
上海交通大学学报(医学版) ›› 2026, Vol. 46 ›› Issue (1): 90-99.doi: 10.3969/j.issn.1674-8115.2026.01.011
收稿日期:2025-08-06
接受日期:2025-09-10
出版日期:2026-01-28
发布日期:2026-01-30
通讯作者:
陈素贞,教授,博士;电子信箱:cszdream@163.com。作者简介:第一联系人:张娴静负责文献检索与撰写,陈素贞负责论文修改与审核。所有作者均阅读并同意了最终稿件的提交。
基金资助:Received:2025-08-06
Accepted:2025-09-10
Online:2026-01-28
Published:2026-01-30
Contact:
Chen Suzhen, E-mail: cszdream@163.com.About author:First author contact:Zhang Xianjing contributed to literature search and writing. Chen Suzhen revised and reviewed the manuscript. Both authors have read the final version of manuscript and consented to its submission.
Supported by:摘要:
糖尿病肾病(diabetic nephropathy,DN)作为糖尿病最严重的微血管并发症之一,其进行性进展可导致不可逆的终末期肾病(end-stage renal disease,ESRD),对全球公共卫生造成重大挑战。肾纤维化作为DN进展的核心病理特征,已成为治疗干预的关键靶点。现行临床管理策略(包括血糖/血压控制、肾素-血管紧张素系统抑制剂应用等)虽能延缓疾病进展,但对于肾纤维化这一关键病理过程的阻断效果仍然有限。近年来研究发现,中药在DN防治领域展现出独特的治疗价值,其通过多组分、多靶点、多通路的协同作用机制,在调节糖脂代谢、抑制炎症反应、减轻氧化应激以及延缓纤维化进程等方面表现出显著优势。临床研究证实,中药方剂及活性成分不仅能有效改善患者临床症状、降低蛋白尿水平,还具有明确的肾功能保护作用和疾病进展延缓效应。基于这些发现,中药调控DN肾纤维化的分子机制已成为新型抗纤维化药物研发的重要研究方向。该综述系统梳理具有明确抗纤维化功效的中药及其活性成分的作用机制,旨在为开发针对DN肾纤维化的创新治疗策略提供科学依据和转化医学思路。
中图分类号:
张娴静, 陈素贞. 中药防治糖尿病肾病肾纤维化的作用机制及治疗进展[J]. 上海交通大学学报(医学版), 2026, 46(1): 90-99.
Zhang Xianjing, Chen Suzhen. Mechanisms and therapeutic progress of Chinese materia medica in the prevention and treatment of renal fibrosis in diabetic nephropathy[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2026, 46(1): 90-99.
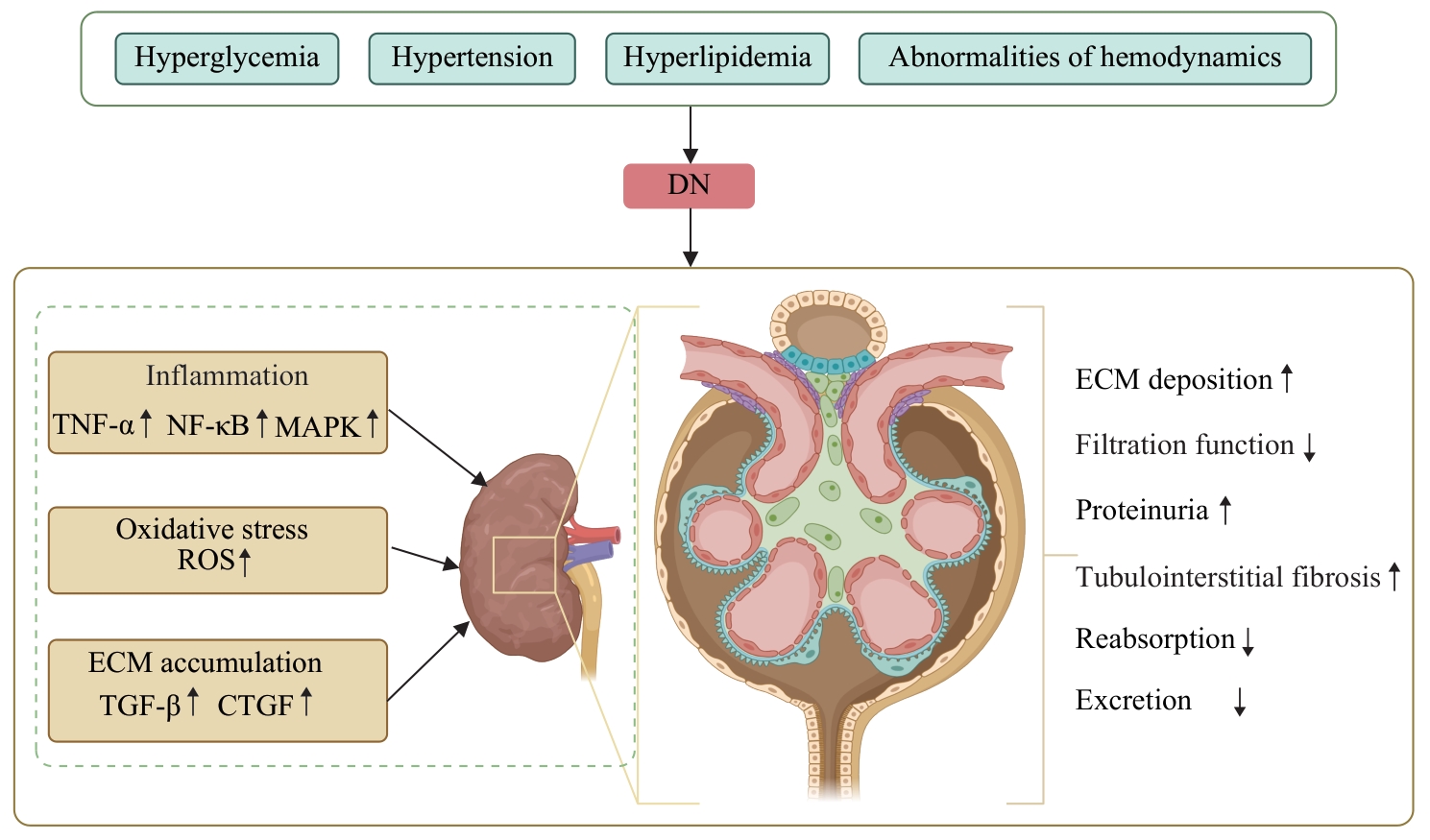
图1 DN肾纤维化的主要发病机制Note: DN—diabetic nephropathy; TNF-α—tumour necrosis factor-α; NF-κB—nuclear factor κB; ROS—reactive oxygen species; TGF-β—transforming growth factor-β; CTGF—connective tissue growth factor; ECM—extracellular matrix.
Fig 1 Main pathogenesis of renal fibrosis in DN
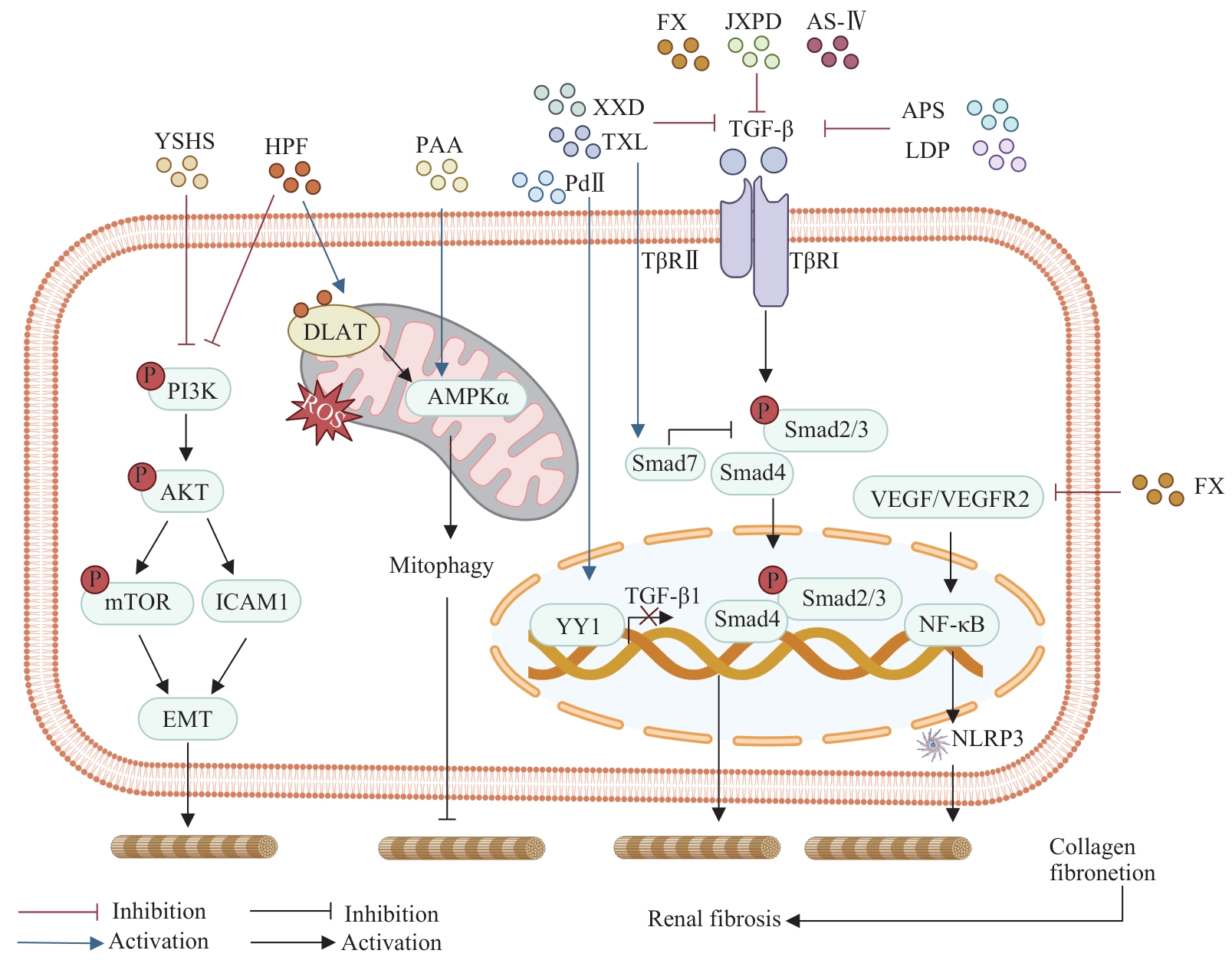
图2 中药抑制DN肾纤维化作用涉及的信号通路Note: YSHS—Yishen Huashi granules; HPF—hyperforin; PAA—Poricoic acid A; XXD—Xiexin decoction; TXL—Tongxinluo; FX—Fuxin granules; JXPD—Jixue Paidu Tang; AS-Ⅳ—Astragaloside Ⅳ; APS—Astragalus polysaccharides; LDP—Liuwei Dihuang pill; PdⅡ—Picroside Ⅱ; TβR—transforming growth factor-β receptor; YY1—Yin Yang1; DLAT—dihydrolipoamide S-acetyltransferase; VEGF—vascular endothelial growth factor; VEGFR2—vascular endothelial growth factor receptor 2; EMT—epithelial-mesenchymal transition; ICAM1—intercellular adhesion molecule 1; NF-κB—nuclear factor κB; PI3K—phosphoinositide 3-kinase; AKT—AKT serine/threonine kinase; mTOR—mammalian target of rapamycin; TGF-β—transforming growth factor-β; ROS—reactive oxygen species; AMPK—AMP-activated protein kinase; NLRP3—NOD-like receptor family pyrin domain containing 3.
Fig 2 Signaling pathways involved in the inhibition of renal fibrosis in DN by Chinese materia medica
| Chinese materia medica | Main crude drug | Design | Subject | Intervention | Effect | Side effect | Reference |
|---|---|---|---|---|---|---|---|
