
上海交通大学学报(医学版) ›› 2024, Vol. 44 ›› Issue (7): 830-838.doi: 10.3969/j.issn.1674-8115.2024.07.004
• 转化医学研究前沿进展专题 • 上一篇
张烨晟1( ), 杨易静1, 黄依雯2, 施珑玙3, 王曼媛4, 陈思思5(
), 杨易静1, 黄依雯2, 施珑玙3, 王曼媛4, 陈思思5( )
)
收稿日期:2024-01-30
接受日期:2024-04-28
出版日期:2024-07-28
发布日期:2024-07-28
通讯作者:
陈思思
E-mail:yesheng_zhang@163.com;sisichen@shsmu.edu.cn
作者简介:张烨晟(2001—),男,博士生;电子信箱:yesheng_zhang@163.com。
基金资助:
ZHANG Yesheng1( ), YANG Yijing1, HUANG Yiwen2, SHI Longyu3, WANG Manyuan4, CHEN Sisi5(
), YANG Yijing1, HUANG Yiwen2, SHI Longyu3, WANG Manyuan4, CHEN Sisi5( )
)
Received:2024-01-30
Accepted:2024-04-28
Online:2024-07-28
Published:2024-07-28
Contact:
CHEN Sisi
E-mail:yesheng_zhang@163.com;sisichen@shsmu.edu.cn
Supported by:摘要:
肿瘤微环境(tumor microenvironment,TME)是肿瘤细胞生存的复杂细胞环境,内含多种类型的细胞和围绕肿瘤细胞的细胞外成分。免疫细胞是TME的关键组成部分,包括肿瘤相关巨噬细胞(tumor-associated macrophages,TAMs)、髓系抑制性细胞(myeloid-derived suppressor cells,MDSCs)、淋巴细胞、调节性T细胞(regulatory T cells,Tregs)、自然杀伤细胞(natural killer cells,NK cells)及树突状细胞(dendritic cells,DCs)等。值得关注的是,目前肿瘤耐药是化学治疗(化疗)、放射治疗(放疗)、靶向治疗及免疫治疗等肿瘤治疗方法疗效受限并导致治疗失败的主要原因。研究发现,肿瘤细胞耐药性的产生是肿瘤细胞与TME相互作用的结果。因此,如何克服TME所致肿瘤耐药被认为是肿瘤治疗的一大难点。近年来,随着对TME中免疫细胞研究的深入,免疫细胞调节肿瘤细胞耐药性的具体机制研究取得重大进展,而靶向相应免疫细胞、信号通路或细胞因子的治疗策略被证实能够有效减少肿瘤耐药并改善肿瘤治疗效果。该文就TME中TAMs、MDSCs、Tregs和NK细胞等在肿瘤耐药中发挥的作用及克服肿瘤耐药的靶向策略的研究进展进行综述,并讨论肿瘤相关中性粒细胞(tumor-associated neutrophils,TANs)和免疫抑制性调节性B细胞(B regulatory cells,Bregs)与肿瘤耐药之间的关系,以期为克服肿瘤耐药和提高抗肿瘤治疗效果提供方向和参考。
中图分类号:
张烨晟, 杨易静, 黄依雯, 施珑玙, 王曼媛, 陈思思. 肿瘤微环境免疫细胞调节肿瘤细胞耐药性的研究进展[J]. 上海交通大学学报(医学版), 2024, 44(7): 830-838.
ZHANG Yesheng, YANG Yijing, HUANG Yiwen, SHI Longyu, WANG Manyuan, CHEN Sisi. Research progress in immune cells regulating drug resistance of tumor cells in tumor microenvironment[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2024, 44(7): 830-838.
