先天免疫系统是人类机体免疫防御的重要组成部分。微生物核酸被一系列宿主编码的模式识别受体(pattern recognition receptor,PRR)识别后,触发细胞内信号级联反应,激活Ⅰ型干扰素(interferon,IFN)以及促炎细胞因子,如肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)、白介素-1(interleukin-1,IL-1)、IL-6等,这一系列过程是引发即时抗病毒反应和适应性免疫的先决条件[1-2]。蛋白质泛素化修饰在调节先天免疫的过程中起关键作用,如抗原呈递、细胞分化、免疫防御和炎症反应等[3]。E3泛素连接酶能够特异性识别靶蛋白,在泛素化途径中具有重要作用,并且参与细胞内的多种免疫调控,如:通过诱导髓样分化因子88(myeloid differentiation factor 88,MyD88)降解和TANK结合激酶1(TANK binding kinase 1,TBK1)活化,抑制Toll样受体(Toll-like receptor,TLR)/核因子κB(nuclear factor κB,NF-κB)信号转导[4];与NF-κB P65亚基结合,促进P65发生泛素化并降解,抑制NF-κB活化[5];促进干扰素基因刺激因子(stimulator of interferon genes,STING)在K150处的泛素化及其降解,抑制干扰素调节因子3(interferon regulatory factor 3,IRF3)依赖性抗病毒信号转导等[6]。
RBX1(ring-box protein 1)是一种RING结构蛋白,与S期激酶相关蛋白1(S-phase kinase-associated protein 1,SKP1)、CUL1(cullin 1)和F-box蛋白一起组成SCF(SKP1-CUL1-F-box)复合体。SCF复合体是一种重要的E3泛素化连接酶[7]。研究[8-12]表明,RBX1在多种肿瘤中表达异常,包括肝癌、胃癌、食管癌、乳腺癌、膀胱癌、肺癌和结肠癌,且与临床不良预后相关。如RBX1的高表达可促进三阴性乳腺癌的转移[13],也是小细胞肺癌患者不良预后的相关因素[14];RBX1低表达促进高级别浆液性卵巢癌前体细胞转变为卵巢癌细胞[15];RBX1可调控细胞周期,在神经胶质瘤细胞中,沉默RBX1可使细胞停滞在G2/M期,并导致细胞衰老和凋亡,从而抑制肿瘤细胞生长[9]。E3泛素化连接酶在免疫调控中发挥着重要作用,但RBX1作为E3泛素化连接酶的重要组成部分,对于肿瘤免疫相关基因的调控作用鲜有报道。
葡萄膜黑色素瘤(uveal melanoma,UVM)是成人最常见的眼内恶性肿瘤,极易发生复发、转移,患者预后差,且目前UVM的靶向治疗效果不佳,需要寻找新的治疗靶点[16]。本研究旨在探索RBX1在UVM中对免疫相关基因的调控作用及可能的机制,为改善UVM肿瘤免疫治疗提供新的研究方向。
1 材料与方法
1.1 实验材料
1.1.1 细胞来源
本研究使用的人UVM细胞系,包括92.1细胞系(上海雅吉生物科技有限公司)、MEL290细胞系和OMM2.3细胞系(2种细胞系均来自中国科学院典型培养物保藏委员会细胞库)。所有细胞系均用含10%胎牛血清的RPMI 1640培养液培养。
1.1.2 主要试剂和仪器
RPMI 1640培养液、Opti-MEM培养液、胎牛血清、0.25%胰蛋白酶、青霉素-链霉素(美国Gibco),LipofectamineTM 2000(美国Thermo Fisher),荧光定量PCR预混液ChamQ SYBR qPCR Master Mix(中国诺唯赞),RBX1抗体(11922S,美国CST),β-肌动蛋白(β-actin)抗体(66009,美国Proteintech),信号转导与转录激活因子1(signal transducer and activator of transcription 1,STAT1)抗体(14994S,美国CST),p-STAT1抗体(9167S,美国CST),荧光二抗(A21206,美国Invitrogen),RNA抽提试剂盒(美国EZBioscience),STAT1活化抑制剂fludarabine(美国Selleck)。
