
上海交通大学学报(医学版) ›› 2025, Vol. 45 ›› Issue (12): 1559-1567.doi: 10.3969/j.issn.1674-8115.2025.12.001
• 论著 · 基础研究 •
李文丽, 钟方元, 赵怡超, 金力行, 雷杰, 石瑶, 卜军, 葛恒( )
)
收稿日期:2025-06-19
接受日期:2025-08-21
出版日期:2025-12-28
发布日期:2025-12-28
通讯作者:
葛 恒,主任医师,博士;电子信箱:dr.geheng@foxmail.com。基金资助:
LI Wenli, ZHONG Fangyuan, ZHAO Yichao, JIN Lixing, LEI Jie, SHI Yao, PU Jun, GE Heng( )
)
Received:2025-06-19
Accepted:2025-08-21
Online:2025-12-28
Published:2025-12-28
Contact:
GE Heng, E-mail: dr.geheng@foxmail.com.Supported by:摘要:
目的·探讨血红蛋白(hemoglobin,Hb)诱导心肌细胞损伤的机制,以及Basigin(BSG)在其中的调控作用。方法·构建体外实验模型,采用不同浓度Hb(0、7.5、15.0、30.0 μmol/L)处理H9c2心肌细胞,并利用WST-1法和流式细胞术检测细胞活性及死亡率;随后,对H9c2心肌细胞进行缺氧/复氧处理,并加入低浓度梯度的Hb(0、2.5、5.0、7.5 μmol/L)模拟缺血再灌注损伤的病理微环境,以进一步验证Hb对心肌细胞的毒性作用。使用多种细胞死亡抑制剂,包括坏死性凋亡抑制剂(necrostatin-1,Nec-1)、自噬抑制剂(3-methyladenine,3-MA)、铁死亡抑制剂(ferrostatin-1,Fer-1)、焦亡抑制剂(VX-765)干预,以探究Hb促进心肌细胞损伤的机制。采用Western blotting及实时荧光定量PCR检测Hb诱导后心肌细胞中Bsg mRNA和蛋白质表达变化。采用siRNA敲低H9c2心肌细胞中Bsg的表达水平,并通过WST-1法和流式细胞术验证BSG在Hb诱导的心肌细胞损伤和铁死亡过程中的作用。结果·无论在常氧还是缺氧/复氧条件下,Hb均对H9c2心肌细胞表现出直接的毒性作用,且该毒性作用呈现浓度依赖性。进一步研究发现,相较于其他细胞死亡抑制剂,铁死亡抑制剂Fer-1能够更显著地减轻Hb诱导的心肌细胞损伤。Western blotting和实时荧光定量PCR结果显示,与对照组相比,Hb处理组H9c2心肌细胞中Bsg的mRNA和蛋白表达水平显著增加。敲低Bsg的表达能够降低铁死亡标志物前列腺素内过氧化物合酶2(prostaglandin-endoperoxide synthase 2,Ptgs2)mRNA的表达,并减轻Hb诱导的心肌细胞损伤和死亡。结论·Hb可能通过诱导心肌细胞铁死亡导致心肌损伤;BSG在此过程中发挥一定作用,抑制其表达能够抵抗Hb诱导的铁死亡和心肌细胞损伤。
中图分类号:
李文丽, 钟方元, 赵怡超, 金力行, 雷杰, 石瑶, 卜军, 葛恒. 血红蛋白诱导心肌细胞铁死亡及Basigin的调控机制研究[J]. 上海交通大学学报(医学版), 2025, 45(12): 1559-1567.
LI Wenli, ZHONG Fangyuan, ZHAO Yichao, JIN Lixing, LEI Jie, SHI Yao, PU Jun, GE Heng. Mechanisms of Basigin regulation in hemoglobin-induced cardiomyocyte ferroptosis[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2025, 45(12): 1559-1567.