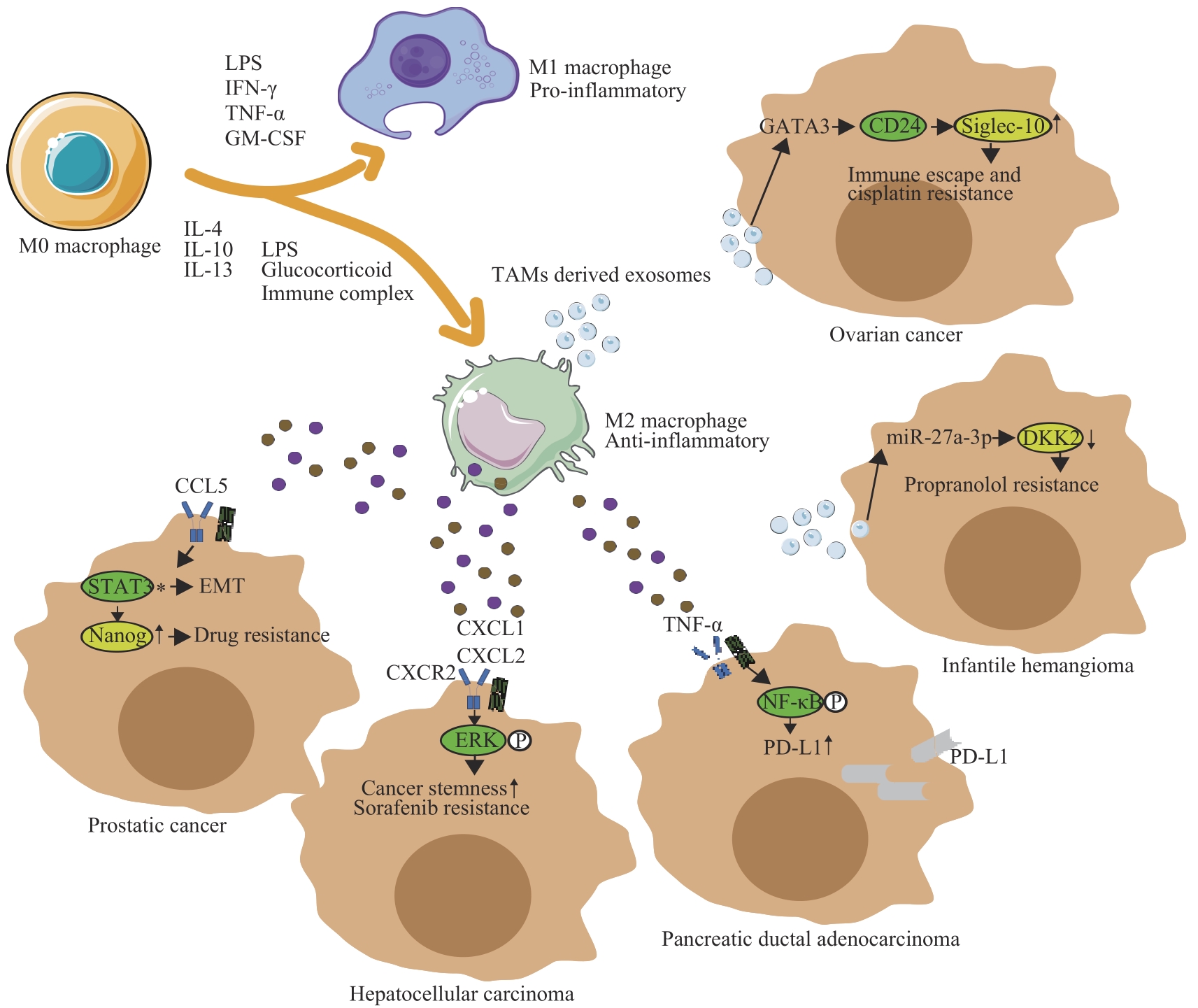
图1 TAMs的异质性及介导肿瘤耐药的相关机制Note: LPS—lipopolysaccharide; IFNγ—interferon-γ; GM-CSF—granulocyte-macrophage colony-stimulating factor; EMT—epithelial-mesenchymal transition.