| YSHS | Ginseng Radix Et Rhizoma, Astragali Radix, Atractylodis Macrocephalae Rhizoma, Poria, Alismatis Rhizoma, Pinelliae Rhizoma, Notopterygii Rhizoma Et Radix, Angelicae Pubescentis Radix | Meta-analysis | A total of 2 416 DN patients from 28 RCTs | C: conventional therapy T: combined YSHS therapy | Reduced UAER, 24 h-UP, BUN, and Scr | Dizziness, headache, dry cough, and postural hypotension | [ |
| LDP | Rehmanniae Radix, Dioscoreae Rhizoma, Alismatis Rhizoma, Moutan Cortex, Poria | Meta-analysis | A total of 1 275 DN patients from 18 RCTs | T: LDP plus Western medicine. C: Western medicine | Reduced UMA, 24 h-UP, UAER, BUN, Scr, FBG, and PBG | Slight cough, hypoglycemia, and gastrointestinal reactions | [ |
| Huangqi | Astragali Radix | Meta-analysis | A total of 1 804 DN patients from 21 RCTs and 4 semi-RCTs | T: Astragalus injection with ACEI/ARB. C: ACEI/ARB/placebo | Improved 24 h-UP, UMA, BUN, Scr, Ccr, and serum albumin | N/R | [ |
| Salvianolate | Salviae Miltiorrhizae Radix Et Rhizoma | Meta-analysis | A total of 1 030 DN patients from 12 RCTs | T: Salvianolate with Western medicine. C: Western medicine alone | Reduced UMA, 24 h-UP, UAER, BUN, and Scr | N/R | [ |
| Dongchongxiacao | Ophiocordyceps | Meta-analysis | A total of 2 198 early-stage DN patients from 26 RCTs | T: Jinshuibao capsule with ARB. C: ARB | Reduced 24 h-UP, UAER, BUN, Scr, and UACR | Emesis | [ |
表1 中药治疗DN的临床疗效
Tab 1 Clinical efficacy of Chinese materia medica in treating DN
| Chinese materia medica | Main crude drug | Design | Subject | Intervention | Effect | Side effect | Reference |
|---|---|---|---|---|---|---|---|
| YSHS | Ginseng Radix Et Rhizoma, Astragali Radix, Atractylodis Macrocephalae Rhizoma, Poria, Alismatis Rhizoma, Pinelliae Rhizoma, Notopterygii Rhizoma Et Radix, Angelicae Pubescentis Radix | Meta-analysis | A total of 2 416 DN patients from 28 RCTs | C: conventional therapy T: combined YSHS therapy | Reduced UAER, 24 h-UP, BUN, and Scr | Dizziness, headache, dry cough, and postural hypotension | [ |
| LDP | Rehmanniae Radix, Dioscoreae Rhizoma, Alismatis Rhizoma, Moutan Cortex, Poria | Meta-analysis | A total of 1 275 DN patients from 18 RCTs | T: LDP plus Western medicine. C: Western medicine | Reduced UMA, 24 h-UP, UAER, BUN, Scr, FBG, and PBG | Slight cough, hypoglycemia, and gastrointestinal reactions | [ |
| Huangqi | Astragali Radix | Meta-analysis | A total of 1 804 DN patients from 21 RCTs and 4 semi-RCTs | T: Astragalus injection with ACEI/ARB. C: ACEI/ARB/placebo | Improved 24 h-UP, UMA, BUN, Scr, Ccr, and serum albumin | N/R | [ |
| Salvianolate | Salviae Miltiorrhizae Radix Et Rhizoma | Meta-analysis | A total of 1 030 DN patients from 12 RCTs | T: Salvianolate with Western medicine. C: Western medicine alone | Reduced UMA, 24 h-UP, UAER, BUN, and Scr | N/R | [ |