| siRNA | Forward | Reverse |
|---|---|---|
| si-NC | UUCUCCGAACGUGUCACGUdTdT | ACGUGACACGUUCGGAGAAdTdT |
| si-Bsg | GAUCAAGGUGGGAAAGAAtt | UUUCUUUCCCACCUUGAUCtt |
表1 siRNA序列(5′→3′)
Tab 1 Sequences of siRNA (5'→3')
| siRNA | Forward | Reverse |
|---|---|---|
| si-NC | UUCUCCGAACGUGUCACGUdTdT | ACGUGACACGUUCGGAGAAdTdT |
| si-Bsg | GAUCAAGGUGGGAAAGAAtt | UUUCUUUCCCACCUUGAUCtt |
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Ptgs2 | TTCCTCCTGTGGCTGATGACTG | AGGTCCTCGCTTCTGATCTGTC |
| Bsg | GCATCTTCCTTCCTGAGCCTGTG | TGGCGTGTTCCGATTTCTTTCCC |
| β-actin | CACTATCGGCAATGAGCGGTTC | CAGCACTGTGTTGGCATAGAGG |
表2 实时荧光定量PCR引物序列(5′→3′)
Tab 2 Primer sequences for real-time quantitative PCR (5′→3′)
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Ptgs2 | TTCCTCCTGTGGCTGATGACTG | AGGTCCTCGCTTCTGATCTGTC |
| Bsg | GCATCTTCCTTCCTGAGCCTGTG | TGGCGTGTTCCGATTTCTTTCCC |
| β-actin | CACTATCGGCAATGAGCGGTTC | CAGCACTGTGTTGGCATAGAGG |
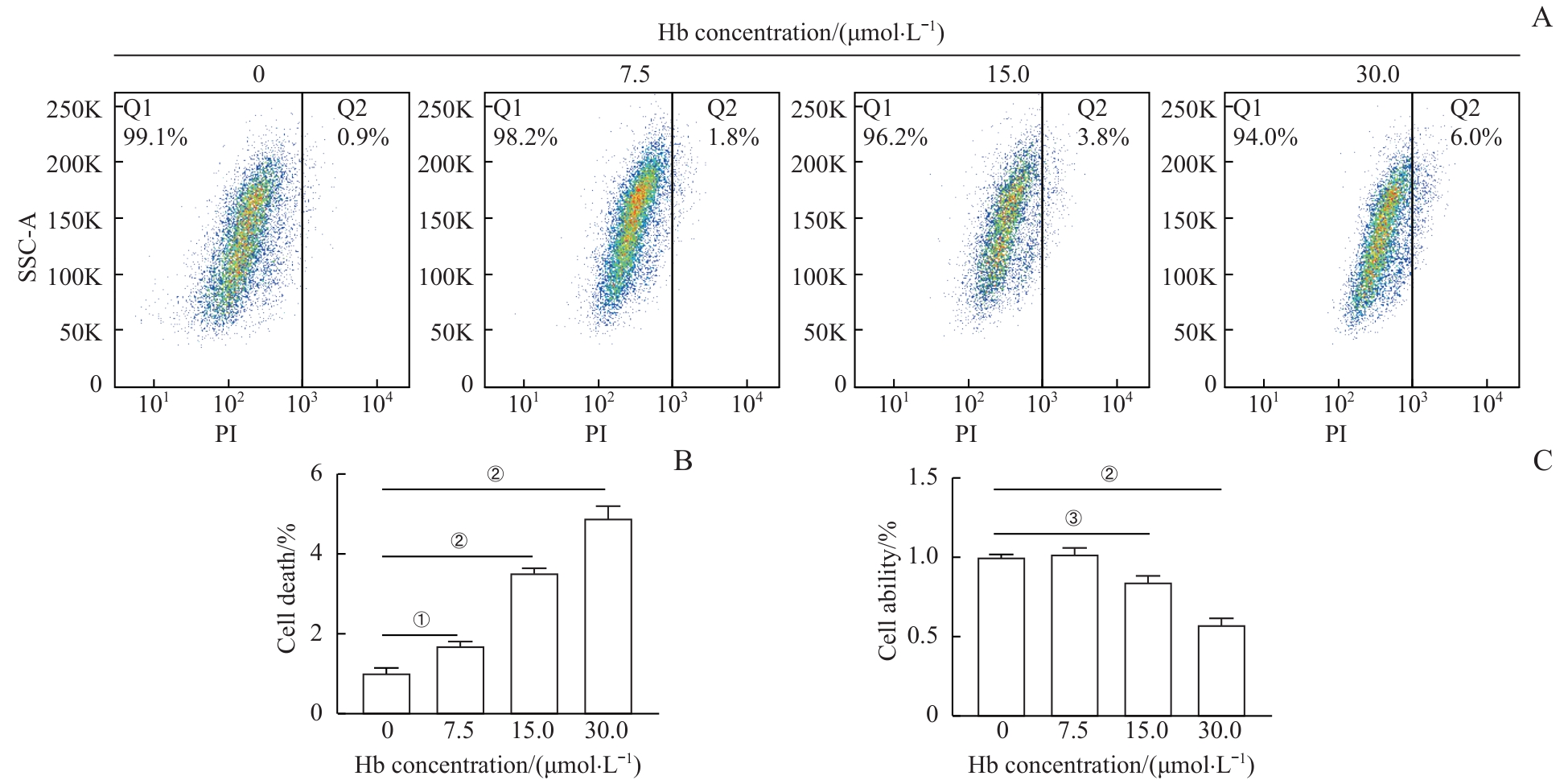
图1 Hb诱导H9c2心肌细胞损伤且呈浓度依赖性Note: A. Flow cytometry analysis of PI-positive H9c2 cardiomyocytes after treatment with different concentrations of Hb for 24 h. B. Quantitative analysis of PI-positive rates in H9c2 cardiomyocytes. C. WST-1 assay to detect the viability of H9c2 cardiomyocytes after treatment with different concentrations of Hb. ①P=0.044, ②P<0.001, ③P=0.020.
