
上海交通大学学报(医学版) ›› 2025, Vol. 45 ›› Issue (12): 1644-1653.doi: 10.3969/j.issn.1674-8115.2025.12.010
• 综述 • 上一篇
收稿日期:2025-08-04
接受日期:2025-09-22
出版日期:2025-12-12
发布日期:2025-12-12
通讯作者:
项耀祖,教授,博士;电子信箱:yaozu.xiang@tongji.edu.cn。基金资助:
JIANG Kai, XU Yue, YANG Xingbo, WANG Dandan, XIANG Yaozu( )
)
Received:2025-08-04
Accepted:2025-09-22
Online:2025-12-12
Published:2025-12-12
Contact:
XIANG Yaozu, E-mail: yaozu.xiang@tongji.edu.cn.Supported by:摘要:
心肌梗死后的心室重构及其伴随的心肌纤维化是心力衰竭进展的核心病理机制。现有研究证实,免疫细胞的时空动态调控在心肌梗死后心室重构中发挥着至关重要的作用,其贯穿心脏炎症扩散、组织修复与纤维化的全过程。心肌梗死不仅诱发局部炎症反应,还可通过炎症因子释放及交感神经激活等途径远程调控骨髓、脾脏等造血器官,驱动造血干/祖细胞向髓系细胞分化倾斜,形成“髓系上升-淋系下降”的造血失衡现象。这一失衡表现为中性粒细胞、单核/巨噬细胞等促炎性髓系细胞过度增殖与释放,而T细胞、B细胞等淋系细胞则相对减少。该系统性免疫失衡重塑了心脏及外周器官的免疫细胞的构成,加剧心脏持续性炎症反应、氧化应激、细胞凋亡及细胞外基质过度沉积,最终导致心脏纤维化加重、心室扩张与收缩功能恶化,是驱动心肌梗死后不良重构与心力衰竭发生的关键环节。该文旨在系统探讨心肌梗死后造血失衡驱动的免疫亚群在时间与空间维度上的动态变化及其功能特征,并进一步提出通过精准调控造血干细胞分化路径与关键免疫细胞亚群,以改善心脏炎症微环境、延缓纤维化进程、抑制不良心室重构的新型干预策略,为心肌梗死后心力衰竭的防治提供潜在靶点。
中图分类号:
姜凯, 徐越, 杨兴博, 王丹丹, 项耀祖. 心肌梗死后造血失衡介导的心室重构:免疫细胞亚群的作用与干预新策略[J]. 上海交通大学学报(医学版), 2025, 45(12): 1644-1653.
JIANG Kai, XU Yue, YANG Xingbo, WANG Dandan, XIANG Yaozu. Hematopoietic imbalance-mediated ventricular remodeling after myocardial infarction: roles of immune cell subsets and emerging therapeutic strategies[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2025, 45(12): 1644-1653.
