七氟烷(sevoflurane)是儿科麻醉中最常用的吸入性全麻药,具有良好的呼吸耐受性,且起效快、恢复快,患者血流动力学稳定。动物研究[6-10]已经证明,重复接受七氟烷麻醉对发育中大脑的长期神经具损伤作用。七氟烷相关神经发育毒性的多种机制也已经被证实,如引起神经细胞死亡,导致神经细胞损伤,影响神经回路可塑性、tau磷酸化和神经内分泌过程等[11]。前额叶皮层(prefrontal cortex,PFC)是关键的记忆与情绪功能脑区。已有研究[12-14]发现,七氟烷麻醉可以通过破坏PFC的兴奋性神经元而诱发行为缺陷、记忆衰退和神经功能异常;重复七氟烷麻醉可引起PFC中N6-甲基腺嘌呤(N6-methyladenosine,m6A)介导的mRNA翻译障碍,这可能与七氟烷引起的精细运动障碍有关[15]。
目前,随着大家对全麻药物影响神经发育的关注增加,七氟烷诱导神经发育毒性机制的相关研究越来越深入。许多新的技术和方法也被开发并应用,比如利用多组学研究七氟烷的神经发育毒性[16-18]。本研究基于新生小鼠单次和重复七氟烷麻醉后PFC单细胞转录组测序(single-cell RNA sequencing,scRNA-seq)结果,探究新生啮齿类动物PFC细胞在七氟烷麻醉后的转录组改变,比较单次和重复七氟烷麻醉后新生啮齿类动物PFC细胞在转录组水平上的差异,研究七氟烷麻醉影响PFC神经发育的潜在相关基因和信号通路,进一步分析PFC中处于不同分化潜能的神经元在不同麻醉次数的情况下产生的转录组水平变化,寻找七氟烷对PFC神经元损伤可能的调控途径,为七氟烷的神经发育毒性预防和治疗提供新的思路。
1 材料与方法
1.1 实验动物
怀孕14.5 d的C57BL/J6母鼠(SPF级)由浙江维通利华实验动物技术有限公司提供,实验动物生产许可证号为SCXK(浙)2019-0001。实验使用的是出生后第6日(postnatal day 6,P6)的新生小鼠。实验小鼠饲养于上海交通大学医学院附属第九人民医院实验动物中心,实验动物使用许可证号为SYXK(沪)2020-0025。小鼠饲养环境温度20~22 ℃,光照12 h/d,母鼠可以自由获得水和饲料,新生小鼠由母鼠哺乳。将新生小鼠分为重复麻醉暴露(Sev3)组、单次麻醉暴露(Sev1)组和对照(Ctrl)组,每组3只。不同性别的小鼠在各组间并未进行等量分配,因此本研究未考虑七氟烷对幼年小鼠神经毒性的性别差异。
1.2 主要试剂和仪器
七氟烷(国药准字H20070172)购自上海恒瑞医药有限公司,MACS组织储存液(130-100-008)、红细胞裂解液(130-094-183)、MACS去死细胞试剂盒(130-090-101)购自德国Miltenyi Biotec公司,磷酸盐缓冲液(PBS,E607008)购自生工生物工程(上海)股份有限公司,胶原酶Ⅰ(LS004194)、脱氧核糖核酸酶Ⅰ(DNase Ⅰ,LS002007)购自美国Worthington Biochemical公司,牛血清白蛋白(BSA,219989625)购自新西兰ChromatoPur公司,AO/PI双染试剂盒(RE010213)购自上海睿钰生物科技有限公司,单细胞基因表达试剂盒(Chromium Single Cell 3' Reagent Kit v2)购自美国10x Genomics公司。
通用型小动物麻醉机(R500,深圳市瑞沃德生命科技有限公司),小动物直肠探针[RET-4,海德创业(北京)生物科技有限公司],手持温度测量仪[TK-8851,海德创业(北京)生物科技有限公司],台式高速冷冻离心机(ST 16R,美国Thermo Fisher Scientific),全自动荧光细胞分析仪(Countstar Rigel S2,上海睿钰生物科技有限公司),Qubit台式荧光仪(Qubit Flex,美国Thermo Fisher Scientific),TapeStation系统(Agilent 2200,美国Agilent Technologies),高通量测序(NGS)仪(NovaSeq 6000,美国Illumina)。
1.3 实验方法
1.3.1 动物麻醉及样本采集
Sev3组的小鼠接受七氟烷麻醉的具体方法为分别在出生后第6日(P6)、第7日(P7)和第8日(P8)于麻醉室中接受3%七氟烷和60%氧气(其余成分为空气)的麻醉,流速为1 L/min,2 h/d。Sev1组的小鼠只在P6当日接受七氟烷麻醉,P7、P8仅接受60%的氧气。Ctrl组的小鼠在P6、P7和P8以相同的流速接受60%的氧气。麻醉室被放置在一个暖箱中,并使用小动物直肠探针和手持温度测量仪间断监测小鼠体温,以保持小鼠的直肠温度为(37.0±0.5)℃。已有研究[19-21]证明,实验中所使用的3%七氟烷加60%的氧气的麻醉方案可导致新生小鼠的认知障碍。实验过程中没有小鼠死亡。小鼠在出生后第9日(P9)经3%七氟烷麻醉3 min后处死,并获取PFC。
1.3.2 单细胞悬液的制备及scRNA-seq
将PFC保存在MACS组织保存液中。组织样本的处理方法如下所述:首先用PBS清洗样本,在冰上切成小块(约1 mm×1 mm×1 mm),用200 U/mL胶原酶Ⅰ和50 U/mL DNase Ⅰ在37 ℃下搅拌45 min进行酶解。消化后,样品通过细胞过滤网过滤,并在300×g下离心5 min。去除上清液后,将细胞悬浮在红细胞裂解液中以裂解红细胞。用含有0.04% BSA的PBS洗涤后,将沉淀的细胞团重新悬浮在含有0.04% BSA的PBS中,并通过孔径35 μm的细胞过滤网重新过滤。然后用AO/PI双染试剂盒对解离的单细胞进行染色,用全自动荧光细胞分析仪检测细胞活性。用MACS去死细胞试剂盒去除死细胞,进一步富集单细胞悬液。