Fig 1 H9c2 cardiomyocyte injury induced by Hb in a concentration-dependent manner
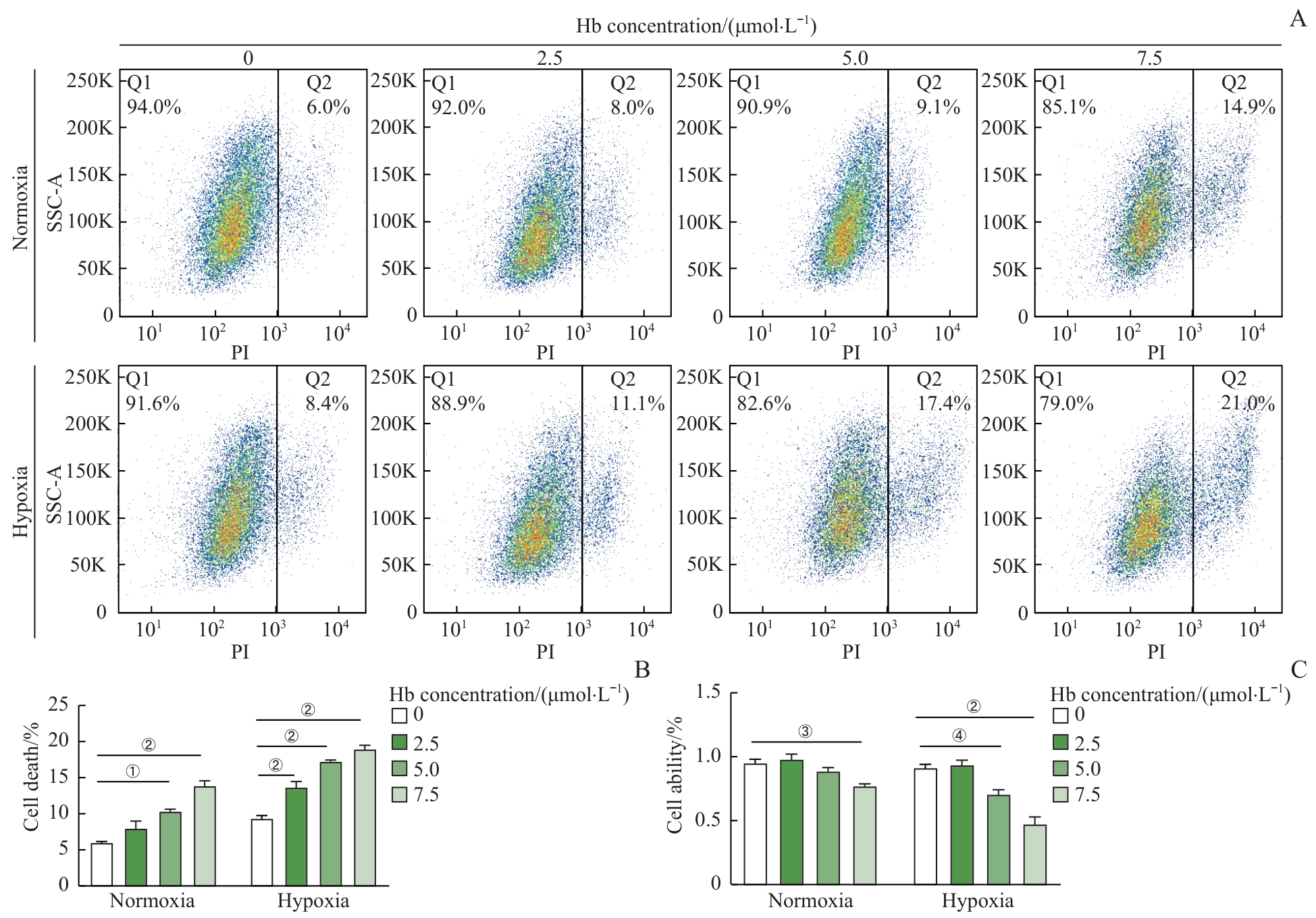
图2 Hb加重缺氧诱导的H9c2心肌细胞损伤Note: A. Flow cytometry analysis of PI-positive H9c2 cardiomyocytes after hypoxia (1% O₂, 6 h) followed by treatment with different concentrations of Hb for 24 h. B. Quantitative analysis of PI-positive rates in H9c2 cardiomyocytes. C. WST-1 assay to detect the viability of H9c2 cardiomyocytes after hypoxia followed by treatment with different concentrations of Hb. ①P=0.032, ②P<0.001, ③P=0.042, ④P=0.010.