Fig 1 Heterogeneity of TAMs and mechanisms underlying TAM-mediated tumor drug resistance
| 1 | HANAHAN D, COUSSENS L M. Accessories to the crime: functions of cells recruited to the tumor microenvironment[J]. Cancer Cell, 2012, 21(3): 309-322. |
| 2 | ANDERSON N M, SIMON M C. The tumor microenvironment[J]. Curr Biol, 2020, 30(16): R921-R925. |
| 3 | FU T, DAI L J, WU S Y, et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response[J]. J Hematol Oncol, 2021, 14(1): 98. |
| 4 | DUAN Q Q, ZHANG H L, ZHENG J N, et al. Turning cold into hot: firing up the tumor microenvironment[J]. Trends Cancer, 2020, 6(7): 605-618. |
| 5 | SUN Y. Tumor microenvironment and cancer therapy resistance[J]. Cancer Lett, 2016, 380(1): 205-215. |
| 6 | MANTOVANI A, ALLAVENA P, SICA A, et al. Cancer-related inflammation[J]. Nature, 2008, 454(7203): 436-444. |
| 7 | SHARMA P, HU-LIESKOVAN S, WARGO J A, et al. Primary, adaptive, and acquired resistance to cancer immunotherapy[J]. Cell, 2017, 168(4): 707-723. |
| 8 | POTT S, LIEB J D. Single-cell ATAC-seq: strength in numbers[J]. Genome Biol, 2015, 16(1): 172. |
| 9 | SOLINAS G, GERMANO G, MANTOVANI A, et al. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation[J]. J Leukoc Biol, 2009, 86(5): 1065-1073. |
| 10 | CHIM L K, WILLIAMS I L, BASHOR C J, et al. Tumor-associated macrophages induce inflammation and drug resistance in a mechanically tunable engineered model of osteosarcoma[J]. Biomaterials, 2023, 296: 122076. |
| 11 | BOUTILIER A J, ELSAWA S F. Macrophage polarization states in the tumor microenvironment[J]. Int J Mol Sci, 2021, 22(13): 6995. |
| 12 | GINHOUX F, GUILLIAMS M. Tissue-resident macrophage ontogeny and homeostasis[J]. Immunity, 2016, 44(3): 439-449. |
| 13 | MILLS C D, KINCAID K, ALT J M, et al. M-1/M-2 macrophages and the Th1/Th2 paradigm[J]. J Immunol, 2000, 164(12): 6166-6173. |
| 14 | WANG L X, ZHANG S X, WU H J, et al. M2b macrophage polarization and its roles in diseases[J]. J Leukoc Biol, 2019, 106(2): 345-358. |
| 15 | JEANNIN P, PAOLINI L, ADAM C, et al. The roles of CSFs on the functional polarization of tumor-associated macrophages[J]. FEBS J, 2018, 285(4): 680-699. |
| 16 | CHRISTOFIDES A, STRAUSS L, YEO A, et al. The complex role of tumor-infiltrating macrophages[J]. Nat Immunol, 2022, 23(8): 1148-1156. |
| 17 | MOSSER D M, EDWARDS J P. Exploring the full spectrum of macrophage activation[J]. Nat Rev Immunol, 2008, 8(12): 958-969. |
| 18 | KHALAF K, HANA D, CHOU J T T, et al. Aspects of the tumor microenvironment involved in immune resistance and drug resistance[J]. Front Immunol, 2021, 12: 656364. |
| 19 | MA J, SHAYITI F, MA J, et al. Tumor-associated macrophage-derived CCL5 promotes chemotherapy resistance and metastasis in prostatic cancer[J]. Cell Biol Int, 2021, 45(10): 2054-2062. |