使用单细胞基因表达试剂盒生成单细胞转录组文库。首先,将细胞悬液浓缩至约1 000个细胞/μL并加载到每个通道中以产生含有单细胞和凝胶微珠的乳滴。在反转录步骤之后,乳滴被裂解,cDNA被纯化和扩增。将扩增后的cDNA打断,含有多聚腺苷酸(poly A)尾的片段加接头后,加入引物进行PCR扩增。使用台式荧光仪对最终文库进行定量,并使用TapeStation系统对文库进行质检。所有文库均由NovaSeq 6000测序系统进行双端的150 bp测序。
1.3.3 scRNA-seq数据分析
scRNA-seq数据分析使用NovelBrain Cloud Analysis Platform(
得到每个样品中的所有细胞比对成功的平均序列数,以平均序列数最低的样品为参照,其他样品随机丢弃掉部分序列,使每个样品中的平均序列数一致,然后进行多样本合并。我们使用DoubletFinder按照8%的比例去除双细胞。接下来,将线粒体基因占比超过20%的细胞和基因数表达低于200的细胞去除,将管家基因占比低于40%的细胞去除,将消化影响基因占比超过20%的细胞去除,将红细胞基因占比超过5%的细胞去除,并去除线粒体基因和红细胞基因,从而形成最终表达矩阵用于后续分析。
Seurat软件包(版本4.0.3,
根据文献[23-27]及测序结果,将新生小鼠PFC细胞分为以下10个类型:① 放射状胶质细胞(radial glial cell,RGC),标志基因为Ascl1、Hes6。② 神经元(neuron),标志基因为Dlx1、Sox11、Tubb3、Stmn2、Igfbpl1、Dcx。③ 少突胶质细胞前体细胞(oligodendrocyte precursor cell,OPC),标志基因为Pdgfra、C1ql1、Olig1、Olig2。④ 具有分化倾向的少突胶质细胞前体(differentiation-committed oligodendrocyte precursor,COP),标志基因为Bcas1、Plp1、Bmp4、Enpp6。⑤ 星形胶质细胞(astrocyte),标志基因为Aldoc、Slc1a3、Aqp4。⑥ 纤毛上皮细胞(ciliated ependymal,Ependymal),标志基因为Ccdc153、Dynlrb2、Riiad1。⑦ 小胶质细胞(microglia),标志基因为Fcrls、Trem2、P2ry12。⑧ 周细胞(pericyte,PER),标志基因为Rgs5、Kcnj8。⑨ 纤维细胞(fibroblast,Fibro),标志基因为Dcn、Col1a1。⑩ 内皮细胞(endothelium,Endo),标志基因为Cldn5、Pglyrp1。
将新生小鼠PFC神经元分为以下8个类型:① 增殖型中间祖细胞1(proliferative intermediate progenitor cell 1,PIPC1),标志基因为Top2a、Pbk、Prc1。② 增殖型中间祖细胞2(proliferative intermediate progenitor cell 2,PIPC2),标志基因为Cenpa、Cdc20、Ccnb2、Ube2c。③ 增殖型中间祖细胞3(proliferative intermediate progenitor cell 3,PIPC3),标志基因为Mcm2、Mcm3、Mcm6、Pcna。④ 中间祖细胞1(intermediate progenitor cell 1,IPC1),标志基因为Htr3a、Gad2、Stmn2、Sp8、Dlx5。⑤ 中间祖细胞2(intermediate progenitor cell 2,IPC2),标志基因为Meg3、Adarb2、Pbx3。⑥ Cajal-Retzius细胞(Cajal-Retzius cell,CRC),标志基因为Reln、Snhg11、Ndnf、Nrn1、Calb2。⑦ 神经颗粒蛋白(neurogranin,NRGN)阳性中间神经元(NRGN+ interneuron,NRGN+ IN),标志基因为Nrgn、Snca、Mef2c、Cck。⑧ 谷氨酰胺投射锥体神经元(glutaminergic projection pyramidal neuron,PyN),标志基因为Neurod6、Neurod1、Sema3c、Neurod2。细胞类型占比统计使用Wilcoxon秩和检验计算Sev3组与Ctrl组、Sev1组与Ctrl组和Sev3组与Sev1组组间差异的P值,阈值设为P<0.05。
1.3.4 RNA速度分析
RNA速度(RNA velocity)分析使用ScanPy Python中的scVelo(Version 0.2.3)软件包[28],以构建细胞动力学判断细胞演化方向。
1.3.5 转录因子分析和差异表达基因分析