Fig 2 Hypoxia-induced H9c2 cardiomyocyte injury exacerbated by Hb
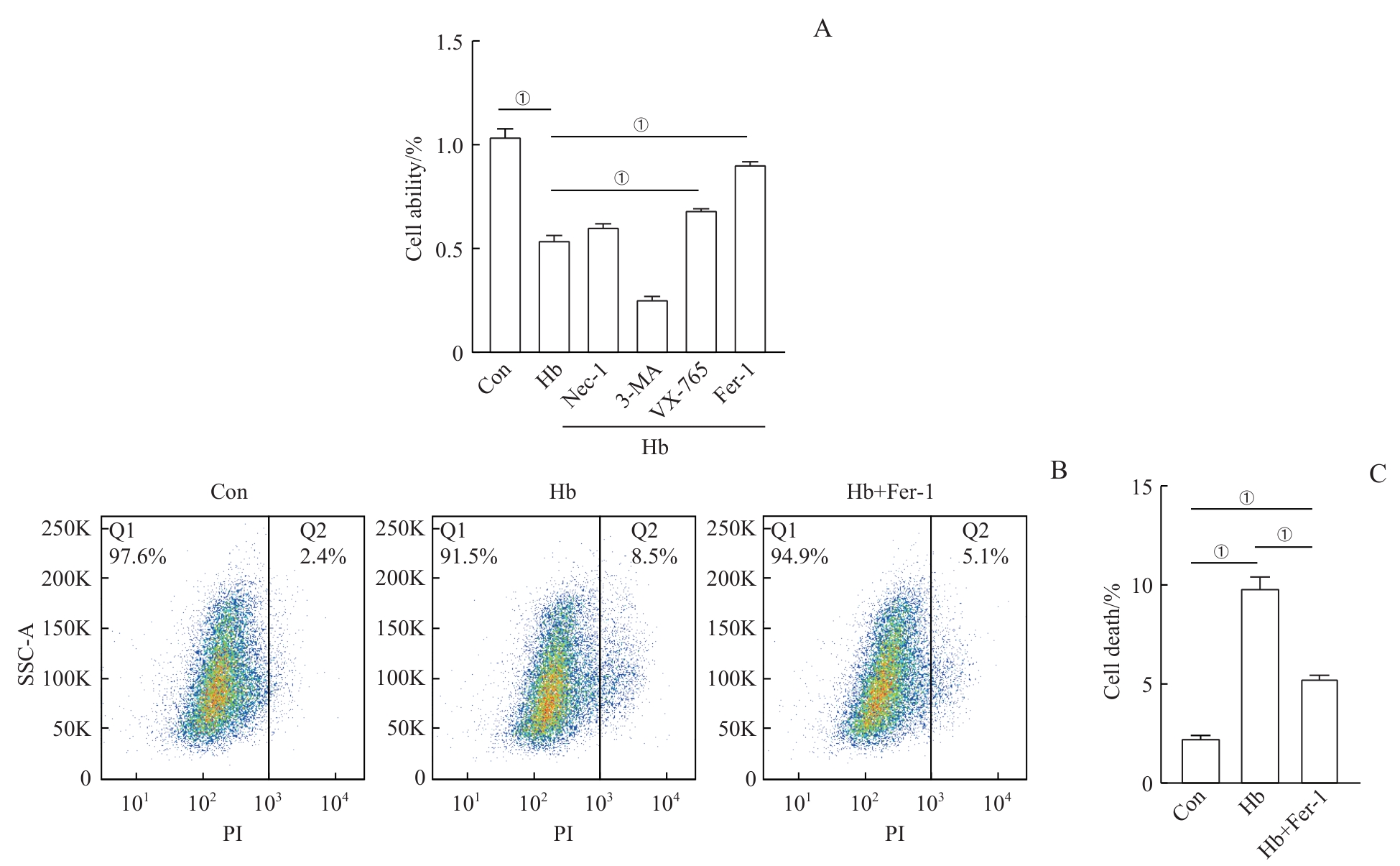
图3 铁死亡抑制剂对Hb诱导的H9c2心肌细胞损伤的影响Note: A. WST-1 assay to detect the effects of different cell death inhibitors on the viability of H9c2 cardiomyocytes induced by Hb. B. Flow cytometry analysis of the effect of Fer-1 on PI-positive rate in Hb-treated H9c2 cardiomyocytes. C. Quantitative analysis of the flow cytometry results. ①P<0.001.