| 20 | WANG H C, HAUNG L Y, WANG C J, et al. Tumor-associated macrophages promote resistance of hepatocellular carcinoma cells against sorafenib by activating CXCR2 signaling[J]. J Biomed Sci, 2022, 29(1): 99. |
| 21 | LI D B, JI H F, NIU X J, et al. Tumor-associated macrophages secrete CC-chemokine ligand 2 and induce tamoxifen resistance by activating PI3K/Akt/mTOR in breast cancer[J]. Cancer Sci, 2020, 111(1): 47-58. |
| 22 | LI H, LUO F, JIANG X Y, et al. CircITGB6 promotes ovarian cancer cisplatin resistance by resetting tumor-associated macrophage polarization toward the M2 phenotype[J]. J Immunother Cancer, 2022, 10(3): e004029. |
| 23 | ZHANG H, LIU L, LIU J B, et al. Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers[J]. Mol Cancer, 2023, 22(1): 58. |
| 24 | TSUKAMOTO M, IMAI K, ISHIMOTO T, et al. PD-L1 expression enhancement by infiltrating macrophage-derived tumor necrosis factor-α leads to poor pancreatic cancer prognosis[J]. Cancer Sci, 2019, 110(1): 310-320. |
| 25 | CHEN Y J, LI G N, LI X J, et al. Targeting IRG1 reverses the immunosuppressive function of tumor-associated macrophages and enhances cancer immunotherapy[J]. Sci Adv, 2023, 9(17): eadg0654. |
| 26 | YUAN S Y, CHEN W J, YANG J, et al. Tumor-associated macrophage-derived exosomes promote EGFR-TKI resistance in non-small cell lung cancer by regulating the AKT, ERK1/2 and STAT3 signaling pathways[J]. Oncol Lett, 2022, 24(4): 356. |
| 27 | LIU C, ZHAO Z L, GUO S K, et al. Exosomal miR-27a-3p derived from tumor-associated macrophage suppresses propranolol sensitivity in infantile hemangioma[J]. Cell Immunol, 2021, 370: 104442. |
| 28 | CHEN C, ZHANG L, RUAN Z Y. GATA3 encapsulated by tumor-associated macrophage-derived extracellular vesicles promotes immune escape and chemotherapy resistance of ovarian cancer cells by upregulating the CD24/siglec-10 axis[J]. Mol Pharm, 2023, 20(2): 971-986. |
| 29 | XIA Y Q, RAO L, YAO H M, et al. Engineering macrophages for cancer immunotherapy and drug delivery[J]. Adv Mater, 2020, 32(40): e2002054. |
| 30 | RODRIGUEZ-GARCIA A, LYNN R C, POUSSIN M, et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy[J]. Nat Commun, 2021, 12(1): 877. |
| 31 | GUNASSEKARAN G R, POONGKAVITHAI VADEVOO S M, BAEK M C, et al. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages[J]. Biomaterials, 2021, 278: 121137. |
| 32 | LI C X, XU X F, WEI S H, et al. Tumor-associated macrophages: potential therapeutic strategies and future prospects in cancer[J]. J Immunother Cancer, 2021, 9(1): e001341. |
| 33 | CASSETTA L, POLLARD J W. A timeline of tumour-associated macrophage biology[J]. Nat Rev Cancer, 2023, 23(4): 238-257. |
| 34 | SIKIC B I, LAKHANI N, PATNAIK A, et al. First-in-human, first-in-class phase Ⅰ trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers[J]. J Clin Oncol, 2019, 37(12): 946-953. |
| 35 | ADVANI R, FLINN I, POPPLEWELL L, et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin′s lymphoma[J]. N Engl J Med, 2018, 379(18): 1711-1721. |