| Dongchongxiacao | Ophiocordyceps | Meta-analysis | A total of 2 198 early-stage DN patients from 26 RCTs | T: Jinshuibao capsule with ARB. C: ARB | Reduced 24 h-UP, UAER, BUN, Scr, and UACR | Emesis | [ |
| [1] | Samsu N. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment[J]. Biomed Res Int, 2021, 2021: 1497449. |
| [2] | Ahmad E, Lim S, Lamptey R, et al. Type 2 diabetes [J]. Lancet, 2022, 400(10365): 1803-1820. |
| [3] | Tang G Y, Li S, Zhang C, et al. Clinical efficacies, underlying mechanisms and molecular targets of Chinese medicines for diabetic nephropathy treatment and management[J]. Acta Pharm Sin B, 2021, 11(9): 2749-2767. |
| [4] | Wang S J, Qin S, Cai B C, et al. Promising therapeutic mechanism for Chinese herbal medicine in ameliorating renal fibrosis in diabetic nephropathy[J]. Front Endocrinol (Lausanne), 2023, 14: 932649. |
| [5] | Talukdar A, Basumatary M. Rodent models to study type 1 and type 2 diabetes induced human diabetic nephropathy[J]. Mol Biol Rep, 2023, 50(9): 7759-7782. |
| [6] | Zhang Y Q, Jin D, Kang X M, et al. Signaling pathways involved in diabetic renal fibrosis[J]. Front Cell Dev Biol, 2021, 9: 696542. |
| [7] | Khalid M, Petroianu G, Adem A. Advanced glycation end products and diabetes mellitus: mechanisms and perspectives[J]. Biomolecules, 2022, 12(4): 542. |
| [8] | Sanajou D, Ghorbani Haghjo A, Argani H, et al. AGE-RAGE axis blockade in diabetic nephropathy: current status and future directions[J]. Eur J Pharmacol, 2018, 833: 158-164. |
| [9] | Ratliff B B, Abdulmahdi W, Pawar R, et al. Oxidant mechanisms in renal injury and disease[J]. Antioxid Redox Signal, 2016, 25(3): 119-146. |
| [10] | Han Y C, Xu X X, Tang C Y, et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis[J]. Redox Biol, 2018, 16: 32-46. |
| [11] | 何其睿, 李杨, 邓文珍, 等. 硫氧还蛋白相互作用蛋白通过诱导氧化应激促进肾脏纤维化的发生[J]. 第三军医大学学报, 2018, 40(22): 2061-2067. |
| He Q R, Li Y, Deng W Z, et al. Thioredoxin-interacting protein promotes renal fibrosis in mice by inducing oxidative stress [J]. Journal of Army Medical University, 2018, 40(22): 2061-2067. | |
| [12] | Wei M M, Li Z G, Xiao L, et al. Effects of ROS-relative NF-κB signaling on high glucose-induced TLR4 and MCP-1 expression in podocyte injury[J]. Mol Immunol, 2015, 68(2 Pt A): 261-271. |
| [13] | Rayego-Mateos S, Rodrigues-Diez R R, Fernandez-Fernandez B, et al. Targeting inflammation to treat diabetic kidney disease: the road to 2030[J]. Kidney Int, 2023, 103(2): 282-296. |
| [14] | Calle P, Hotter G. Macrophage phenotype and fibrosis in diabetic nephropathy [J]. Int J Mol Sci, 2020, 21(8):2806. |
| [15] | Liu Y P, Su Y Y, Yang Q, et al. Stem cells in the treatment of renal fibrosis: a review of preclinical and clinical studies of renal fibrosis pathogenesis[J]. Stem Cell Res Ther, 2021, 12(1): 333. |
| [16] | Huang R, Fu P, Ma L. Kidney fibrosis: from mechanisms to therapeutic medicines[J]. Signal Transduct Targe Ther, 2023, 8(1): 129. |