Fig 3 Effect of the ferroptosis inhibitor on Hb-induced H9c2 cardiomyocyte injury
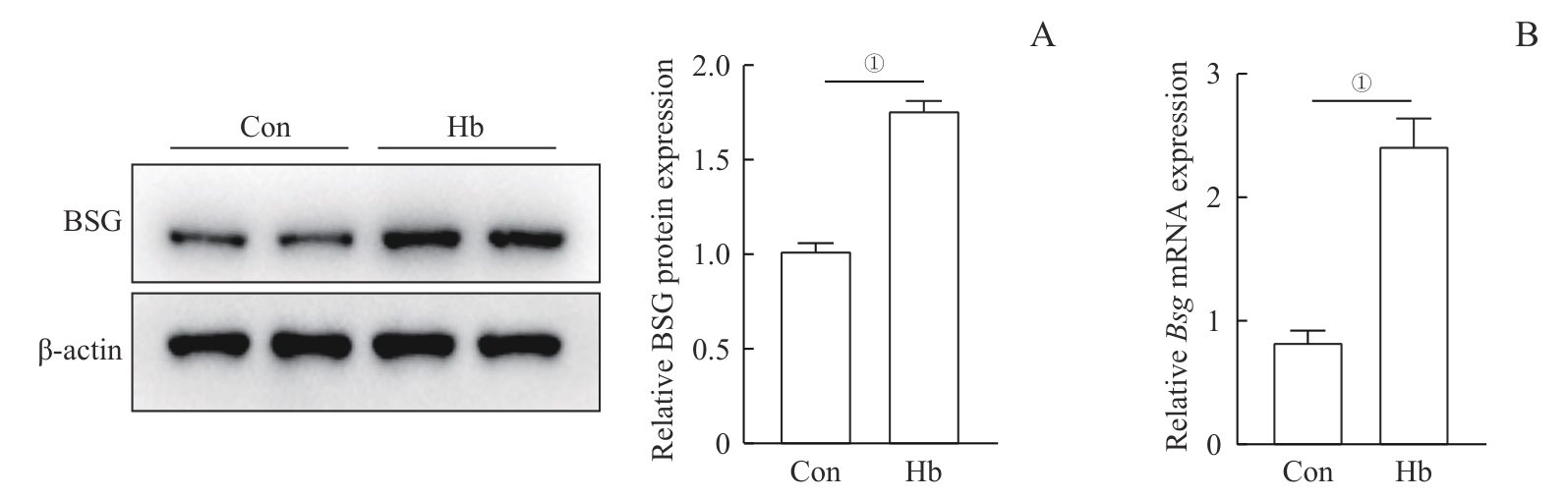
图4 Hb诱导的H9c2心肌细胞中BSG表达变化Note: A. Western blotting analysis of BSG protein expression levels in two groups of H9c2 cardiomyocytes. B. Real-time quantitative PCR analysis of Bsg mRNA expression levels in two groups of H9c2 cardiomyocytes. ①P<0.001.
Fig 4 Hb-induced changes in BSG expression in H9c2 cardiomyocytes
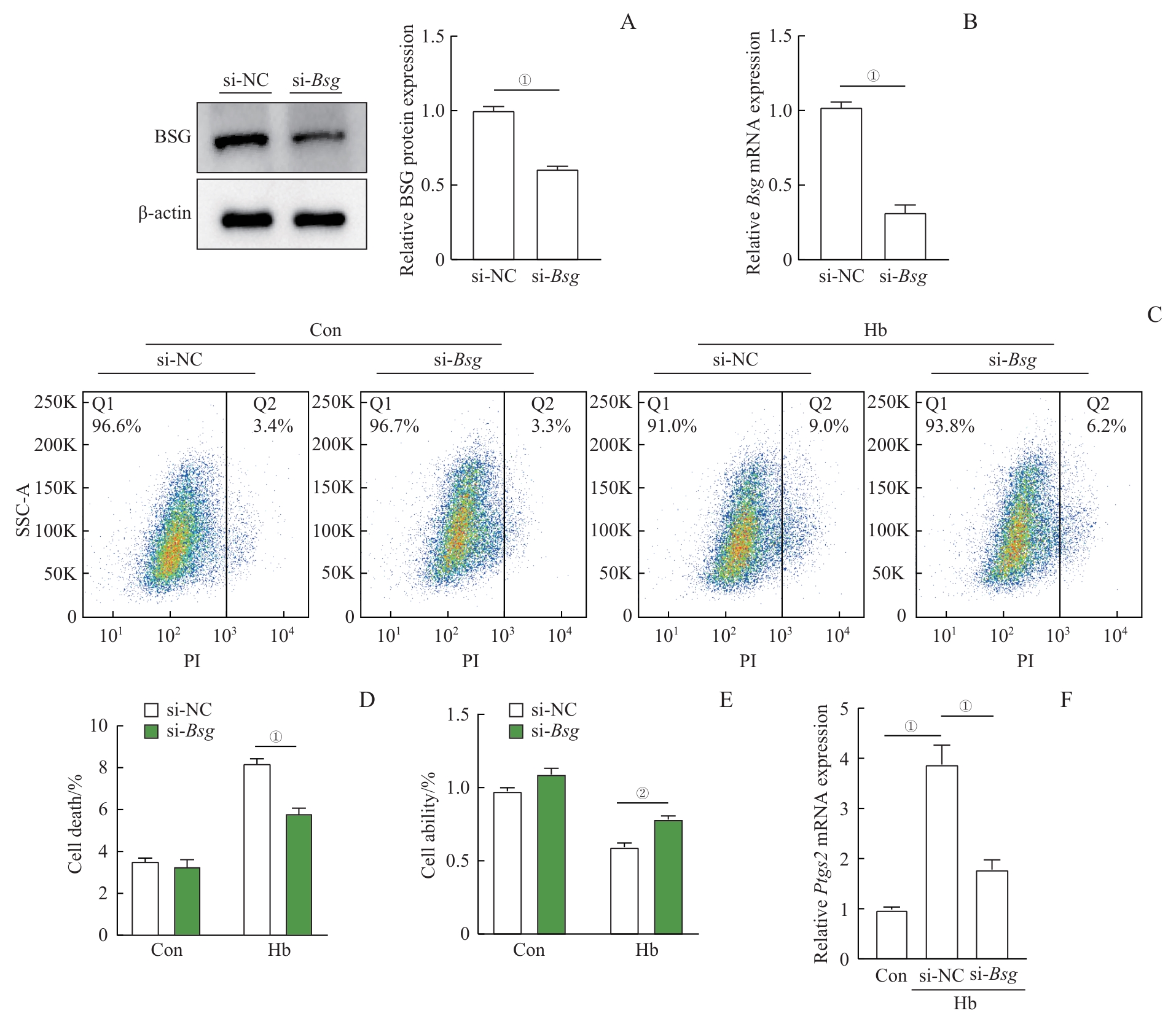
图5 敲低 Bsg 对Hb诱导的H9c2心肌细胞损伤的影响Note: A. Western blotting analysis of BSG protein expression levels after si-NC or si-Bsg transfection. B. Real-time quantitative PCR analysis of Bsg mRNA expression levels after si-NC or si-Bsg transfection. C. Flow cytometry analysis of the effect of Bsg silencing on the PI-positive rate in Hb-treated H9c2 cardiomyocytes. D. Quantitative analysis of the flow cytometry results. E. WST-1 assay to detect the viability of H9c2 cardiomyocytes in the indicated groups. F. Real-time quantitative PCR analysis of Ptgs2 mRNA expression in Hb-treated H9c2 cardiomyocytes after Bsg silencing. ①P<0.001, ②P=0.011.
Fig 5 Effects of knocking down Bsg on Hb-induced H9c2 cardiomyocyte injury
| [1] | DAMLUJI A A, VAN DIEPEN S, KATZ J N, et al. Mechanical complications of acute myocardial infarction: a scientific statement from the American Heart Association[J]. Circulation, 2021, 144(2): e16-e35. |
| [2] | ZEYMER U, LUDMAN P, DANCHIN N, et al. Reperfusion therapies and in-hospital outcomes for ST-elevation myocardial infarction in Europe: the ACVC-EAPCI EORP STEMI Registry of the European Society of Cardiology[J]. Eur Heart J, 2021, 42(44): 4536-4549. |
| [3] | EZEKOWITZ J A, KAUL P, BAKAL J A, et al. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction[J]. J Am Coll Cardiol, 2009, 53(1): 13-20. |
| [4] | DOCHERTY K F, JACKSON A M, MACARTNEY M, et al. Declining risk of heart failure hospitalization following first acute myocardial infarction in Scotland between 1991‒2016[J]. Eur J Heart Fail, 2023, 25(8): 1213-1224. |
| [5] | BULLUCK H, ROSMINI S, ABDEL-GADIR A, et al. Residual myocardial iron following intramyocardial hemorrhage during the convalescent phase of reperfused ST-segment-elevation myocardial infarction and adverse left ventricular remodeling[J]. Circ Cardiovasc Imaging, 2016, 9(10): e004940. |