为了评估转录因子调控强度,我们应用了单细胞调控网络推理和聚类(single-cell regulatory network inference and clustering,SCENIC)分析软件(v0.9.5)[29],使用RcisTarget和GRNboost的2万个基序(motif)数据库,使用AUCell评估每个细胞中每个调节子(regulon)的活性。具体而言,我们在单细胞表达矩阵的基础上利用SCENIC分析工具计算了每个细胞中转录因子的AUC值,取AUC值>0.05,同时标准化的富集分数(normalized enrichment score,NES)按从大到小排序后排名前5 000个且NES值>3.0的转录因子,进而得到每个细胞转录因子的AUC值矩阵。将Sev3组与Ctrl组、Sev1组与Ctrl组每个细胞的单个转录因子的AUC值进行比较,采用Wilcoxon秩和检验计算P值。
为确认不同组间的差异表达基因,使用FindAllMarkers函数和Wilcoxon秩和检验计算出差异基因,筛选标准:① 差异倍数log2FC>0.25。② P值<0.05。③ 最低表达百分比min.pct>0.1。
1.3.6 基因功能富集分析和通路富集分析
进行基因本体论(Gene Ontology,GO)分析[30],以阐明差异表达基因的生物学意义。从NCBI(
根据京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)数据库,使用通路富集分析来找出差异表达基因的富集通路。采用Fisher精确检验来计算每个通路的P值和FDR值[31]。阈值设为P<0.05。
1.3.7 QuSAGE分析
为描述细胞周期和Hippo信号通路基因集的相对激活情况,我们进行了QuSAGE(quantitative set analysis for gene expression,版本2.16.1)分析[32]。QuSAGE分析,即基因集表达激活度定量分析是一种基于基因集和基因表达的富集度定量分析策略,它采用方差膨胀因子(variance inflation factor)算法对基因集进行富集度分析,采用t检验比较不同基因cluster的富集度差异,P值采用BH算法校正后得到FDR值。
1.3.8 CytoTRACE分析
基于每个细胞转录本的丰富度,利用CytoTRACE(cellular trajectory reconstruction analysis using gene counts and expression)分析来计算每个细胞的信息熵,以此评估每个细胞的干性。
1.3.9 拟时序分析
基于表达矩阵和如前所述获得的高可变基因,利用Monocle2(
2 结果
2.1 新生小鼠七氟烷麻醉后PFC细胞图谱、样本分布和细胞占比
取Ctrl组、Sev1组和Sev3组P9新生小鼠的PFC(每组3只),进行10x Genomics单细胞转录组测序(图1A),经质控后共获得40 061个细胞用于进一步分析,其中Ctrl组13 620个、Sev1组12 424个、Sev3组14 017个。单个细胞基因数均值为2 675,UMI均值为6 918。使用Seurat软件包通过UMAP分析对新生小鼠PFC的主要细胞类型分类,利用RNA速度分析判断细胞演化方向,同时参考相关文献中细胞类型分类的标志基因[23-27],共确定了10种细胞类型,分别是放射状胶质细胞1 806个、神经元8 224个、少突胶质前体细胞8 264个、具有分化倾向的少突胶质细胞前体1 388个、星形胶质细胞10 681个、纤毛上皮细胞495个、小胶质细胞3 974个、周细胞2 406个、纤维细胞496个、内皮细胞2 327个(图1B~D)。3组新生小鼠样本细胞类型分布(图1E)及占比(图1F)差异均无统计学意义(均P>0.05)。
图1
图1
各组小鼠PFC细胞图谱、细胞类型分布及占比
Note: A. Experimental procedures. The PFC cells acquired on postnatal day 9 (P9) from control (Ctrl) group, single anesthesia exposure (Sev1) group, and multiple anesthesia exposure (Sev3) group were prepared in single-cell suspension for 10x Genomics scRNA-seq. B. UMAP cluster showing PFC scRNA-seq results. C. Clustering visualization of RNA velocity analysis results of PFC cells. D. Bubble map of sorting gene markers in PFC cells. Bubble size represents the percentage of cells with the gene expressed in the population, and bubble colour represents the average value of the gene expressed in the population. E. Distribution of 3 groups of sample cells on the UMAP plot. F. Bar graphs of the percentages of cell types in 9 samples.