CO2培养箱(美国Thermo Fisher),电泳装置、ChemiDoc凝胶成像仪(美国Bio-Rad),离心机(德国Eppendorf),Light Cycler 480Ⅱ定量PCR仪(美国Roche)。
1.2 实验方法
1.2.1 数据库分析
使用GEPIA数据库(
1.2.2 小干扰RNA转染
转染前24 h提前将状态良好的细胞均匀接种到6孔板中,使转染时细胞密度为50%,转染时使用无抗生素培养液。在50 μL Opti-MEM培养液中加入200 μmol 小干扰RNA(small interfering RNA,siRNA),轻轻吹打混匀;在50 μL Opti-MEM 培液中加入5.0 μL LipofectamineTM 2000试剂,轻轻吹打混匀,并且室温静置5 min。将含有转染试剂和siRNA的培养液混合,轻轻吹打混匀,并室温静置15~20 min。将静置后的转染培养液加入6孔细胞板中,轻摇混匀。将细胞板置于37 ℃、5%CO2培养箱中培养6~8 h后更换新鲜培养液,继续培养48 h后进行验证。所用siRNA序列见表1,序列设计及合成由吉满生物科技(上海)有限公司完成。
表1 siRNA序列
Tab 1
| Primer name | Sequence (5'→3') |
|---|---|
| siRBX1-1 | GGGAUAUUGUGGUUGAUAAtt |
| siRBX1-2 | CAGACCGUGUGUUUCCAAAtt |
1.2.3 实时荧光定量PCR
用RNA抽提试剂盒提取细胞RNA,取1 μg RNA进行反转录,得到cDNA。10 μL PCR反应体系:ChamQ SYBR qPCR Master Mix 5 μL,cDNA模板5 μL,上、下游引物(10 μmol/L)各1 μL。每个样本设置3个复孔。上样结束后贴上透明膜并刮除气泡,离心60 s后放入定量PCR仪中按照设定程序扩增。以β-actin作为内参计算mRNA相对表达量。实时荧光定量PCR(qPCR)的引物序列见表2。
表2 qPCR检验的引物序列
Tab 2
| Gene | Forward (5'→3') | Reverse (5'→3') |
|---|---|---|
| β-actin | CATGTACGTTGCTATCCAGGC | CTCCTTAATGTCACGCACGAT |
| RBX1 | TTGTGGTTGATAACTGTGCCAT | GACGCCTGGTTAGCTTGACAT |
| STAT1 | CAGCTTGACTCAAAATTCCTGGA | TGAAGATTACGCTTGCTTTTCCT |
| CXCL9 | TGAGAAAGGGTCGCTGTTCC | TCAAACTGCTTGGCTCACCA |
| CXCL10 | GCTTCCAAGGATGGACCACA | GCAGGGTCAGAACATCCACT |
1.2.4 Western blotting
用胰酶消化细胞,在4 ℃离心机中离心收集细胞沉淀,经PBS缓冲液清洗后用Urea蛋白裂解液在冰上裂解30 min,在4 ℃下离心获得蛋白上清液,用酶标仪进行蛋白定量后与十二烷基磺酸钠(sodium dodecyl sulfate,SDS)一起煮沸,获得蛋白样品。取30 μg的蛋白样品上样后行蛋白凝胶电泳100 min,然后将蛋白质转膜到聚偏二氟乙烯(PVDF)膜上。用5%牛血清白蛋白溶液室温封闭1 h,洗膜后滴加一抗,4 ℃孵育过夜。次日洗膜后滴加二抗,在室温下孵育30 min,洗膜后在凝胶成像仪中成像并记录。