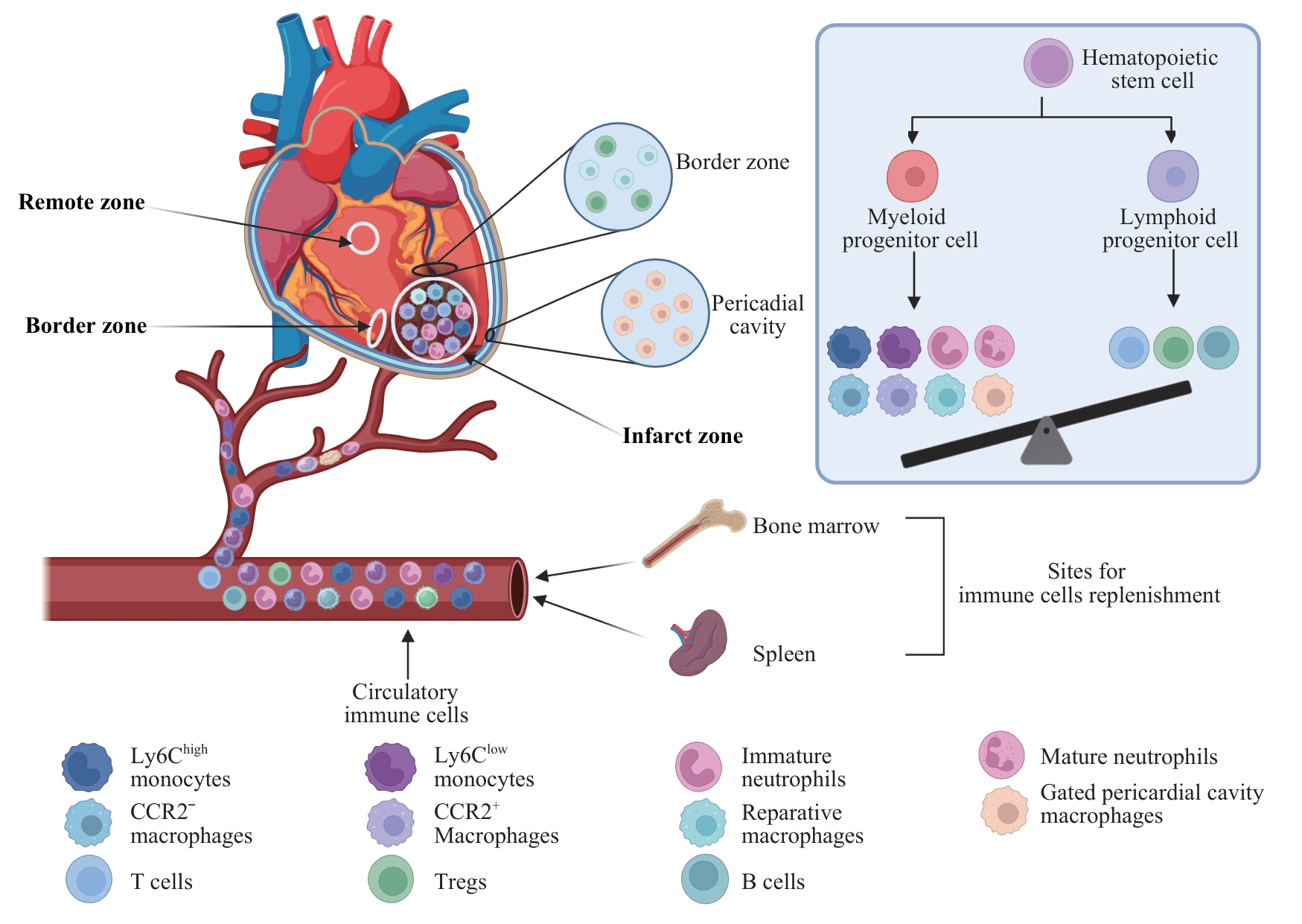
图1 MI后免疫细胞来源与在心脏不同区域的分布Note: HSC—hematopoietic stem cell; CCR2—C-C chemokine receptor type 2; Ly6Chigh/low—Ly6C high/low monocytes; Treg—regulatory T cell.