| [6] | D'ENTREMONT M A, ALAZZONI A, DZAVIK V, et al. No-reflow after primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction: an angiographic core laboratory analysis of the TOTAL Trial[J]. EuroIntervention, 2023, 19(5): e394-e401. |
| [7] | LECHNER I, REINDL M, STIERMAIER T, et al. Clinical outcomes associated with various microvascular injury patterns identified by CMR after STEMI[J]. J Am Coll Cardiol, 2024, 83(21): 2052-2062. |
| [8] | VORA K P, KUMAR A, KRISHNAM M S, et al. Microvascular obstruction and intramyocardial hemorrhage in reperfused myocardial infarctions: pathophysiology and clinical insights from imaging[J]. JACC Cardiovasc Imaging, 2024, 17(7): 795-810. |
| [9] | LIU T, HOWARTH A G, CHEN Y Y, et al. Intramyocardial hemorrhage and the "wave front" of reperfusion injury compromising myocardial salvage[J]. J Am Coll Cardiol, 2022, 79(1): 35-48. |
| [10] | LIU Y, QI L X, LI Z, et al. Crosstalk between matrix metalloproteinases and their inducer EMMPRIN/CD147: a promising therapeutic target for intracerebral hemorrhage[J]. Transl Stroke Res, 2025, 16(2): 557-567. |
| [11] | SEIZER P, OCHMANN C, SCHÖNBERGER T, et al. Disrupting the EMMPRIN (CD147)-cyclophilin A interaction reduces infarct size and preserves systolic function after myocardial ischemia and reperfusion[J]. Arterioscler Thromb Vasc Biol, 2011, 31(6): 1377-1386. |
| [12] | REDDY V S, PRABHU S D, MUMMIDI S, et al. Interleukin-18 induces EMMPRIN expression in primary cardiomyocytes via JNK/Sp1 signaling and MMP-9 in part via EMMPRIN and through AP-1 and NF-κB activation[J]. Am J Physiol Heart Circ Physiol, 2010, 299(4): H1242-H1254. |
| [13] | SUZUKI K, SATOH K, IKEDA S, et al. Basigin promotes cardiac fibrosis and failure in response to chronic pressure overload in mice[J]. Arterioscler Thromb Vasc Biol, 2016, 36(4): 636-646. |
| [14] | YOON Y W, KWON H M, HWANG K C, et al. Upstream regulation of matrix metalloproteinase by EMMPRIN; extracellular matrix metalloproteinase inducer in advanced atherosclerotic plaque[J]. Atherosclerosis, 2005, 180(1): 37-44. |
| [15] | TIMMERS L, PASTERKAMP G, DE HOOG V C, et al. The innate immune response in reperfused myocardium[J]. Cardiovasc Res, 2012, 94(2): 276-283. |
| [16] | VYAS R, CHANGAL K H, BHUTA S, et al. Impact of intramyocardial hemorrhage on clinical outcomes in ST-elevation myocardial infarction: a systematic review and meta-analysis[J]. J Soc Cardiovasc Angiogr Interv, 2022, 1(6): 100444. |