1.2.5 转录组测序
转录组测序(RNA sequencing,RNA-seq)由上海派森诺生物科技股份有限公司完成,每组设置2个生物学重复。使用TRIzol法提取siRBX1干预组(转染siRBX1-1或siRBX1-2)和对照组(转染siNC)92.1细胞的RNA,每个样品约需要1 μg的总RNA。在转录组文库构建完成并质检合格后上机测序,对各组样本进行基因表达水平定量。计算每个基因的每千个碱基的转录每百万映射读取的片段数(fragments per kilobase of exon model per million mapped fragments,FPKM)值,对差异表达的基因进行基因集富集分析(gene set enrichment analysis,GSEA)。
1.2.6 药物干预
用fludarabine抑制细胞STAT1活性。以DMSO为溶剂,分别按fludarabine终浓度5 nmol/L和10 nmol/L加入细胞培养液,培养48 h后进行检测。对照组加入等体积DMSO。
1.3 统计学分析
应用GraphPad Prism 8软件进行数据的分析和处理。符合正态分布、方差齐性的定量资料的多组间比较采用方差分析,两两比较采用双尾非配对t检验。P<0.05表示差异具有统计学意义。
2 结果
2.1 RBX1在肿瘤中的临床意义
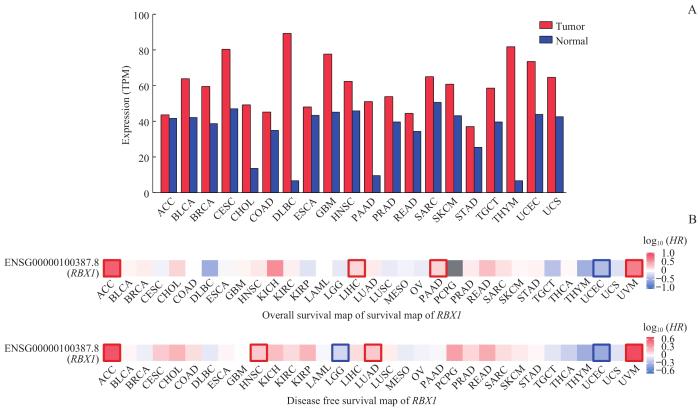
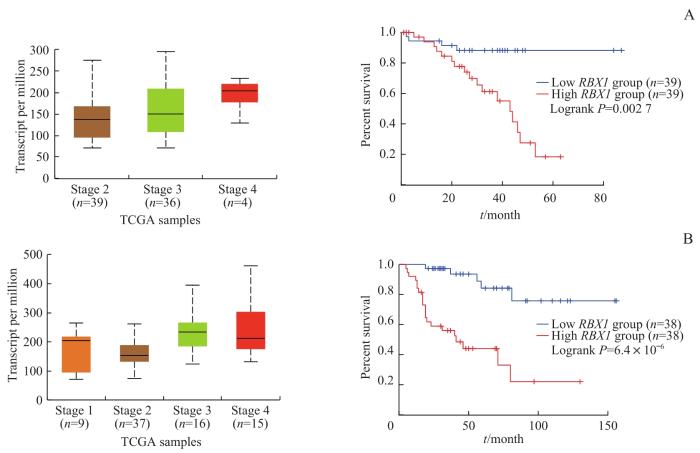
为探索RBX1在肿瘤免疫中的作用,我们首先在GEPIA数据库中检索了RBX1基因在各种肿瘤中的表达情况。图1A显示RBX1基因在多种肿瘤中高表达。分析RBX1基因的表达与各肿瘤患者总生存率(overall survival)和无病生存率(disease free survival)的关系,结果(图1B)显示RBX1的高表达是UVM和肾上腺皮质癌(adrenocortical carcinoma,ACC)患者生存的显著不利因素。为探索RBX1与肿瘤的关系,我们通过UALCAN网站检索了TCGA数据库并进行分析,结果(图2)显示UVM和ACC临床分期较晚的患者,RBX1表达水平相对更高,且RBX1高表达的患者总生存期更短。
图1
图1
RBX1 在肿瘤中的表达水平及对患者生存的影响
Note: A. Search for RBX1 expression in different tumors in GEPIA database. B. Overall survival heat map (above) and disease free survival heat map (below) of RBX1 in patients with various tumors. TPM—transcript per million; ACC—adrenocortical carcinoma; BLCA—bladder urothelial carcinoma; BRCA—breast invasive carcinoma; CESC—cervical squamous cell carcinoma and endocervical adenocarcinoma; CHOL—cholangiocarcinoma; COAD—colon adenocarcinoma; DLBC—lymphoid neoplasm diffuse large B-cell lymphoma; ESCA—esophageal carcinoma; GBM—glioblastoma multiforme; HNSC—head and neck squamous cell carcinoma; PAAD—pancreatic adenocarcinoma; PRAD—prostate