Fig 1
Cellular profiles, and cell type distribution and proportion in the PFCs of three groups of mice
2.2 单次和重复七氟烷麻醉对新生小鼠PFC细胞转录组水平的影响
2.2.1 新生小鼠七氟烷麻醉后PFC细胞差异表达基因功能注释
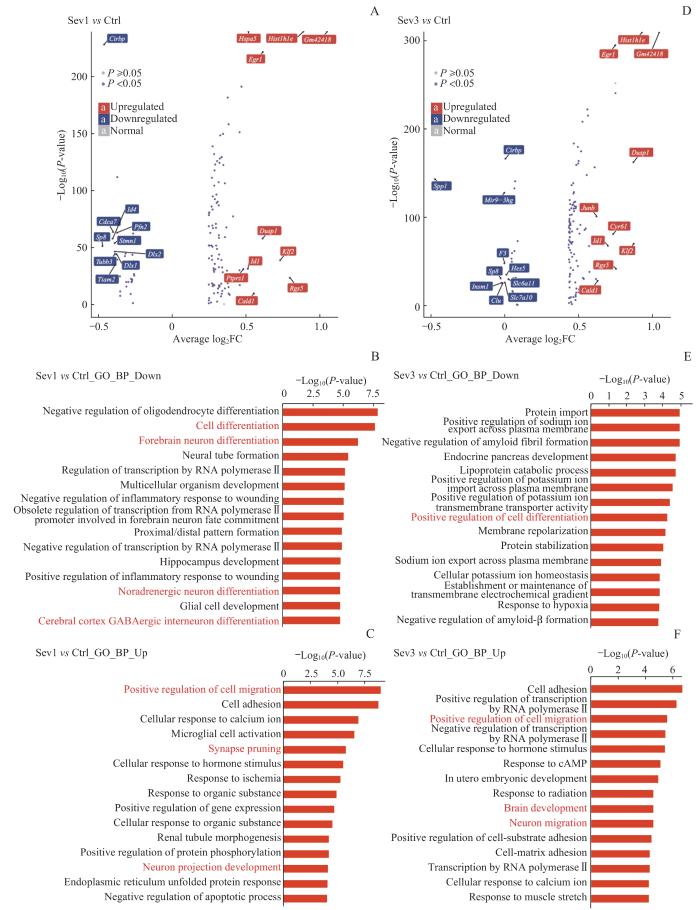
为分析新生小鼠单次七氟烷麻醉暴露后PFC细胞转录组改变,进行差异表达基因分析及功能注释(图2A~C)。对单次麻醉暴露后下调的基因进行GO功能富集分析,结果显示与PFC神经发生相关的生物学过程包括细胞分化、前脑神经元分化、去甲肾上腺素能神经元分化和大脑皮层γ-氨基丁酸(γ-aminobutyric acid,GABA)能中间神经元分化。单次麻醉暴露后上调的基因GO功能富集分析结果显示,与PFC神经发生相关的生物学过程包括细胞迁移的正向调节、突触修剪和神经元投射发育。
图2
图2
新生小鼠七氟烷麻醉后PFC细胞转录组变化和功能注释分析
Note: A. Volcano map showing differentially expressed genes between Sev1 group and Ctrl group. B/C. Top 15 enrichment entries of down-regulated genes (B) and up-regulated genes (C) in the GO-BP database between Sev1 group and Ctrl group. D. Volcano map showing differentially expressed genes between Sev3 group and Ctrl group. E/F. Top 15 enrichment entries of down-regulated genes (E) and up-regulated genes (F) in the GO-BP database between Sev3 group and Ctrl group. Entries highlighted in red represent biological processes associated with PFC neurogenesis.