| [17] | Rockey D C, Darwin Bell P, Hill J A. Fibrosis: a common pathway to organ injury and failure[J]. N Engl J Med, 2015, 372(12): 1138-1149. |
| [18] | Lin Y C, Chang Y H, Yang S Y, et al. Update of pathophysiology and management of diabetic kidney disease[J]. J Formos Med Assoc, 2018, 117(8): 662-675. |
| [19] | Liu Z J, Nan P, Gong Y H, et al. Endoplasmic reticulum stress-triggered ferroptosis via the XBP1-Hrd1-Nrf2 pathway induces EMT progression in diabetic nephropathy[J]. Biomed Pharmacother, 2023, 164: 114897. |
| [20] | Qi W E, Keenan H A, Li Q, et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction[J]. Nat Med, 2017, 23(6): 753-762. |
| [21] | Wu Q R, Huang F J. Targeting ferroptosis as a prospective therapeutic approach for diabetic nephropathy[J]. Ann Med, 2024, 56(1): 2346543. |
| [22] | Dai B, Chen Y X, Song C Q, et al. Efficacy and safety of Yishen Huashi granules combined with conventional therapy in the treatment of diabetic kidney disease: a systematic review and meta-analysis[J]. Heliyon, 2024, 10(20): e39213. |
| [23] | Zhao T T, Xiang Q, Lie B F, et al. Yishen Huashi granule modulated lipid metabolism in diabetic nephropathy via PI3K/AKT/mTOR signaling pathways[J]. Heliyon, 2023, 9(3): e14171. |
| [24] | Liang M Z, Zhu X D, Zhang D, et al. Yi-Shen-Hua-Shi granules inhibit diabetic nephropathy by ameliorating podocyte injury induced by macrophage-derived exosomes[J]. Front Pharmacol, 2022, 13: 962606. |
| [25] | Wu J S, Shi R, Zhong J, et al. Renal protective role of Xiexin decoction with multiple active ingredients involves inhibition of inflammation through downregulation of the nuclear factor-κB pathway in diabetic rats[J]. Evid Based Complement Alternat Med, 2013, 2013: 715671. |
| [26] | Wu J S, Shi R, Lu X, et al. Combination of active components of Xiexin decoction ameliorates renal fibrosis through the inhibition of NF-κB and TGF-β1/Smad pathways in db/db diabetic mice[J]. PLoS One, 2015, 10(3): e0122661. |
| [27] | Wu T X, Harrison R A, Chen X Y, et al. Tongxinluo (Tong Xin Luo or Tong-Xin-Luo) capsule for unstable angina pectoris[J]. Cochrane Database Syst Rev, 2006, 2006(4): CD004474. |
| [28] | Xie X S, Liu H C, Yang M, et al. Ginsenoside Rb1, a panoxadiol saponin against oxidative damage and renal interstitial fibrosis in rats with unilateral ureteral obstruction[J]. Chin J Integr Med, 2009, 15(2): 133-140. |
| [29] | 袁国强, 吴以岭, 贾振华,等. 通心络对大脑中动脉闭塞模型大鼠脑缺血后神经细胞凋亡的影响[J]. 中国中西医结合杂志, 2007, 27(8): 720-723. |
| Yuan G Q, Wu Y L, Jia Z H. Experimental study on effect of Tongxinluo on nerve cell apoptosis after cerebral ischemia in middle cerebral arterial obstructive model rats[J]. Chinese Journal of Integrated Traditional and Western Medicine, 2007, 27(8): 720-723. | |