adenocarcinoma; READ—rectum adenocarcinoma; SARC—sarcoma; SKCM—skin cutaneous melanoma; STAD—stomach adenocarcinoma; TGCT—testicular germ cell tumor; THYM—thymoma; UCEC—uterine corpus endometrial carcinoma; UCS—uterine carcinosarcoma; PCPG—pheochromocytoma and paraganglioma; KICH—kidney chromophobe; KIRC—kidney renal clear cell carcinoma; KIRP—kidney renal papillary cell carcinoma; LAML—acute myeloid leukemia; LGG—brain lower grade glioma; LIHC—liver hepatocellular carcinoma; LUAD—lung adenocarcinoma; LUSC—lung squamous cell carcinoma; MESO—mesothelioma; OV—ovarian serous cystadenocarcinoma; THCA—thyroid carcinoma; UVM—uveal melanoma.
Fig 1
Expression of RBX1 in tumors and its effect on patients' survival
图2
图2
RBX1 表达水平与UVM和ACC患者分期及总生存期的关系
Note: A. RBX1 expression levels in different stages of UVM patients (left) and overall survival curves of high expression and low expression patients (right). B. RBX1 expression levels in different stages of ACC patients (left) and overall survival curves of high expression and low expression patients (right).
Fig 2
Relationships of RBX1 expression level with stages and overall survivals of UVM and ACC patients
2.2 细胞系转染siRNA后 RBX1 的表达变化
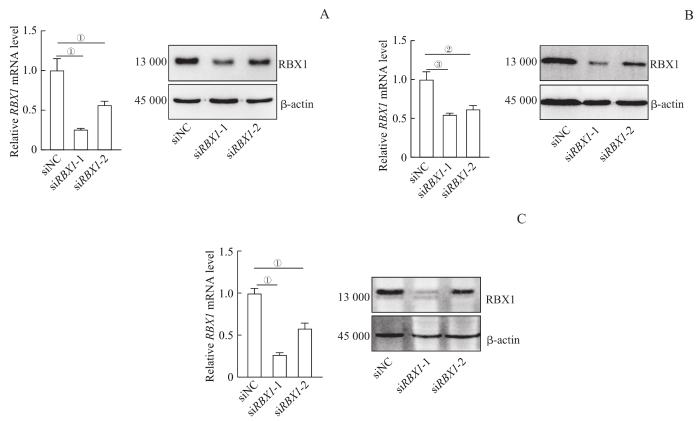
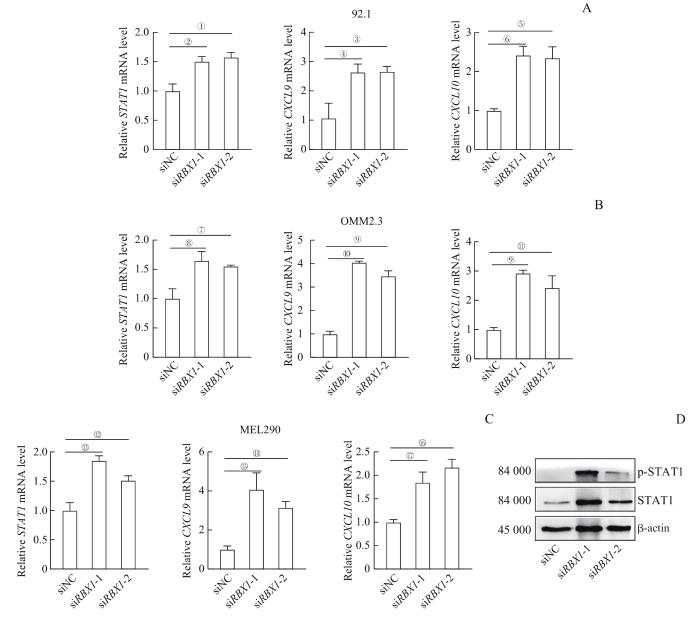
为探索RBX1在肿瘤免疫中的调控作用,我们选用了人UVM 92.1细胞、OMM2.3细胞和MEL290细胞为研究体系,转染siRNA,瞬时敲低RBX1。qPCR和Western blotting验证结果显示,siRBX1-1和siRBX1-2转染后92.1细胞、OMM2.3细胞和MEL290细胞中RBX1的mRNA及蛋白质表达水平均下降(图3)。
图3
图3
转染siRNA后UVM细胞系中 RBX1 mRNA及蛋白的表达变化
Note:A. Expression of RBX1 in 92.1 cell line. B. Expression of RBX1 in OMM2.3 cell line. C. Expression of RBX1 in Mel290 cell line. ①P=0.011, ②P=0.016, ③P=0.020.