Fig 1 Origins and spatial distribution of immune cells in different cardiac regions after MI
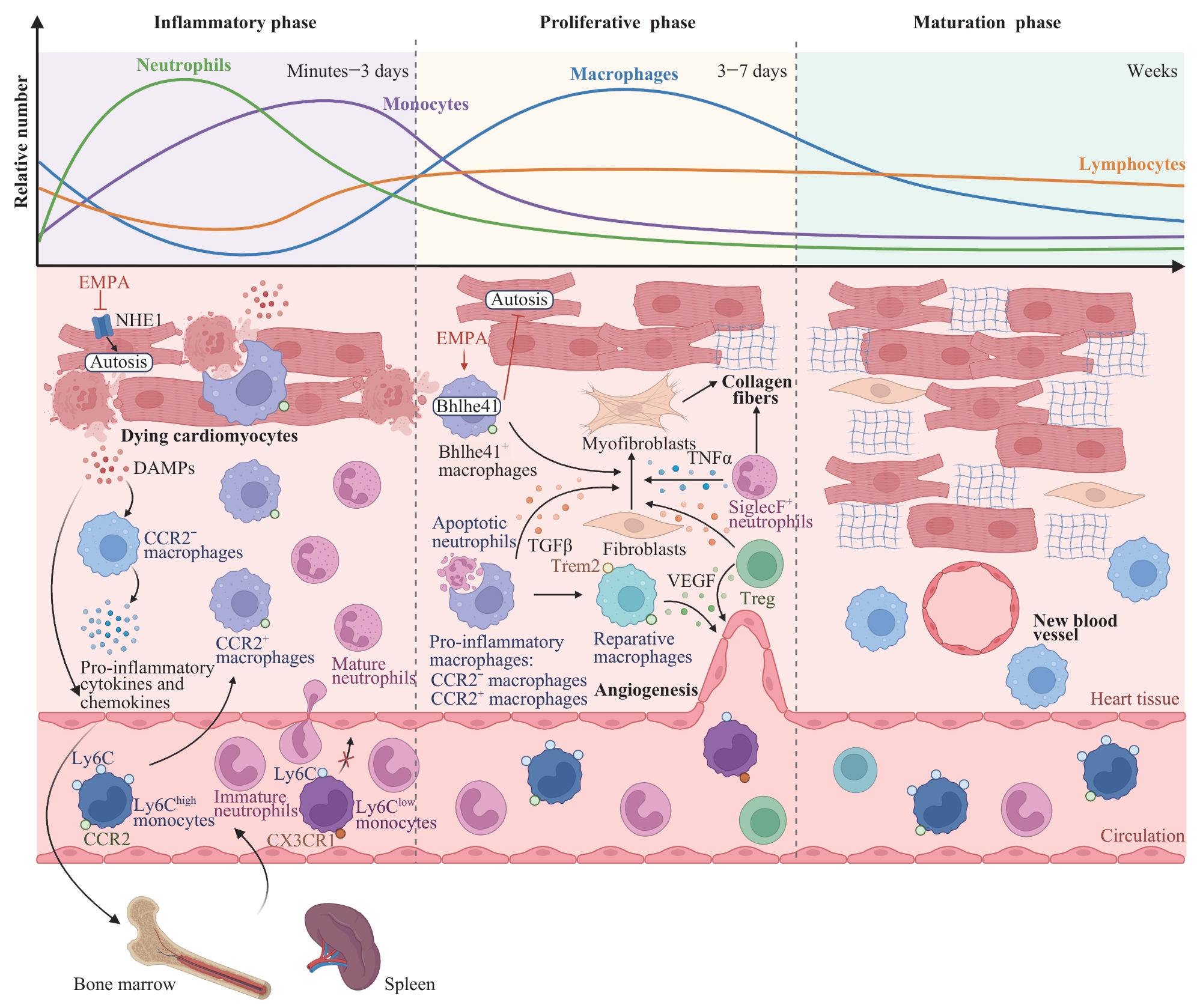
图2 MI后免疫细胞在炎症、修复与成熟阶段的动态变化和功能Note: DAMPs—damage-associated molecular patterns; EMPA—empagliflozin; NHE1—Na+/H+ exchanger 1; Ly6Chigh/low—Ly6C high/low monocytes; CX3CR1—C-X3-C chemokine receptor 1; SiglecF—sialic acid-binding immunoglobulin-type lectin F; TGF-β—transforming growth factor-β; VEGF—vascular endothelial growth factor; TNF-α—tumor necrosis factor-α; Trem2—triggering receptor expressed on myeloid cells 2; Treg—regulatory T cell.