| [30] | Zhong X, Chung A C K, Chen H Y, et al. Smad3-mediated upregulation of miR-21 promotes renal fibrosis[J]. J Am Soc Nephrol, 2011, 22(9): 1668-1681. |
| [31] | Wang Y H, Qian P G, Liu P, et al. Effects of Panax notoginseng flower extract on the TGF-β/Smad signal transduction pathway in heart remodeling of human chymase transgenic mice[J]. Mol Med Rep, 2012, 5(6): 1443-1448. |
| [32] | Wang J Y, Gao Y B, Zhang N, et al. Tongxinluo ameliorates renal structure and function by regulating miR-21-induced epithelial-to-mesenchymal transition in diabetic nephropathy[J]. Am J Physiol Renal Physiol, 2014, 306(5): F486-F495. |
| [33] | Xu Z J, Shu S, Li Z J, et al. Liuwei Dihuang pill treats diabetic nephropathy in rats by inhibiting of TGF-β/SMADS, MAPK, and NF-κB and upregulating expression of cytoglobin in renal tissues[J]. Medicine (Baltimore), 2017, 96(3): e5879. |
| [34] | Zheng W W, Qian C, Xu F M, et al. Fuxin Granules ameliorate diabetic nephropathy in db/db mice through TGF-β1/Smad and VEGF/VEGFR2 signaling pathways[J]. Biomed Pharmacother, 2021, 141: 111806. |
| [35] | Miao X J, Bi T T, Tang J M, et al. Regulatory mechanism of TGF-β1/SGK1 pathway in tubulointerstitial fibrosis of diabetic nephropathy[J]. Eur Rev Med Pharmacol Sci, 2019, 23(23): 10482-10488. |
| [36] | Jin J, Zhang Z, Chen J W, et al. Jixuepaidu Tang-1 inhibits epithelial-mesenchymal transition and alleviates renal damage in DN mice through suppressing long non-coding RNA LOC498759[J]. Cell Cycle, 2019, 18(22): 3125-3136. |
| [37] | Adesso S, Russo R, Quaroni A, et al. Astragalus membranaceus extract attenuates inflammation and oxidative stress in intestinal epithelial cells via NF-κB activation and Nrf2 response[J]. Int J Mol Sci, 2018, 19(3): 800. |
| [38] | Farag M R, Elhady W M, Ahmed S Y A, et al. Astragalus polysaccharides alleviate tilmicosin-induced toxicity in rats by inhibiting oxidative damage and modulating the expressions of HSP70, NF-kB and Nrf2/HO-1 pathway[J]. Res Vet Sci, 2019, 124: 137-148. |
| [39] | Li M X, Wang W X, Xue J, et al. Meta-analysis of the clinical value of Astragalus membranaceus in diabetic nephropathy[J]. J Ethnopharmacol, 2011, 133(2): 412-419. |
| [40] | Szrejder M, Piwkowska A. AMPK signalling: implications for podocyte biology in diabetic nephropathy[J]. Biol Cell, 2019, 111(5): 109-120. |
| [41] | Meng X, Wei M M, Wang D, et al. Astragalus polysaccharides protect renal function and affect the TGF- β/Smad signaling pathway in streptozotocin-induced diabetic rats[J]. J Int Med Res, 2020, 48(5): 300060520903612. |
| [42] | Du N, Xu Z P, Gao M Y, et al. Combination of Ginsenoside Rg1 and Astragaloside Ⅳ reduces oxidative stress and inhibits TGF-β1/Smads signaling cascade on renal fibrosis in rats with diabetic nephropathy[J]. Drug Des Devel Ther, 2018, 12: 3517-3524. |