Fig 3
Expression changes of RBX1 mRNA and protein in UVM cell lines transfected with siRNA
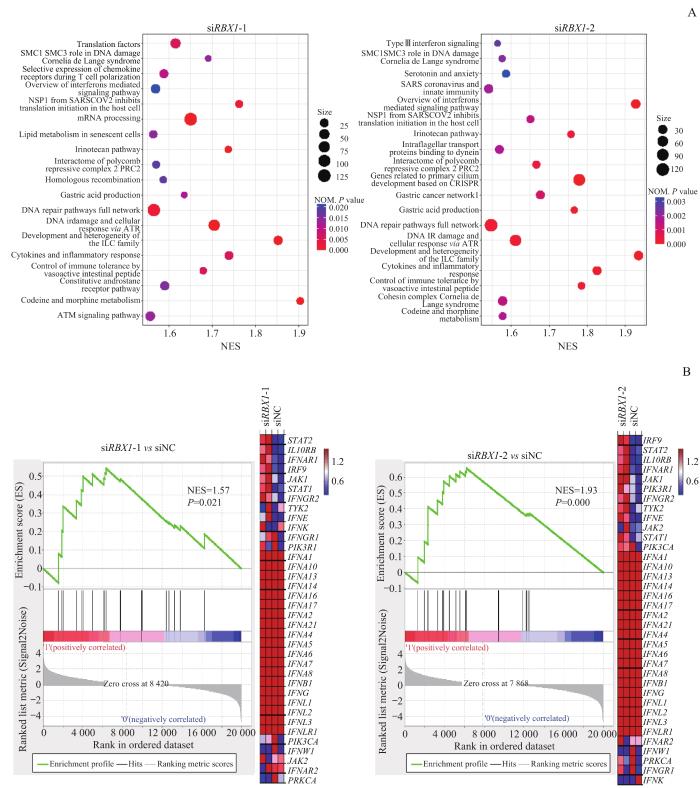
2.3 RBX1对免疫相关基因表达的调控
图4
图4
92.1细胞系中敲低 RBX1 对免疫相关通路的影响
Note: A. Differential gene enrichment analysis after siRBX1-1 (left) or siRBX1-2 (right) intervention compared with siNC. B. Differential genes enriched in the interferon-mediated signaling pathway after siRBX1-1 (left) or siRBX1-2 (right) intervention compared with siNC, and the associated differential genes heat map. NES—normalized enrichment score; STAT2—signal transducer and activator of transcription 2; IL10RB—interleukin 10 receptor subunit β; IFNAR1—interferon α and β receptor subunit 1; IRF9—interferon regulatory factor 9; JAK1—Janus kinase 1; IFNGR2—interferon γ receptor 2; TYK2—tyrosine kinase 2; IFNE—interferon ε; IFNK—interferon κ; IFNGR1—interferon γ receptor 1; PIK3R1—phosphoinositide-3-kinase regulatory subunit 1; IFNA1—interferon α1; IFNA10—interferon α10; IFNA13—interferon α13; IFNA14—interferon α14; IFNA16—interferon α16; IFNA17—interferon α17; IFNA2—interferon α2; IFNA21—interferon α21; IFNA4—interferon α4; IFNA5—interferon α5; IFNA6—interferon α6; IFNA7—interferon α7; IFNA8—interferon α8; IFNB1—interferon β1; IFNG—interferon γ; IFNL1—interferon λ1; IFNL2—interferon λ2; IFNL3—interferon λ3; IFNLR1—interferon λ receptor 1; PIK3CA—phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α; IFNW1—interferon ω1; JAK2—Janus kinase 2; IFNAR2—interferon α and β receptor subunit 2; PRKCA—protein kinase Cα.