Fig 2
Analysis of transcriptome changes and functional annotation of PFC cells in neonatal mice after sevoflurane anesthesia
对新生小鼠重复七氟烷麻醉暴露后的PFC细胞进行差异表达基因分析及功能注释(图2D~F)。重复麻醉暴露后下调基因的GO功能富集分析结果发现,与PFC神经发生相关的生物学过程是细胞分化的正向调节。重复麻醉暴露后上调的基因富集的生物学过程中与PFC神经发生相关的包括对细胞迁移的正向调节、大脑发育和神经元迁移。
2.2.2 新生小鼠七氟烷麻醉后PFC细胞差异表达基因富集通路
图3
图3
新生小鼠七氟烷麻醉后PFC细胞差异表达基因的通路分析
Note: A/B. Top 10 enrichment pathway entries of down-regulated genes (A) and up-regulated genes (B) in the KEGG database between Sev1 group and Ctrl group. C/D. Top 10 enrichment pathway entries of down-regulated genes (C) and up-regulated genes (D) in the KEGG database between Sev3 group and Ctrl group. Entries marked in red represent pathways associated with PFC neurogenesis.
Fig 3
Pathway analysis of differential expressed genes in PFC cells in neonatal mice after sevoflurane anesthesia
2.2.3 新生小鼠七氟烷麻醉后PFC细胞转录因子变化
新生小鼠单次和重复七氟烷麻醉后PFC细胞中部分转录因子表达水平发生变化(图4)。其中转录因子早期生长反应因子1(early growth response 1,Egr1)、SRY-box转录因子7(SRY-box transcription factor 7,Sox7)在单次麻醉暴露和重复麻醉暴露后与Ctrl组相比均上调,且差异均具有统计学意义(均P<0.01)。转录因子HES家族bHLH转录因子6(HES family bHLH transcription factor 6,Hes6)、NKX同源框-1基因(NK2 homeobox 1,Nkx2-1)在单次麻醉暴露后下调,与Ctrl组相比差异具有统计学意义(均P<0.01);重复麻醉暴露后与Ctrl组相比变化不明显。
图4
图4
新生小鼠七氟烷麻醉后PFC细胞转录因子变化
Note: A. AUCell values of Egr1. B. AUCell values of Sox7. C. AUCell values of Hes6. D. AUCell values of Nkx2-1. ①P=0.000, ②P=0.009. ns—not significant.
Fig 4
Transcription factor changes in PFC cells in neonatal mice after sevoflurane anesthesia
2.2.4 新生小鼠七氟烷麻醉后PFC细胞基因集激活
使用QuSAGE分析鉴定新生小鼠七氟烷麻醉后细胞周期和Hippo信号通路基因集的激活情况(图5)。麻醉后放射状胶质细胞和神经元与细胞周期相关的基因集激活度增加,并且重复麻醉暴露后激活度增加更明显。重复麻醉暴露后神经元与Hippo信号通路相关的基因集由抑制变为激活。
图5
图5
新生小鼠七氟烷麻醉后PFC细胞细胞周期和Hippo信号通路的基因集激活情况
Note: QuSAGE analysis of differentially expressed genes after exposure to sevoflurane anesthesia. A. Cell cycle pathway gene set activation analysis. B. Hippo signaling pathway-multiple species gene set activation analysis.
Fig 5
Gene set activation of cell cycle pathways and Hippo signaling pathways in PFC cells in neonatal mice after sevoflurane anesthesia
2.3 新生小鼠七氟烷麻醉后PFC神经元图谱
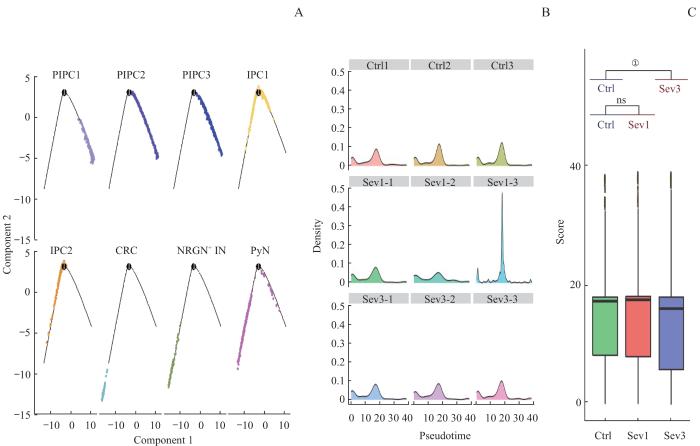