| [43] | Sun L, Li W P, Li W Z, et al. Astragaloside IV prevents damage to human mesangial cells through the inhibition of the NADPH oxidase/ROS/Akt/NF-κB pathway under high glucose conditions[J]. Int J Mol Med, 2014, 34(1): 167-176. |
| [44] | Shao M H, Ye C Y, Bayliss G, et al. New insights into the effects of individual Chinese herbal medicines on chronic kidney disease[J]. Front Pharmacol, 2021, 12: 774414. |
| [45] | Chen K J. Blood stasis syndrome and its treatment with activating blood circulation to remove blood stasis therapy[J]. Chin J Integr Med, 2012, 18(12): 891-896. |
| [46] | Shen Y H, Wang S L, Liu Y Y, et al. The effects of salvianolate combined with western medicine on diabetic nephropathy: a systematic review and meta-analysis[J]. Front Pharmacol, 2020, 11: 851. |
| [47] | Bergman M E, Davis B, Phillips M A. Medically useful plant terpenoids: biosynthesis, occurrence, and mechanism of action[J]. Molecules, 2019, 24(21): 3961. |
| [48] | Pang H Q, Wu L, Tang Y P, et al. Chemical analysis of the herbal medicine salviae miltiorrhizae Radix et rhizoma (Danshen)[J]. Molecules, 2016, 21(1): 51. |
| [49] | Cai L Q, Chen Y, Xue H Z, et al. Effect and pharmacological mechanism of Salvia miltiorrhiza and its characteristic extracts on diabetic nephropathy[J]. J Ethnopharmacol, 2024, 319(Pt 3): 117354. |
| [50] | Zhou Y, Li J S, Zhang X, et al. Ursolic acid inhibits early lesions of diabetic nephropathy[J]. Int J Mol Med, 2010, 26(4): 565-570. |
| [51] | Qi M Y, Wang X T, Xu H L, et al. Protective effect of ferulic acid on STZ-induced diabetic nephropathy in rats[J]. Food Funct, 2020, 11(4): 3706-3718. |
| [52] | Lin C Y, Tsai S J, Huang C S, et al. Antiglycative effects of protocatechuic acid in the kidneys of diabetic mice[J]. J Agric Food Chem, 2011, 59(9): 5117-5124. |
| [53] | Kumari S, Kamboj A, Wanjari M, et al. Nephroprotective effect of Vanillic acid in STZ-induced diabetic rats[J]. J Diabetes Metab Disord, 2021, 20(1): 571-582. |
| [54] | Ma T K, Xu L, Lu L X, et al. Ursolic acid treatment alleviates diabetic kidney injury by regulating the ARAP1/AT1R signaling pathway[J]. Diabetes Metab Syndr Obes, 2019, 12: 2597-2608. |
| [55] | Xu L H, Shen P Q, Bi Y L, et al. Danshen injection ameliorates STZ-induced diabetic nephropathy in association with suppression of oxidative stress, pro-inflammatory factors and fibrosis[J]. Int Immunopharmacol, 2016, 38: 385-394. |
| [56] | Xia J, Zhang L, Zhang X X, et al. Effect of large dosage of Fuling on urinary protein of diabetic nephropathy: a protocol of systematic review and meta-analysis of randomized clinical trials[J]. Medicine (Baltimore), 2020, 99(40): e22377. |
| [57] | Wang Y Z, Zhang J, Zhao Y L, et al. Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb.: a review[J]. J Ethnopharmacol, 2013, 147(2): 265-276. |
| [58] | Wu Y W, Deng H H, Sun J Z, et al. Poricoic acid A induces mitophagy to ameliorate podocyte injury in diabetic kidney disease via downregulating FUNDC1[J]. J Biochem Mol Toxicol, 2023, 37(12): e23503. |