Fig 4
Effect of RBX1 knockdown on immune-related pathways in 92.1 cell line
图5
图5
RBX1 对UVM细胞中 STAT1 及相关基因的表达调控作用
Note:A. mRNA expression of STAT1, CXCL9, and CXCL10 in 92.1 cell line after siRBX1 intervention detected by qPCR. B. mRNA expression of STAT1, CXCL9, and CXCL10 in OMM2.3 cell line after siRBX1 intervention detected by qPCR. C. mRNA expression of STAT1, CXCL9, and CXCL10 in MEL290 cell line after siRBX1 intervention detected by qPCR. D. STAT1 and p-STAT1 protein expression levels in 92.1 cell line after siRBX1 intervention detected by Western blotting. ①P=0.029, ②P=0.009, ③P=0.048, ④P=0.045, ⑤P=0.017, ⑥P=0.006, ⑦P=0.028, ⑧P=0.047, ⑨P=0.001, ⑩P=0.000, 11P=0.018, 12P=0.043, 13P=0.019, 14P=0.002, 15P=0.025, 16P=0.012, 17P=0.033.
Fig 5
Regulation of RBX1 on STAT1 and related genes expressions in UVM cell lines
2.4 抑制STAT1后 CXCL9 和 CXCL10 的表达下降
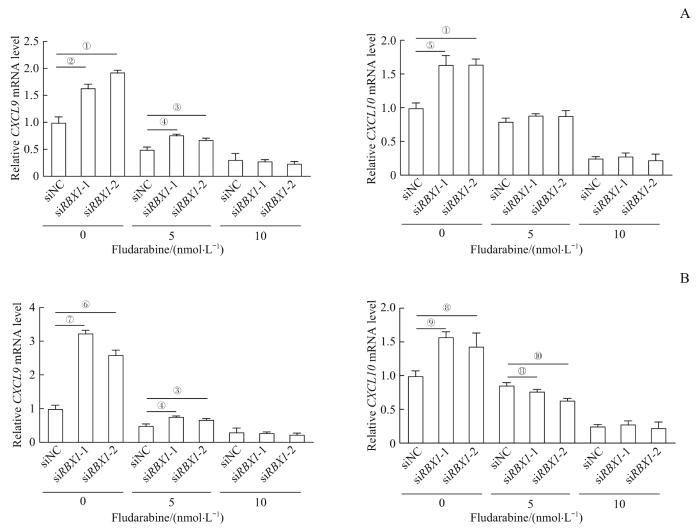
为进一步探索RBX1是否通过STAT1来调控CXCL9和CXCL10的表达,使用STAT1抑制剂fludarabine进行研究。在6孔板中分别接种相同数量的OMM2.3细胞和MEL290细胞并转染siRNA干扰RBX1表达,干扰24 h后加入fludarabine(终浓度分别为5 nmol/L和10 nmol/L),并设置DMSO组作为对照组,处理48 h后通过qPCR检测STAT1下游基因CXCL9和CXCL10的mRNA表达。结果(图6)显示,加入STAT1抑制剂后,siNC组的CXCL9和CXCL10的表达被抑制,而siRBX1干预组的CXCL9和CXCL10被抑制程度更加显著,且在高浓度fludarabine(10 nmol/L)处理下,siRBX1干预组与siNC组的CXCL9和CXCL10表达水平基本一致。表明RBX1可能通过调控STAT1影响CXCL9和CXCL10的表达。
图6
图6
STAT1抑制剂对 RBX1 敲低后的UVM细胞中 CXCL9 和 CXCL10 mRNA表达的影响
Note: The RBX1 knockdown cell lines of OMM2.3 (A) and MEL290 (B) were treated with 5 nmol/L and 10 nmol/L fludarabine. The samples were collected after 48 h of dosing and qPCR was performed. ①P=0.002, ②P=0.003, ③P=0.041, ④P=0.006, ⑤P=0.004, ⑥P=0.007, ⑦P=0.000, ⑧P=0.027, ⑨P=0.012, ⑩P=0.008, 11P=0.035.