| 36 | VEGLIA F, SANSEVIERO E, GABRILOVICH D I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity[J]. Nat Rev Immunol, 2021, 21(8): 485-498. |
| 37 | RODRÍGUEZ P C, OCHOA A C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives[J]. Immunol Rev, 2008, 222: 180-191. |
| 38 | CIMEN BOZKUS C, ELZEY B D, CRIST S A, et al. Expression of cationic amino acid transporter 2 is required for myeloid-derived suppressor cell-mediated control of T cell immunity[J]. J Immunol, 2015, 195(11): 5237-5250. |
| 39 | BAUMANN T, DUNKEL A, SCHMID C, et al. Regulatory myeloid cells paralyze T cells through cell-cell transfer of the metabolite methylglyoxal[J]. Nat Immunol, 2020, 21(5): 555-566. |
| 40 | ANTONIOS J P, SOTO H, EVERSON R G, et al. Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma[J]. Neuro Oncol, 2017, 19(6): 796-807. |
| 41 | PICO DE COAÑA Y, POSCHKE I, GENTILCORE G, et al. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production[J]. Cancer Immunol Res, 2013, 1(3): 158-162. |
| 42 | DOLCETTI L, PERANZONI E, UGEL S, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF[J]. Eur J Immunol, 2010, 40(1): 22-35. |
| 43 | MILLRUD C R, BERGENFELZ C, LEANDERSSON K. On the origin of myeloid-derived suppressor cells[J]. Oncotarget, 2017, 8(2): 3649-3665. |
| 44 | NEFEDOVA Y, NAGARAJ S, ROSENBAUER A, et al. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the Janus-activated kinase 2/signal transducers and activators of transcription 3 pathway[J]. Cancer Res, 2005, 65(20): 9525-9535. |
| 45 | NI X L, HU G H, CAI X. The success and the challenge of all-trans retinoic acid in the treatment of cancer[J]. Crit Rev Food Sci Nutr, 2019, 59(sup1): S71-S80. |
| 46 | FUJITA M, KOHANBASH G, FELLOWS-MAYLE W, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells[J]. Cancer Res, 2011, 71(7): 2664-2674. |
| 47 | AL-KHAMI A A, ZHENG L Q, DEL VALLE L, et al. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells[J]. Oncoimmunology, 2017, 6(10): e1344804. |
| 48 | JIAN S L, CHEN W W, SU Y C, et al. Glycolysis regulates the expansion of myeloid-derived suppressor cells in tumor-bearing hosts through prevention of ROS-mediated apoptosis[J]. Cell Death Dis, 2017, 8(5): e2779. |
| 49 | ZITVOGEL L, APETOH L, GHIRINGHELLI F, et al. Immunological aspects of cancer chemotherapy[J]. Nat Rev Immunol, 2008, 8(1): 59-73. |
| 50 | ERIKSSON E, WENTHE J, IRENAEUS S, et al. Gemcitabine reduces MDSCs, Tregs and TGFβ-1 while restoring the Teff/Treg ratio in patients with pancreatic cancer[J]. J Transl Med, 2016, 14(1): 282. |
| 51 | SAKAGUCHI S, SAKAGUCHI N, ASANO M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases[J]. J Immunol, 1995, 155(3): 1151-1164. |
| 52 | EXPOSITO F, REDRADO M, HOURY M, et al. PTEN loss confers resistance to anti-PD-1 therapy in non-small cell lung cancer by increasing tumor infiltration of regulatory T cells[J]. Cancer Res, 2023, 83(15): 2513-2526. |