| [59] | Wu Y W, Xu Y C, Deng H H, et al. Poricoic acid a ameliorates high glucose-induced podocyte injury by regulating the AMPKα/FUNDC1 pathway[J]. Mol Biol Rep, 2024, 51(1): 1003. |
| [60] | Wang Q, Pang Y R, Yang H, et al. Investigating the mechanism of Fuling-Banxia-Dafupi in the treatment of diabetic kidney disease using network pharmacology and molecular docking[J]. Nat Prod Res, 2025, 39(17): 5109-5114. |
| [61] | Zhou P H, Wang N, Lu S J, et al. Dihydrolipoamide S-acetyltransferase activation alleviates diabetic kidney disease via AMPK-autophagy axis and mitochondrial protection[J]. Transl Res, 2024, 274: 81-100. |
| [62] | Ghosian Moghaddam M H, Roghani M, Maleki M. Effect of Hypericum perforatum aqueous extracts on serum lipids, aminotransferases, and lipid peroxidation in hyperlipidemic rats[J]. Res Cardiovasc Med, 2016, 5(2): e31326. |
| [63] | Abd El Motteleb D M, Abd El Aleem D I. Renoprotective effect of Hypericum perforatum against diabetic nephropathy in rats: insights in the underlying mechanisms[J]. Clin Exp Pharmacol Physiol, 2017, 44(4): 509-521. |
| [64] | Paterniti I, Briguglio E, Mazzon E, et al. Effects of Hypericum Perforatum, in a rodent model of periodontitis[J]. BMC Complement Altern Med, 2010, 10: 73. |
| [65] | Mozaffari S, Esmaily H, Rahimi R, et al. Effects of Hypericum perforatum extract on rat irritable bowel syndrome[J]. Pharmacogn Mag, 2011, 7(27): 213-223. |
| [66] | Chen S Z, Liu X X, Peng C, et al. The phytochemical hyperforin triggers thermogenesis in adipose tissue via a Dlat-AMPK signaling axis to curb obesity[J]. Cell Metab, 2021, 33(3): 565-580.e7. |
| [67] | Lu S J, Jiang Q X, Zhou P H, et al. Targeting Dlat-Trpv3 pathway by hyperforin elicits non-canonical promotion of adipose thermogenesis as an effective anti-obesity strategy[J]. J Adv Res, 2025, 75: 793-809. |
| [68] | Yang S B, Zhong S, Deng Z J, et al. Hyperforin regulates renal fibrosis via targeting the PI3K-AKT/ICAM1 axis[J]. Cell Signal, 2023, 108: 110691. |
| [69] | Gao P, Li L L, Yang L, et al. Yin Yang 1 protein ameliorates diabetic nephropathy pathology through transcriptional repression of TGFβ1[J]. Sci Transl Med, 2019, 11(510): eaaw2050. |
| [70] | Zhang X J, Zhang J R, Xu X J, et al. Picroside Ⅱ alleviates renal fibrosis through YY1-dependent transcriptional inhibition of TGFβ1[J]. Metabol Open, 2024, 23: 100316. |
| [71] | Xu G K, Sun C Y, Qin X Y, et al. Effects of ethanol extract of Bombax ceiba leaves and its main constituent mangiferin on diabetic nephropathy in mice[J]. Chin J Nat Med, 2017, 15(8): 597-605. |
| [72] | Liu W W, Liang L M, Zhang Q, et al. Effects of andrographolide on renal tubulointersticial injury and fibrosis. Evidence of its mechanism of action[J]. Phytomedicine, 2021, 91: 153650. |