Fig 6
Effect of STAT1 inhibitor on the mRNA expression of CXCL9 and CXCL10 in UVM cell lines after RBX1 knockdown
3 讨论
在本研究中,对TCGA数据库的分析结果显示RBX1在UVM中高表达,且显著影响患者的总生存期和无病生存期,在晚期的UVM患者中表达水平更高,因此推测RBX1在UVM中可能起重要作用。沉默92.1细胞的RBX1基因后,对RNA-seq结果进行GSEA分析,结果发现RBX1除了参与细胞周期和DNA损伤修复相关通路外,在免疫相关通路上也存在调控作用。研究[4-6]表明,E3泛素化连接酶参与多种免疫调控,鉴于RBX1是E3泛素化连接酶的重要组成部分,我们推测RBX1在肿瘤免疫调节中也可能发挥重要功能。分析显示,92.1细胞中的RBX1被沉默后,STAT1的表达水平升高,qPCR和Western blotting的结果与RNA-seq的结果一致,提示STAT1的活性增强。在Janus激酶(Janus kinase,JAK)/STAT信号通路中,转录因子STAT1是IFN的关键效应因子,参与多种免疫反应[17]。在经典免疫通路中IFN-γ通过JAK使STAT1活化,激活下游免疫通路[21]。趋化因子CXCL9、CXCL10是介导T淋巴细胞迁移的最重要的趋化因子,已有研究[19-20]表明STAT1可调控CXCL9和CXCL10的表达。但RBX1沉默后CXCL9和CXCL10的mRNA表达升高的机制仍不清楚。为进一步证实RBX1是否通过STAT1调控CXCL9和CXCL10的表达,我们利用STAT1的活化抑制剂fludarabine进行验证,结果显示在RBX1沉默的细胞中,原本升高的CXCL9和CXCL10 mRNA在fludarabine的作用下mRNA表达被抑制,表明RBX1可通过STAT1调控CXCL9和CXCL10的表达。由于本研究仅在转录水平上进行验证,因此仍不能确定RBX1是否通过其泛素化作用调控STAT1,未来仍需进一步研究。另外,本研究为体外细胞实验,RBX1在体内对免疫相关基因的调控作用仍需进一步验证。
综上所述,RBX1在UVM细胞中可能通过STAT1调控CXCL9和CXCL10的表达,在免疫调控中有重要作用,为UVM的靶向治疗提供了潜在的选择。
作者贡献声明
周晓雯、李倩负责实验操作,张哲负责数据分析,沈键锋负责实验设计,周晓雯、沈键锋、范先群参与论文的写作和修改。所有作者均阅读并同意了最终稿件的提交。
AUTHOR's CONTRIBUTIONS
The experiments were completed by ZHOU Xiaowen and LI Qian. Data was analyzed by ZHANG Zhe. The study was designed by SHEN Jianfeng. ZHOU Xiaowen, SHEN Jianfeng and FAN Xianqun drafted and revised the manuscript. All the authors have read the last version of paper and consented for submission.
利益冲突声明
所有作者声明不存在利益冲突。
COMPETING INTERESTS
All authors disclose no relevant conflict of interests.
参考文献