Fig 2 Dynamic changes and roles of immune cells during inflammatory, reparative, and maturation phases after MI
| [1] | ANDERSON J L, MORROW D A. Acute myocardial infarction[J]. N Engl J Med, 2017, 376(21): 2053-2064. |
| [2] | KRITTANAWONG C, KHAWAJA M, TAMIS-HOLLAND J E, et al. Acute myocardial infarction: etiologies and mimickers in young patients[J]. J Am Heart Assoc, 2023, 12(18): e029971. |
| [3] | SQUIRE I B, SZE S. Prognosis following acute myocardial infarction[J]. Eur Heart J, 2025, 46(16): 1551-1553. |
| [4] | FRANGOGIANNIS N G. The inflammatory response in myocardial injury, repair, and remodelling[J]. Nat Rev Cardiol, 2014, 11(5): 255-265. |
| [5] | JIANG K, HWA J, XIANG Y Z. Novel strategies for targeting neutrophil against myocardial infarction[J]. Pharmacol Res, 2024, 205: 107256. |
| [6] | KING K R, AGUIRRE A D, YE Y X, et al. IRF3 and type Ⅰ interferons fuel a fatal response to myocardial infarction[J]. Nat Med, 2017, 23(12): 1481-1487. |
| [7] | PEET C, IVETIC A, BROMAGE D I, et al. Cardiac monocytes and macrophages after myocardial infarction[J]. Cardiovasc Res, 2020, 116(6): 1101-1112. |
| [8] | SWIRSKI F K, NAHRENDORF M. Cardioimmunology: the immune system in cardiac homeostasis and disease[J]. Nat Rev Immunol, 2018, 18(12): 733-744. |
| [9] | XU Y, JIANG K, CHEN F, et al. Bone marrow-derived naïve B lymphocytes improve heart function after myocardial infarction: a novel cardioprotective mechanism for empagliflozin[J]. Basic Res Cardiol, 2022, 117(1): 47. |
| [10] | FRANGOGIANNIS N G. Chemokines in the ischemic myocardium: from inflammation to fibrosis[J]. Inflamm Res, 2004, 53(11): 585-595. |
| [11] | YANG S, PENNA V, LAVINE K J. Functional diversity of cardiac macrophages in health and disease[J]. Nat Rev Cardiol, 2025, 22(6): 431-442. |
| [12] | HOFMANN U, FRANTZ S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction[J]. Circ Res, 2015, 116(2): 354-367. |
| [13] | ZOUGGARI Y, AIT-OUFELLA H, BONNIN P, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction[J]. Nat Med, 2013, 19(10): 1273-1280. |
| [14] | NESTOROWA S, HAMEY F K, PIJUAN SALA B, et al. A single-cell resolution map of mouse hematopoietic stem and progenitor cell differentiation[J]. Blood, 2016, 128(8): e20-31. |
| [15] | HEUSCH G, KLEINBONGARD P. The spleen in ischaemic heart disease[J]. Nat Rev Cardiol, 2025, 22(7): 497-509. |
| [16] | LV H Z, WANG C C, LIU Z N, et al. Suppression of the prostaglandin I2-type 1 interferon axis induces extramedullary hematopoiesis to promote cardiac repair after myocardial infarction[J]. Circulation, 2025, 151(24): 1730-1747. |
| [17] | NINH V K, CALCAGNO D M, YU J D, et al. Spatially clustered type Ⅰ interferon responses at injury borderzones[J]. Nature, 2024, 633(8028): 174-181. |