| [73] | Menzies R I, Booth J W R, Mullins J J, et al. Hyperglycemia-induced renal P2X7 receptor activation enhances diabetes-related injury[J]. EBioMedicine, 2017, 19: 73-83. |
| [74] | Hou Y, Lin S X, Qiu J, et al. NLRP3 inflammasome negatively regulates podocyte autophagy in diabetic nephropathy[J]. Biochem Biophys Res Commun, 2020, 521(3): 791-798. |
| [75] | Wang C, Hou X X, Rui H L, et al. Artificially cultivated Ophiocordyceps sinensis alleviates diabetic nephropathy and its podocyte injury via inhibiting P2X7R expression and NLRP3 inflammasome activation[J]. J Diabetes Res, 2018, 2018: 1390418. |
| [76] | Yoon J J, Park J H, Lee Y J, et al. Protective effects of ethanolic extract from rhizome of Polygoni avicularis against renal fibrosis and inflammation in a diabetic nephropathy model[J]. Int J Mol Sci, 2021, 22(13): 7230. |
| [77] | Lin L, Wang Q H, Yi Y X, et al. Liuwei Dihuang pills enhance the effect of western medicine in treating diabetic nephropathy: a meta-analysis of randomized controlled trials[J]. Evid Based Complement Alternat Med, 2016, 2016: 1509063. |
| [78] | Lu Q, Li C L, Chen W W, et al. Clinical efficacy of Jinshuibao capsules combined with angiotensin receptor blockers in patients with early diabetic nephropathy: a meta-analysis of randomized controlled trials[J]. Evid Based Complement Alternat Med, 2018, 2018: 6806943. |
| [1] | 韩珍, 王浩, 苏秀秀, 方际. 金丝桃素通过抑制NF-κB信号通路减轻糖尿病肾病足细胞损伤[J]. 上海交通大学学报(医学版), 2026, 46(1): 43-53. |
| [2] | 魏云鑫, 蒋绪顺, 蔡梦瑶, 温睿智, 杜晓刚. COMP与糖尿病肾病自噬相关性分析及其功能验证[J]. 上海交通大学学报(医学版), 2024, 44(7): 847-858. |
| [3] | 王莹, 平立风, 刘彤彤, 刘珊珊, 刘磊. 甲基莲心碱调节SDF-1/CXCR4信号通路对糖尿病肾病的影响[J]. 上海交通大学学报(医学版), 2024, 44(2): 183-195. |
| [4] | 贾君杰, 邢海帆, 张群子, 刘奇烨, 汪年松, 范瑛. 缺氧诱导因子-1α抑 制剂YC-1改善糖尿病肾病小鼠肾脏损伤的机制研究[J]. 上海交通大学学报(医学版), 2023, 43(9): 1089-1098. |
| [5] | 吴佳晋, 钟晨, 李大伟, 陈若洋, 瞿俊文, 张明. 甲基转移酶3调控pri-miR-21甲基化修饰在糖尿病肾病肾脏纤维化中的作用[J]. 上海交通大学学报(医学版), 2023, 43(1): 1-7. |
| [6] | 邢海帆, 范瑛. 单细胞RNA测序应用于肾小球疾病研究的进展[J]. 上海交通大学学报(医学版), 2022, 42(10): 1458-1465. |
| [7] | 张佳思, 邹春波, 卢宇, 陈茜, 张伟亚, 何姣姣. 血脂蛋白磷脂酶A2和中性粒细胞明胶酶相关脂质运载蛋白在诊断早期糖尿病肾病中的价值[J]. 上海交通大学学报(医学版), 2021, 41(6): 770-775. |
| [8] | 姜梦迪, 张文. 糖尿病肾病中的组蛋白修饰与靶向干预的研究进展[J]. 上海交通大学学报(医学版), 2021, 41(1): 103-107. |
| [9] | 王婷婷 1, 2,李明杰 1,林宁 1,钮忆欣 1,简蔚霞 1,苏青 1. 血清高尿酸水平与住院糖尿病患者白蛋白尿短期进展的关系研究[J]. 上海交通大学学报(医学版), 2019, 39(7): 754-. |
| [10] | 祝利民,沈克平. 中药对大肠癌干细胞干预作用的研究进展[J]. 上海交通大学学报(医学版), 2017, 37(2): 258-. |
| [11] | 李昀昊,杨宏杰,何燕铭,等. 中药复方对桥本甲状腺炎患者细胞因子和抗体的影响[J]. 上海交通大学学报(医学版), 2015, 35(8): 1174-. |
| [12] | 赵明明,刘丽梅. 蛋白激酶C-β与糖尿病肾病研究的进展[J]. 上海交通大学学报(医学版), 2014, 34(4): 551-. |
| [13] | 陈 珏, 张路路, 严玉澄,等. 雷帕霉素对糖尿病肾病大鼠足细胞损伤的作用[J]. 上海交通大学学报(医学版), 2013, 33(9): 1197-. |
| [14] | 吴景程, 李晓华, 彭永德. 2型糖尿病患者血清胆红素水平与糖尿病肾病的关系[J]. 上海交通大学学报(医学版), 2013, 33(6): 813-. |
| [15] | 王 袁, 吴琪俊, 施 榕. 糖尿病肾病与载脂蛋白E基因多态性相关性的Meta分析[J]. , 2011, 31(5): 625-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