| 53 | MARSHALL L A, MARUBAYASHI S, JORAPUR A, et al. Tumors establish resistance to immunotherapy by regulating Treg recruitment via CCR4[J]. J Immunother Cancer, 2020, 8(2): e000764. |
| 54 | GAO Y N, YOU M J, FU J L, et al. Intratumoral stem-like CCR4+ regulatory T cells orchestrate the immunosuppressive microenvironment in HCC associated with hepatitis B[J]. J Hepatol, 2022, 76(1): 148-159. |
| 55 | D'ALISE A M, LEONI G, LUCIA M D, et al. Maximizing cancer therapy via complementary mechanisms of immune activation: PD-1 blockade, neoantigen vaccination, and Tregs depletion[J]. J Immunother Cancer, 2021, 9(11): e003480. |
| 56 | FONG W, LI Q, JI F F, et al. Lactobacillus gallinarum-derived metabolites boost anti-PD1 efficacy in colorectal cancer by inhibiting regulatory T cells through modulating IDO1/Kyn/AHR axis[J]. Gut, 2023, 72(12): 2272-2285. |
| 57 | IMBERT C, MONTFORT A, FRAISSE M, et al. Resistance of melanoma to immune checkpoint inhibitors is overcome by targeting the sphingosine kinase-1[J]. Nat Commun, 2020, 11(1): 437. |
| 58 | LI Z T, DENG Y Y, SUN H H, et al. Redox modulation with a perfluorocarbon nanoparticle to reverse Treg-mediated immunosuppression and enhance anti-tumor immunity[J]. J Control Release, 2023, 358: 579-590. |
| 59 | PIPER M, VAN COURT B, MUELLER A, et al. Targeting Treg-expressed STAT3 enhances NK-mediated surveillance of metastasis and improves therapeutic response in pancreatic adenocarcinoma[J]. Clin Cancer Res, 2022, 28(5): 1013-1026. |
| 60 | REVENKO A, CARNEVALLI L S, SINCLAIR C, et al. Direct targeting of FOXP3 in Tregs with AZD8701, a novel antisense oligonucleotide to relieve immunosuppression in cancer[J]. J Immunother Cancer, 2022, 10(4): e003892. |
| 61 | AMOOZGAR Z, KLOEPPER J, REN J, et al. Targeting Treg cells with GITR activation alleviates resistance to immunotherapy in murine glioblastomas[J]. Nat Commun, 2021, 12(1): 2582. |
| 62 | TANG F, LI J H, QI L, et al. A pan-cancer single-cell panorama of human natural killer cells[J]. Cell, 2023, 186(19): 4235-4251.e20. |
| 63 | LI L, MOHANTY V, DOU J Z, et al. Loss of metabolic fitness drives tumor resistance after CAR-NK cell therapy and can be overcome by cytokine engineering[J]. Sci Adv, 2023, 9(30): eadd6997. |
| 64 | MYERS J A, MILLER J S. Exploring the NK cell platform for cancer immunotherapy[J]. Nat Rev Clin Oncol, 2021, 18(2): 85-100. |
| 65 | FANTINI M, ARLEN P M, TSANG K Y. Potentiation of natural killer cells to overcome cancer resistance to NK cell-based therapy and to enhance antibody-based immunotherapy[J]. Front Immunol, 2023, 14: 1275904. |
| 66 | LUO H Y, ZHOU Y H, ZHANG J, et al. NK cell-derived exosomes enhance the anti-tumor effects against ovarian cancer by delivering cisplatin and reactivating NK cell functions[J]. Front Immunol, 2022, 13: 1087689. |