| [18] | CHEN R K, ZHANG H R, TANG B T, et al. Macrophages in cardiovascular diseases: molecular mechanisms and therapeutic targets[J]. Signal Transduct Target Ther, 2024, 9(1): 130. |
| [19] | JIANG K, TU Z Z, CHEN K, et al. Gasdermin D inhibition confers antineutrophil-mediated cardioprotection in acute myocardial infarction[J]. J Clin Invest, 2022, 132(1): e151268. |
| [20] | SHI J J, ZHAO Y, WANG K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death[J]. Nature, 2015, 526(7575): 660-665. |
| [21] | CALCAGNO D M, ZHANG C, TOOMU A, et al. SiglecFHI marks late-stage neutrophils of the infarcted heart: a single-cell transcriptomic analysis of neutrophil diversification[J]. J Am Heart Assoc, 2021, 10(4): e019019. |
| [22] | PFIRSCHKE C, ENGBLOM C, GUNGABEESOON J, et al. Tumor-promoting ly-6G+ SiglecFhigh cells are mature and long-lived neutrophils[J]. Cell Rep, 2020, 32(12): 108164. |
| [23] | ENGBLOM C, PFIRSCHKE C, ZILIONIS R, et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils[J]. Science, 2017, 358(6367): eaal5081. |
| [24] | RYU S, SHIN J W, KWON S, et al. Siglec-F-expressing neutrophils are essential for creating a profibrotic microenvironment in renal fibrosis[J]. J Clin Invest, 2022, 132(12): e156876. |
| [25] | CALCAGNO D M, TAGHDIRI N, NINH V K, et al. Single-cell and spatial transcriptomics of the infarcted heart define the dynamic onset of the border zone in response to mechanical destabilization[J]. Nat Cardiovasc Res, 2022, 1(11): 1039-1055. |
| [26] | WANG X K, XU Y, YU C Q, et al. Periodontitis-related myocardial fibrosis by expansion of collagen-producing SiglecF+ neutrophils[J]. Eur Heart J, 2025, 46(23): 2223-2238. |
| [27] | THORP E B. Cardiac macrophages and emerging roles for their metabolism after myocardial infarction[J]. J Clin Invest, 2023, 133(18): e171953. |
| [28] | WANG W F, LI X, DING X N, et al. Lymphatic endothelial transcription factor Tbx1 promotes an immunosuppressive microenvironment to facilitate post-myocardial infarction repair[J]. Immunity, 2023, 56(10): 2342-2357.e10. |
| [29] | WANG Q X, ISMAHIL M A, ZHU Y J, et al. CD206+ IL-4Rα+ macrophages are drivers of adverse cardiac remodeling in ischemic cardiomyopathy[J]. Circulation, 2025, 152(4): 257-273. |
| [30] | ISMAHIL M A, ZHOU G H, RAJASEKAR S, et al. Splenic CD169+ Tim4+ marginal metallophilic macrophages are essential for wound healing after myocardial infarction[J]. Circulation, 2025, 151(24): 1712-1729. |
| [31] | XU Y, JIANG K, SU F H, et al. A transient wave of Bhlhe41+ resident macrophages enables remodeling of the developing infarcted myocardium[J]. Cell Rep, 2023, 42(10): 113174. |