由于新生小鼠七氟烷麻醉后PFC神经元细胞周期和发育相关通路基因集明显激活,为进一步分析新生小鼠七氟烷麻醉后PFC神经元的转录组改变,将8 224个神经元单独进行亚群分析(图6A)。新生小鼠单次和重复七氟烷麻醉后神经元类型分布(图6B)及占比(图6C)均无明显变化(P>0.05)。利用RNA速度分析判断细胞演化方向,结合SCENIC分析,同时参考相关文献[23,25-26]中细胞类型分类的标志基因,共确定了8种神经元谱系细胞,分别是增殖型中间祖细胞1(1 583个)、增殖型中间祖细胞2(679个)、增殖型中间祖细胞3(727个)、中间祖细胞1(3 044个)、中间祖细胞2(1 463个)、Cajal-Retzius细胞(252个)、NRGN阳性中间神经元(88个)、谷氨酰胺投射锥体神经元(388个)(图6D~F)。对新生小鼠PFC神经元进行CytoTRACE分析,Cajal-Retzius细胞和NRGN阳性中间神经元转录本丰富度较高、干性较高,中间祖细胞1和中间祖细胞2转录本丰富度较低、干性较低(图6G);并且CytoTRACE评分表明,麻醉后除谷氨酰胺投射锥体神经元和中间祖细胞2,其他神经元谱系细胞的转录本丰富度增加,干性增加,神经元发育迟滞(图6H)。
图6
图6
各组小鼠PFC神经元图谱、类型分布及干性分析
Note: A. UMAP cluster showing the scRNA-seq results of PFC cells. B. Distribution of 3 groups of sample cells on the UMAP plot. C. Bar graphs of the percentages of neuron subdivisions in 9 samples. D. Clustering visualization of RNA velocity analysis results of PFC neurons. E. SCENIC analysis. F. Bubble map of sorting gene markers in PFC neurons. Bubble size represents the percentage of cells with the gene expressed in the population, and bubble colour represents the average value of the gene expressed in the population. G. CytoTRACE score. The color represents the score, and the higher the score, the higher the cell stemness. H. CytoTRACE box plot.
Fig 6
PFC neuron profiles, type distribution and stemness analysis of mice in 3 groups
2.4 新生小鼠七氟烷麻醉后PFC神经元拟时序分析
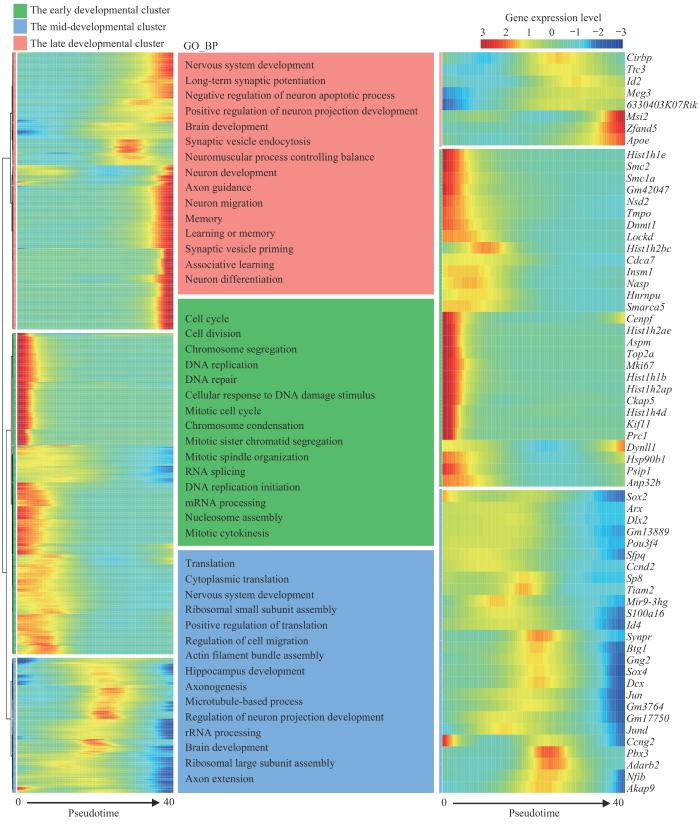
为确定新生小鼠七氟烷麻醉后PFC神经元分化轨迹,使用拟时序分析鉴定出神经元发育的3个阶段,其中3种增殖型中间祖细胞为发育早期,2种中间祖细胞处于发育中期,Cajal-Retzius细胞、NRGN阳性中间神经元和谷氨酰胺投射锥体神经元处于发育晚期较成熟阶段(图7A)。拟时序分析得到新生小鼠七氟烷麻醉后PFC神经元拟时间密度曲线图(图7B);重复七氟烷麻醉暴露后,PFC神经元发育拟时间与Ctrl组相比发生后退,且差异具有统计学意义(P=0.000,图7C)。这提示重复七氟烷麻醉后新生小鼠PFC神经元更多停留在早期阶段,即发育呈现迟缓趋势。为进一步理解不同发育阶段的神经元谱系细胞的转录组特点,通过BEAM分析对发育节点进行解析,以确定决定细胞不同命运的关键基因,并在GO-BP数据库进行富集,取前15条通路展示。神经元发育早期关键基因富集在细胞周期和有丝分裂等与细胞增殖相关的通路;神经元发育中期关键基因富集在神经元投射发育的调节、轴突发生和轴突延伸等通路;发育较成熟阶段关键基因富集在神经元发育、神经元迁移及神经元分化后的功能和生理作用等通路,如学习记忆等。值得注意的是,BEAM分析得到的前1 000个决定细胞命运的关键基因中63个同时也是3组小鼠神经元差异表达的基因(图8)。
图7
图7
七氟烷麻醉后新生小鼠PFC神经元谱系细胞拟时序分析
Note: A. Cell trajectory skeleton plots. B. Density profiles. The horizontal axis represents the proposed time, and the vertical axis represents the density of cell distribution. C. Boxplot of the pseudotime of PFC neuron development in 3 groups. ①P=0.000.