| 67 | NAKAMURA T, SATO T, ENDO R, et al. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation[J]. J Immunother Cancer, 2021, 9(7): e002852. |
| 68 | VALERI A, GARCÍA-ORTIZ A, CASTELLANO E, et al. Overcoming tumor resistance mechanisms in CAR-NK cell therapy[J]. Front Immunol, 2022, 13: 953849. |
| 69 | ZUO H, YANG M J, JI Q, et al. Targeting neutrophil extracellular traps: a novel antitumor strategy[J]. J Immunol Res, 2023, 2023: 5599660. |
| 70 | MOUSSET A, LECORGNE E, BOURGET I, et al. Neutrophil extracellular traps formed during chemotherapy confer treatment resistance via TGF-β activation[J]. Cancer Cell, 2023, 41(4): 757-775.e10. |
| 71 | FLORES-BORJA F, BOSMA A, NG D, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation[J]. Sci Transl Med, 2013, 5(173): 173ra23. |
| 72 | ZHOU X, SU Y X, LAO X M, et al. CD19+IL-10+ regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4+ T cells to CD4+Foxp3+ regulatory T cells[J]. Oral Oncol, 2016, 53: 27-35. |
| 73 | LI S R, MIRLEKAR B, JOHNSON B M, et al. STING-induced regulatory B cells compromise NK function in cancer immunity[J]. Nature, 2022, 610(7931): 373-380. |
| 74 | BARTOSIŃSKA J, PURKOT J, KARCZMARCZYK A, et al. Differential function of a novel population of the CD19+CD24hiCD38hi Bregs in psoriasis and multiple myeloma[J]. Cells, 2021, 10(2): 411. |
| [1] | 冯昫皎, 刘健悦, 戚炀炀, 孙晶, 沈蕾. 结直肠癌中自然杀伤细胞表型及功能初探[J]. 上海交通大学学报(医学版), 2024, 44(6): 713-722. |
| [2] | 刘林楠, 冯莉, 王龙, 刘嘉寅, 范志松. 多能蛋白聚糖在恶性肿瘤中的表达及生物学作用的研究进展[J]. 上海交通大学学报(医学版), 2024, 44(4): 525-530. |
| [3] | 周婉桢, 滕银成. 非经典Wnt通路在卵巢癌中的作用与潜在治疗意义研究进展[J]. 上海交通大学学报(医学版), 2023, 43(8): 1056-1063. |
| [4] | 崔芷嫣, 陈尧, 陶悦, 沈树红, 李慧. PRPS1 I72位点突变对急性淋巴细胞白血病耐药性的影响及其机制研究[J]. 上海交通大学学报(医学版), 2023, 43(8): 977-987. |
| [5] | 魏兰懿, 薛晓川, 陈君君, 杨全军, 王梦月, 韩永龙. 骨肉瘤免疫微环境中肿瘤相关巨噬细胞及其靶向治疗的研究进展[J]. 上海交通大学学报(医学版), 2023, 43(5): 624-630. |
| [6] | 赵富茂, 彭玫, 彭晓露, 舒韦韦, 彭丽. 鲍曼不动杆菌在环境美罗培南浓度变化时耐药性的改变及其机制[J]. 上海交通大学学报(医学版), 2023, 43(11): 1396-1407. |
| [7] | 吴瑞芳, 冯明, 孟健. 脂肪酸结合蛋白4在肥胖相关肿瘤中的作用综述[J]. 上海交通大学学报(医学版), 2023, 43(10): 1311-1316. |
| [8] | 马芳芳, 秦洁洁, 任灵杰, 唐笑梅, 刘佳, 施敏敏, 蒋玲曦. 基于水凝胶微球建立胰腺癌原代细胞的3D培养模型[J]. 上海交通大学学报(医学版), 2023, 43(1): 79-87. |
| [9] | 汤开然, 吴琼, 黄思佳, 邱旭东, 李文彦, 邓华云, 黄雷. 与MUC1共同调控肿瘤化疗耐药的MUCIN家族成员的筛选[J]. 上海交通大学学报(医学版), 2022, 42(9): 1288-1295. |
| [10] | 韩婷, 吕纯鑫, 卓萌, 夏青, 刘腾飞, 吴秀奇, 林晓琳, 肖秀英. 进展期胃癌免疫治疗不良反应的相关因素及预后分析[J]. 上海交通大学学报(医学版), 2022, 42(8): 1053-1061. |
| [11] | 林家俞, 秦洁洁, 蒋玲曦. 肿瘤微环境中免疫细胞的代谢研究进展[J]. 上海交通大学学报(医学版), 2022, 42(8): 1122-1130. |
| [12] | 阿婷曦, 邵春益, 傅瑶. 调节性T细胞在眼表疾病中作用的研究进展[J]. 上海交通大学学报(医学版), 2022, 42(8): 1145-1150. |
| [13] | 廖雅慧, 刘丽云, 朱泓睿, 林厚文, 严继舟, 孙凡. 海绵来源的smenospongine通过抑制非小细胞肺癌细胞中的EGFR-Akt-ABCG2信号通路抑制顺铂耐药[J]. 上海交通大学学报(医学版), 2022, 42(8): 997-1007. |
| [14] | 李聪聪, 姚玉峰, 张传珍. 铜绿假单胞菌对环丙沙星异质性耐药的研究[J]. 上海交通大学学报(医学版), 2022, 42(7): 839-845. |
| [15] | 王雨心, 孙瑞琪, 刘坚华, 何伟娜. 开发用于肿瘤微环境成像的pH敏感荧光探针[J]. 上海交通大学学报(医学版), 2022, 42(7): 875-884. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||