| [32] | PORSCH F, MALLAT Z, BINDER C J. Humoral immunity in atherosclerosis and myocardial infarction: from B cells to antibodies[J]. Cardiovasc Res, 2021, 117(13): 2544-2562. |
| [33] | HORCKMANS M, BIANCHINI M, SANTOVITO D, et al. Pericardial adipose tissue regulates granulopoiesis, fibrosis, and cardiac function after myocardial infarction[J]. Circulation, 2018, 137(9): 948-960. |
| [34] | MARTÍN P, SÁNCHEZ-MADRID F. T cells in cardiac health and disease[J]. J Clin Invest, 2025, 135(2): e185218. |
| [35] | ZHUANG R L, MENG Q S, MA X X, et al. CD4+ FoxP3+ CD73+ regulatory T cell promotes cardiac healing post-myocardial infarction[J]. Theranostics, 2022, 12(6): 2707-2721. |
| [36] | WEIRATHER J, HOFMANN U D W, BEYERSDORF N, et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation[J]. Circ Res, 2014, 115(1): 55-67. |
| [37] | XIA N, LU Y Z, GU M Y, et al. A unique population of regulatory T cells in heart potentiates cardiac protection from myocardial infarction[J]. Circulation, 2020, 142(20): 1956-1973. |
| [38] | BLANCO-DOMÍNGUEZ R, DE LA FUENTE H, RODRÍGUEZ C, et al. CD69 expression on regulatory T cells protects from immune damage after myocardial infarction[J]. J Clin Invest, 2022, 132(21): e152418. |
| [39] | YAN X X, SHICHITA T, KATSUMATA Y, et al. Deleterious effect of the IL-23/IL-17A axis and γδT cells on left ventricular remodeling after myocardial infarction[J]. J Am Heart Assoc, 2012, 1(5): e004408. |
| [40] | JAISWAL S, FONTANILLAS P, FLANNICK J, et al. Age-related clonal hematopoiesis associated with adverse outcomes[J]. N Engl J Med, 2014, 371(26): 2488-2498. |
| [41] | RETTKOWSKI J, ROMERO-MULERO M C, SINGH I, et al. Modulation of bone marrow haematopoietic stem cell activity as a therapeutic strategy after myocardial infarction: a preclinical study[J]. Nat Cell Biol, 2025, 27(4): 591-604. |
| [42] | ANZAI A, CHOI J L, HE S, et al. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes[J]. J Exp Med, 2017, 214(11): 3293-3310. |
| [43] | HAMILTON J A. GM-CSF in inflammation[J]. J Exp Med, 2020, 217(1): e20190945. |
| [44] | RIDKER P M, EVERETT B M, THUREN T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease[J]. N Engl J Med, 2017, 377(12): 1119-1131. |
| [45] | TOLDO S, ABBATE A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases[J]. Nat Rev Cardiol, 2024, 21(4): 219-237. |
| [46] | TOLDO S, ABBATE A. The NLRP3 inflammasome in acute myocardial infarction[J]. Nat Rev Cardiol, 2018, 15(4): 203-214. |
| [47] | SAGER H B, HEIDT T, HULSMANS M, et al. Targeting interleukin-1β reduces leukocyte production after acute myocardial infarction[J]. Circulation, 2015, 132(20): 1880-1890. |