Fig 7
Pseudo‐time analysis of PFC neuronal lineage cells in neonatal mice after sevoflurane anesthesia
图8
图8
七氟烷麻醉后新生小鼠PFC神经元谱系细胞BEAM分析热图
Note: Left: heat map of top 1 000 genes that determine cell fate. Middle: top 15 enrichment entries of the 1 000 genes that determine cell fate in the GO-BP database. Right: heat map of Beam analysis of genes with significant differences in PFC neurons after sevoflurane anesthesia. The horizontal axis represents the proposed time, and the vertical axis represents the clustering of genes. The early developmental cluster included PIPC1‒3, the mid-developmental cluster included IPC1‒2, and the late developmental cluster included CRC, NRGN+ IN, and PyN.
Fig 8
BEAM analysis heat map of PFC neuronal lineage cells in neonatal mice after sevoflurane anesthesia
2.5 新生小鼠七氟烷麻醉后PFC神经元差异表达基因及功能注释
为分析新生小鼠单次七氟烷麻醉后PFC神经元转录组改变,进行差异表达基因分析及功能注释(图9A~C)。对单次麻醉暴露后下调的基因进行GO功能富集分析结果发现,与PFC神经元发生相关的生物学过程包括细胞周期蛋白依赖性蛋白丝氨酸/苏氨酸激酶活性的调节、有丝分裂细胞周期相变、细胞分化、长期记忆和有丝分裂细胞周期G1/S转换;单次麻醉暴露后上调的基因在GO-BP数据库中富集的且与PFC神经元发生相关的生物学过程是神经元投射发育。
图9
图9
新生小鼠七氟烷麻醉后PFC神经元的转录组变化和功能注释分析
Note:A. Volcano map showing differentially expressed genes between Sev1 group and Ctrl group. B/C. Top 15 enrichment entries of down-regulated genes (B) and up-regulated genes (C) in the GO-BP database between Sev1 group and Ctrl group. D. Volcano map showing differentially expressed genes between Sev3 group and Ctrl group. E/F. Top 15 enrichment entries of down-regulated genes (E) and up-regulated genes (F) in the GO-BP database between Sev3 group and Ctrl group. Entries highlighted in red represent biological processes associated with PFC neurogenesis.
Fig 9
Analysis of transcriptome changes and functional annotation of PFC neurons in neonatal mice after sevoflurane anesthesia
为分析新生小鼠重复七氟烷麻醉后PFC神经元转录组改变,进行差异表达基因分析及功能注释(图9D~F)。重复麻醉暴露后下调的基因富集的生物学过程中与PFC神经元发生相关的包括细胞群增殖的负调控、细胞分化的正向调节、前脑神经元的分化、典型Wnt信号通路的正向调控和细胞分化;重复麻醉暴露后上调的基因经GO功能富集分析发现,与PFC神经元发生相关的生物学过程包括细胞周期、细胞分裂、大脑发育和神经元迁移。
3 讨论