| [17] | BETGEM R P, DE WAARD G A, NIJVELDT R, et al. Intramyocardial haemorrhage after acute myocardial infarction[J]. Nat Rev Cardiol, 2015, 12(3): 156-167. |
| [18] | CHEN Y F, LI X T, WANG S Y, et al. Targeting iron metabolism and ferroptosis as novel therapeutic approaches in cardiovascular diseases[J]. Nutrients, 2023, 15(3): 591. |
| [19] | FAN X B, LI A L, YAN Z P, et al. From iron metabolism to ferroptosis: pathologic changes in coronary heart disease[J]. Oxid Med Cell Longev, 2022, 2022: 6291889. |
| [20] | COKIC I, CHAN S F, GUAN X M, et al. Intramyocardial hemorrhage drives fatty degeneration of infarcted myocardium[J]. Nat Commun, 2022, 13(1): 6394. |
| [21] | CHEN R D, ZHANG Y Q, ZHANG H R, et al. SGLT2 inhibitor dapagliflozin alleviates intramyocardial hemorrhage and adverse ventricular remodeling via suppressing hepcidin in myocardial ischemia-reperfusion injury[J]. Eur J Pharmacol, 2023, 950: 175729. |
| [22] | REINSTADLER S J, STIERMAIER T, REINDL M, et al. Intramyocardial haemorrhage and prognosis after ST-elevation myocardial infarction[J]. Eur Heart J Cardiovasc Imaging, 2019, 20(2): 138-146. |
| [23] | JIANG X J, STOCKWELL B R, CONRAD M. Ferroptosis: mechanisms, biology and role in disease[J]. Nat Rev Mol Cell Biol, 2021, 22(4): 266-282. |
| [24] | LI J Y, LIU S Q, YAO R Q, et al. A novel insight into the fate of cardiomyocytes in ischemia-reperfusion injury: from iron metabolism to ferroptosis[J]. Front Cell Dev Biol, 2021, 9: 799499. |
| [25] | MA J D, ZHANG H Q, CHEN Y F, et al. The role of macrophage iron overload and ferroptosis in atherosclerosis[J]. Biomolecules, 2022, 12(11): 1702. |
| [26] | BEHROUZI B, WEYERS J J, QI X L, et al. Action of iron chelator on intramyocardial hemorrhage and cardiac remodeling following acute myocardial infarction[J]. Basic Res Cardiol, 2020, 115(3): 24. |
| [27] | WU J, CHEN L, QIN C, et al. CD147 contributes to SARS-CoV-2-induced pulmonary fibrosis[J]. Signal Transduct Target Ther, 2022, 7(1): 382. |
| [28] | LV J J, WANG H, ZHANG C, et al. CD147 sparks atherosclerosis by driving M1 phenotype and impairing efferocytosis[J]. Circ Res, 2024, 134(2): 165-185. |
| [29] | LIU Y, BAI Q, YONG V W, et al. EMMPRIN promotes the expression of MMP-9 and exacerbates neurological dysfunction in a mouse model of intracerebral hemorrhage[J]. Neurochem Res, 2022, 47(8): 2383-2395. |
| [30] | BIAN H J, CHEN L, ZHANG Z, et al. Meplazumab, a CD147 antibody, for severe COVID-19: a double-blind, randomized, placebo-controlled, phase 3 clinical trial[J]. Signal Transduct Target Ther, 2025, 10(1): 119. |