| [48] | LI Y J, TU Z Z, CHEN F, et al. Anti-inflammatory effect of Danhong injection through inhibition of GSDMD-mediated pyroptosis[J]. Phytomedicine, 2023, 113: 154743. |
| [49] | LI J, LI S H, WU J, et al. Young bone marrow Sca-1 cells rejuvenate the aged heart by promoting epithelial-to-mesenchymal transition[J]. Theranostics, 2018, 8(7): 1766-1781. |
| [50] | KIM C, KIM H, SIM W S, et al. Spatiotemporal control of neutrophil fate to tune inflammation and repair for myocardial infarction therapy[J]. Nat Commun, 2024, 15(1): 8481. |
| [51] | WANG L, YU C J, YOU T, et al. Injection of ROS-responsive hydrogel Loaded with IL-1β-targeted nanobody for ameliorating myocardial infarction[J]. Bioact Mater, 2025, 46: 273-284. |
| [52] | JIANG K, XU Y, WANG D D, et al. Cardioprotective mechanism of SGLT2 inhibitor against myocardial infarction is through reduction of autosis[J]. Protein Cell, 2022, 13(5): 336-359. |
| [53] | LYTVYN Y, BJORNSTAD P, UDELL J A, et al. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials[J]. Circulation, 2017, 136(17): 1643-1658. |
| [1] | 杨晨蝶, 胡长青, 袁贺, TAY Guan Poh, 阿布力克木·阿木提, 张瑞岩, 王晓群. 无糖尿病病史患者胰岛素抵抗水平与急性ST段抬高型心肌梗死后左室重构的相关性[J]. 上海交通大学学报(医学版), 2025, 45(3): 292-300. |
| [2] | 李文丽, 金力行, 赵怡超, 钟方元, 石瑶, 雷杰, 卜军, 葛恒. 基于心脏磁共振评估左心室心肌应变损伤对STEMI急性期继发性三尖瓣反流的影响[J]. 上海交通大学学报(医学版), 2025, 45(12): 1578-1588. |
| [3] | 阮青青, 苏树智, 李延婷, 任渊, 戴勇, 乔增勇. 急性心肌梗死介入治疗并发症风险预测模型构建[J]. 上海交通大学学报(医学版), 2025, 45(12): 1589-1597. |
| [4] | 刘雨婷, 俞莞琦, 洪雯, 康桑, 李歆旎, 旦增曲央, 肖活源, 潘静薇. 临床衰弱指数对急性心肌梗死患者在院心脏康复后远期预后的预测价值[J]. 上海交通大学学报(医学版), 2024, 44(5): 599-605. |
| [5] | 郑梦奕, 毛家亮, 邹治国, 张瑞雷, 张厚, 李世光. 全身免疫炎症指数及躯体化症状评分对首发心梗PCI术后发生院内主要不良心血管事件的预测价值[J]. 上海交通大学学报(医学版), 2024, 44(3): 334-341. |
| [6] | 胡晓, 张鑫, 谷阳. 体质量与C1q肿瘤坏死因子相关蛋白1在心肌梗死患者中的交互作用[J]. 上海交通大学学报(医学版), 2022, 42(6): 786-791. |
| [7] | 许莉, 杨艳, 陈菡芬, 姜萌, 卜军. 急性心肌梗死患者于心脏康复中心就诊的影响因素及效果评价[J]. 上海交通大学学报(医学版), 2022, 42(5): 646-652. |
| [8] | 胡培堃, 何杰, 吴连明, 葛恒, 许建荣, 卜军. ST段抬高型心肌梗死患者微血管阻塞对左室功能及预后的影响[J]. 上海交通大学学报(医学版), 2021, 41(2): 173-179. |
| [9] | 董建勋, 魏莱, 何杰, 孔令璁, 葛恒, 卜军. 心脏磁共振评估左心室机械不同步的研究进展[J]. 上海交通大学学报(医学版), 2021, 41(12): 1698-1702. |
| [10] | 高亚洁, 马文坤, 高程洁, 周翌, 潘静薇. 心肌应变对急性ST段抬高型心肌梗死后心室重构的预测价值探讨[J]. 上海交通大学学报(医学版), 2021, 41(11): 1478-1484. |
| [11] | 冯泽豪1*,张 清1*,柴烨子1,苏 璇1,孙宝航行1,刘启明1,严福华2,姜 萌1#,卜 军1#. 吸烟对急性ST段抬高型心肌梗死急性期心肌损伤及预后的影响[J]. 上海交通大学学报(医学版), 2020, 40(5): 573-582. |
| [12] | 唐冬娟,薛晓梅,何 斌. miR-133a对急性心肌梗死的早期诊断及预后评估价值[J]. 上海交通大学学报(医学版), 2020, 40(3): 339-. |
| [13] | 苗雨桐 1,沈兰 1, 2,何奔 1. 心肌梗死后心脏损伤的影像学评估[J]. 上海交通大学学报(医学版), 2019, 39(4): 436-. |
| [14] | 夏智丽 1,高程洁 2,高亚洁 1,陶逸菁 1,万青 1,吴昊 1,魏钧伯 1,周翌 1,潘静薇 1. 应激性血糖升高比值对急性心肌梗死患者预后的评估价值[J]. 上海交通大学学报(医学版), 2019, 39(3): 309-. |
| [15] | 苏海霞,朱雅琴,张天贶,张绘莉,顾 俊. 冠状动脉粥样硬化性心脏病患者碎裂QRS波的分布特征及其与左心室重构的关系[J]. 上海交通大学学报(医学版), 2019, 39(10): 1162-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||