本研究使用生物信息学,基于单细胞转录组测序数据,对新生小鼠七氟烷麻醉后PFC细胞进行了详细分析,发现麻醉次数对新生小鼠PFC细胞造成不同程度的转录组改变。单次七氟烷麻醉后诱导神经元分化相关的转录因子Hes6和Nkx2-1明显下调[33-34],但是重复七氟烷麻醉后这2种转录因子并未发生明显变化。研究表明:Hes6的过度表达在有丝分裂后神经元中表现出凋亡诱导活性[35];Nkx2-1能够激活中间神经元迁移所需的基因,内侧神经节隆起(medial ganglionic eminence,MGE)衍生的中间神经元向皮层迁移需要下调Nkx2-1[23]。另一方面,七氟烷单次麻醉暴露和重复麻醉暴露后,调节突触可塑性的转录因子Egr1和促进神经元凋亡的转录因子Sox7均明显上调[36-37]。单次麻醉可能作为一种急性的应激,引起的基因的变化较为剧烈和明显,但随着麻醉的消退,变化的基因又有回调。而重复的麻醉可能使机体有了一个适应的过程,所以变化的基因相对较少且集中在神经元凋亡和突触可塑性等。
七氟烷单次麻醉暴露后上调的基因功能富集在细胞迁移和神经元投射发育等生物学过程,KEGG通路富集的PI3K-AKT信号通路和Apelin信号通路均为神经保护作用机制[38-39]。同样,七氟烷重复麻醉暴露后上调的基因除了富集在细胞迁移的正向调节之外,还包括大脑发育和神经元迁移,KEGG通路依旧富集在神经保护相关的信号通路。已经证实七氟烷可以通过抑制神经凋亡、抑制过度活跃的自噬和在病理条件下以时间和剂量依赖的方式抑制炎症来缓解神经缺陷[40-41]。七氟烷后处理可通过丝络蛋白(reelin)-DAB1(disabled 1)蛋白通路改善神经元迁移障碍,并改善新生大鼠在缺氧缺血性损伤后的长期认知[42]。并且,神经凋亡和神经元迁移已被证明在麻醉暴露后继续发展[43-44]。
单次和重复七氟烷麻醉暴露后下调的基因功能富集分析发现与多种类型神经元分化的生物学过程相关。单次麻醉暴露下调基因的KEGG通路富集在TGF-β信号通路,该通路受损会抑制神经亚群的发育[45]。七氟烷重复麻醉后下调的基因与PFC神经发生相关的KEGG富集通路是Notch信号通路。已有研究[46]证明Notch信号通路是七氟烷的下游作用靶点。新生小鼠七氟烷麻醉后细胞周期和Hippo信号通路基因集的激活情况也表明神经元的增殖和分化受到七氟烷麻醉次数的影响。Hippo信号通路高度保守,能够调节静止的神经细胞再激活和控制神经细胞发生末期分化的时间,从而保持神经发生及时进入和退出,限制神经细胞细胞周期速度,确定细胞大小,并改变胚胎后神经发生过程中产生的神经元类型的比例[47]。
通过对神经元的单细胞转录组数据进一步分析发现,七氟烷麻醉后神经元干性增加;重复七氟烷麻醉暴露后,PFC神经元发育拟时间发生后退,神经元发育迟滞。拟时序分析将新生小鼠PFC神经元谱系分为3个发育时期,处于发育早期的神经元具有增殖性,处于发育中期的神经元主要发生投射发育的调节、轴突发生和轴突延伸等生物学过程,而发育较成熟的神经元已可以进行迁移,并具备一定的生理作用。七氟烷麻醉后这些决定神经元命运的部分关键基因出现差异表达,如Top2a、Mki67、Hist1h1e、Insm1、Sp8和Cirbp等。拟时序分析进一步提示,七氟烷麻醉抑制了PFC神经元的分化。
本研究表明单次和重复七氟烷麻醉均促进新生小鼠PFC神经祖细胞增殖和神经元迁移,但只有重复七氟烷麻醉抑制了PFC神经祖细胞向神经元分化。这可能与新生小鼠重复麻醉暴露后的远期认知发育障碍有关,我们将在后续研究中进一步探索单次和重复七氟烷麻醉后新生小鼠PFC细胞转录组改变对远期认知发育影响的机制。同时,已有研究[50]证实新生小鼠重复七氟烷麻醉后单细胞转录组学存在显著的性别差异。本研究仅对新生小鼠在单次和重复七氟烷麻醉暴露后PFC转录组改变进行分析,没有在实验组或对照组中分配同等数量的雌性和雄性小鼠,也未考虑幼年小鼠的麻醉神经毒性的性别依赖影响。因此,七氟烷麻醉次数对新生小鼠PFC发育影响的长期差异机制仍需要进一步的探索。
综上,我们使用单细胞转录组测序比较了单次和重复七氟烷麻醉后新生啮齿类动物PFC细胞在转录组水平上的差异,并分析了PFC中处于不同分化潜能的神经元在不同麻醉次数的情况下产生的转录组水平变化及潜在靶点,发现七氟烷麻醉抑制PFC神经祖细胞向神经元分化的潜在机制。本研究可为七氟烷的神经发育毒性的预防和治疗提供新的基础。
作者贡献声明
刘思雨、张磊参与实验设计;刘思雨完成实验操作、数据分析和测序结果可视化;刘思雨、张磊参与论文的写作和修改。2位作者均阅读并同意了最终稿件的提交。
AUTHOR's CONTRIBUTIONS
The study was designed by LIU Siyu and ZHANG Lei. The experimental manipulation, data analysis and visualisation of sequencing results were completed by LIU Siyu. The manuscript was drafted and revised by LIU Siyu and ZHANG Lei. Both authors have read the last version of the paper and consented to submission.
利益冲突声明
2位作者声明不存在利益冲突。
COMPETING INTERESTS
Both authors disclose no relevant conflict of interests.
参考文献