| [31] | ZHANG H, YANG X M, XUE Y, et al. A basigin antibody modulates MCTs to impact tumor metabolism and immunity[J]. Cell Discov, 2025, 11(1): 44. |
| [32] | WANG R F, ZONG K X, SONG J, et al. Inhibitor of CD147 suppresses T cell activation and recruitment in CVB3-induced acute viral myocarditis[J]. Viruses, 2023, 15(5): 1137. |
| [33] | YUAN S Q, WANG L P, CHEN X X, et al. Triptolide inhibits the migration and invasion of human prostate cancer cells via Caveolin-1/CD147/MMPs pathway[J]. Biomed Pharmacother, 2016, 84: 1776-1782. |
| [34] | ZHOU J, LI S Z, YANG Y T, et al. Triptolide alleviates acute lung injury by reducing mitochondrial dysfunction mediated ferroptosis through the STAT3/P53 pathway[J]. Free Radic Biol Med, 2025, 230: 79-94. |
| [1] | 王静怡, 邓佳丽, 朱仪, 丁心怡, 郭嘉婧, 王中领. 新型pH响应性锰基纳米探针用于乳腺癌铁死亡及磁共振成像实验研究[J]. 上海交通大学学报(医学版), 2025, 45(9): 1183-1193. |
| [2] | 万宏劲, 胡逸斌, 王昕, 张凯, 秦安, 马培翔, 马辉, 赵杰. 甲基莲心碱通过KEAP1/NRF2/GPX4和NF-κB信号通路减轻椎间盘退行性变[J]. 上海交通大学学报(医学版), 2025, 45(3): 261-270. |
| [3] | 李广慧, 冯晓玲. 复发性流产中胎盘细胞铁死亡的研究进展[J]. 上海交通大学学报(医学版), 2025, 45(10): 1383-1389. |
| [4] | 谢滨, 白蒙, 吴妍, 沃璐璐, 黄莺, 张晶. SUMO特异性蛋白酶1在铁死亡中的潜在作用[J]. 上海交通大学学报(医学版), 2025, 45(1): 11-19. |
| [5] | 吴凌恒, 陈建雄, 张梦娇, 沙蕾, 曹萌萌, 沈崔琴, 杜联芳, 李朝军. 血糖控制不理想对2型糖尿病患者亚临床心肌收缩功能的影响研究[J]. 上海交通大学学报(医学版), 2023, 43(8): 1024-1031. |
| [6] | 陈晨, 程卓安, 王存, 夏强. 铁死亡调控在肝脏疾病治疗中的研究进展[J]. 上海交通大学学报(医学版), 2023, 43(3): 365-373. |
| [7] | 徐斐翔, 汪升, 薛明明, 童朝阳, 陈玉梅. 长链非编码RNA-B230352I09表达改变对H9C2心肌细胞增殖及周期的影响[J]. 上海交通大学学报(医学版), 2022, 42(5): 578-582. |
| [8] | 王也飞, 张悦民, 吴蓓颖, 夏文权. 1例血红蛋白M病Boston型报道及文献回顾[J]. 上海交通大学学报(医学版), 2022, 42(4): 551-556. |
| [9] | 杜玉婷, 张婧, 黄莺, 张晶. 铁死亡对肌肉损伤后再生能力的影响[J]. 上海交通大学学报(医学版), 2022, 42(3): 298-306. |
| [10] | 何烨, 方芳. 非体外循环冠状动脉搭桥患者术前糖化血红蛋白水平与术后谵妄的关系[J]. 上海交通大学学报(医学版), 2022, 42(1): 21-27. |
| [11] | 王建茹, 彭广操, 朱明军. 基于GEO数据库和生物信息学分析筛选小鼠心肌缺血再灌注损伤相关的潜在枢纽基因[J]. 上海交通大学学报(医学版), 2022, 42(1): 51-62. |
| [12] | 魏倩, 张颖婷, 林龙帅, 何恩俊, 何咏元, 苏滢泓, 段澄澄, 王斯源, 赵庆华, 赵倩, 贺明. 结合珠蛋白通过抑制ERK1/2减轻肝细胞铁死亡[J]. 上海交通大学学报(医学版), 2021, 41(8): 999-1008. |
| [13] | 韦亚忠, 薛晓梅, 何斌. 活性氧介导心肌缺血再灌注损伤的研究进展[J]. 上海交通大学学报(医学版), 2021, 41(6): 826-829. |
| [14] | 冯泽豪1*,张 清1*,柴烨子1,苏 璇1,孙宝航行1,刘启明1,严福华2,姜 萌1#,卜 军1#. 吸烟对急性ST段抬高型心肌梗死急性期心肌损伤及预后的影响[J]. 上海交通大学学报(医学版), 2020, 40(5): 573-582. |
| [15] | 许书琳,吴 京,娄加陶. 肺癌和贫血的相关性分析[J]. 上海交通大学学报(医学版), 2020, 40(11): 1500-1504. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||