学学报(医学版), 2024, 44(5): 606-616. |
| [4] | 杨瑞君, 吕书红, 刘志业, 张新, 刘志浩. 中国5省初中生视屏时间和饮食行为与抑郁症状的关联[J]. 上海交通大学学报(医学版), 2024, 44(3): 358-364. |
| [5] | 胡灿芳, 钟传钰, 曹立. 神经调控技术在帕金森病治疗中的应用研究进展[J]. 上海交通大学学报(医学版), 2024, 44(2): 258-263. |
| [6] | 马文琳, 林元杰, 金婷婷, 石薇, 蒋莉华, 赵莉. 初中生自我中心主义与非自杀性自伤的关系研究[J]. 上海交通大学学报(医学版), 2023, 43(8): 971-976. |
| [7] | 李偲媛, 和申, 李华芳. 重度抑郁症中自噬通路及其关键标志物的研究进展[J]. 上海交通大学学报(医学版), 2023, 43(10): 1324-1331. |
| [8] | 吴侠霏, 方婕, 漆洪波, 余昕烊. 妊娠期糖尿病对C57BL/6J子代成年鼠神经精神功能的影响[J]. 上海交通大学学报(医学版), 2022, 42(4): 422-432. |
| [9] | 李欣, 范青. 机器学习在抑郁症患者面部特征研究中的应用进展[J]. 上海交通大学学报(医学版), 2022, 42(1): 124-129. |
| [10] | 沈梦婷, 张选红, 钱禛颖, 李惠, 盛建华, 王继军, 唐莺莹. 精神分裂症与抑郁症失匹配负波异常的对照研究[J]. 上海交通大学学报(医学版), 2021, 41(8): 1041-1045. |
| [11] | 王毅, 程诚, 沈红艳, 高红艳, 戴悦宁, 易正辉. 经颅磁刺激对阿尔茨海默病患者认知功能及伴痴呆的行为精神症状疗效的meta分析[J]. 上海交通大学学报(医学版), 2021, 41(7): 931-941. |
| [12] | 耿瑞杰, 姚琳, 黄欣欣, 禹顺英, 苑成梅, 洪武, 吕钦谕, 王庆中, 易正辉, 方贻儒. 基于加权基因共表达网络分析识别抑郁症的差异表达基因模块[J]. 上海交通大学学报(医学版), 2021, 41(6): 724-731. |
| [13] | 吴静, 李学义, 陈京红, 王泽剑. 抑郁模型小鼠海马中胆汁酸受体变化的研究[J]. 上海交通大学学报(医学版), 2021, 41(12): 1628-1634. |
| [14] | 沈琳洁, 黄雨欣, 王勇, 金华. 非侵入性脑刺激在抑郁障碍躯体症状治疗中的应用综述[J]. 上海交通大学学报(医学版), 2021, 41(11): 1535-1539. |
| [15] | 施波, 陈建民, 赵俊雄, 唐伟, 范卫星, 张程赪, 张晨. CREB1基因与抑郁症和双相Ⅱ型障碍的关联研究[J]. 上海交通大学学报(医学版), 2021, 41(10): 1303-1307. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||