
上海交通大学学报(医学版) ›› 2022, Vol. 42 ›› Issue (12): 1757-1765.doi: 10.3969/j.issn.1674-8115.2022.12.015
收稿日期:2022-07-12
接受日期:2022-11-28
出版日期:2022-12-28
发布日期:2022-12-28
通讯作者:
秦金红,电子信箱:jinhongqin@sjtu.edu.cn。作者简介:姜春宇(1997—),女,博士生;电子信箱:jiangchunyu@sjtu.edu.cn。
基金资助:
JIANG Chunyu1( ), GUO Xiaokui2, QIN Jinhong1(
), GUO Xiaokui2, QIN Jinhong1( )
)
Received:2022-07-12
Accepted:2022-11-28
Online:2022-12-28
Published:2022-12-28
Contact:
QIN Jinhong, E-mail: jinhongqin@sjtu.edu.cn.Supported by:摘要:
肺炎克雷伯菌(Klebsiella pneumoniae)在自然界中广泛分布,致病性肺炎克雷伯菌在临床上可以引起广泛感染,如呼吸系统感染、血流感染、肝脓肿及泌尿系统感染等。肺炎克雷伯菌是著名的“质粒收集器”,其基因组可以同时携带多种不同类型的质粒,从而导致临床中不断出现耐药菌株。尤其是近年来高毒力多重耐药菌株亦不断出现,给临床治疗工作带来了极大挑战。因此肺炎克雷伯菌对外源基因特别是耐药以及毒力相关基因的获取能力引起了广大学者的关注。作为细菌的获得性免疫系统,活跃的常间回文重复序列丛集/常间回文重复序列丛集关联蛋白(clustered regularly interspaced palindromic repeats/CRISPR-associated proteins,CRISPR-Cas)系统可以有效阻碍肺炎克雷伯菌基因组中可移动元件的水平转移,特别是接合型质粒的转移。近年来发现一些接合型质粒通过携带anti-CRISPR(Acr)蛋白抑制宿主菌编码的CRISPR-Cas系统活性,逃逸宿主的免疫识别,进而可以有效进行转移。分析数据库中已知肺炎克雷伯菌基因序列结果表明,其基因组中主要的CRISPR-Cas系统类型为I-E型及亚型(I-E*)。研究肺炎克雷伯菌基因组中CRISPR-Cas系统与质粒的分布及转移关系,并研究Acr蛋白在调控CRISPR-Cas系统活性中发挥作用的机制,将为揭示其基因组进化的动力及方向提供线索,为防控高毒力多重耐药菌株提供临床指导。
中图分类号:
姜春宇, 郭晓奎, 秦金红. 肺炎克雷伯菌CRISPR-Cas系统及anti-CRISPR蛋白家族研究进展[J]. 上海交通大学学报(医学版), 2022, 42(12): 1757-1765.
JIANG Chunyu, GUO Xiaokui, QIN Jinhong. Research advances in CRISPR-Cas systems and anti-CRISPR protein families in Klebsiella pneumoniae[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2022, 42(12): 1757-1765.
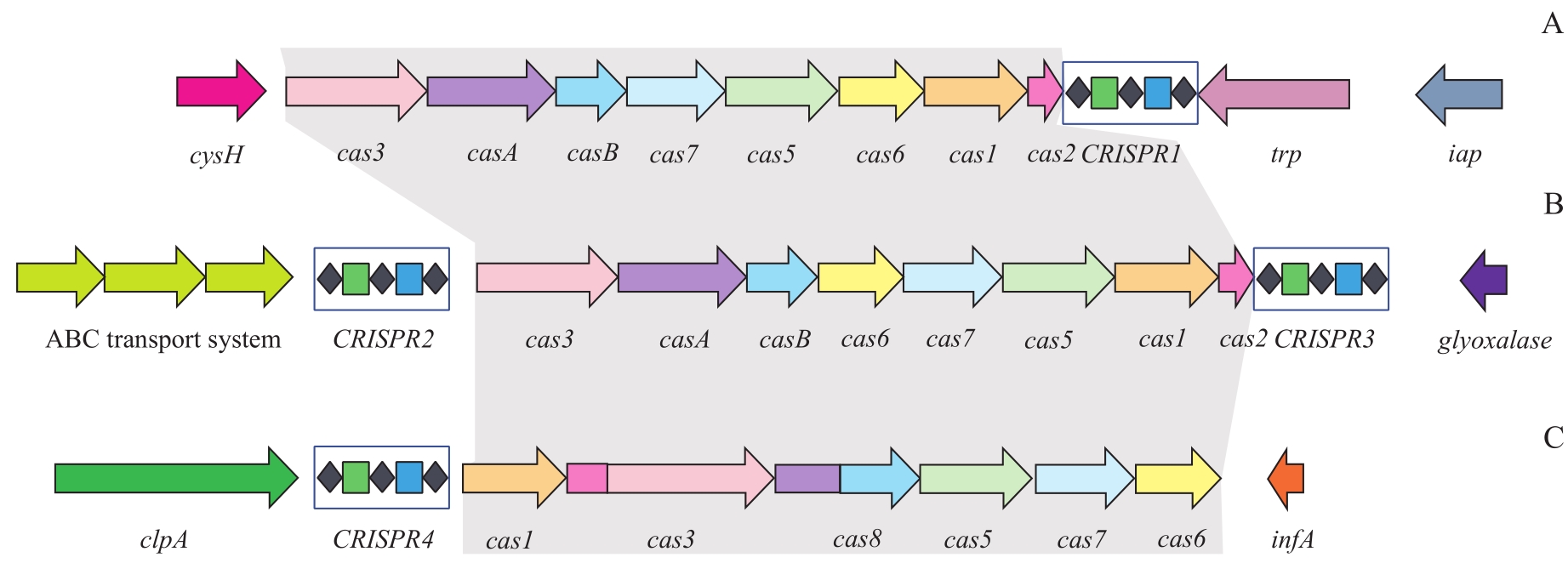
图1 肺炎克雷伯菌基因组中CRISPR-Cas系统类型示意图Note: A. I-E CRISPR-Cas system. B. I-E* CRISPR-Cas system. C. I-F CRISPR-Cas system. The same colored Cas proteins share functional similarity; gray shading indicates cas gene family cluster.
Fig 1 Schematic diagram of CRISPR-Cas system in klebsiella pneumoniae genome
| 1 | BARRANGOU R, FREMAUX C, DEVEAU H, et al. CRISPR provides acquired resistance against viruses in prokaryotes[J]. Science, 2007, 315(5819): 1709-1712. |
| 2 | LI H Y, KAO C Y, LIN W H, et al. Characterization of CRISPR-cas systems in clinical Klebsiella pneumoniae isolates uncovers its potential association with antibiotic susceptibility[J]. Front Microbiol, 2018, 9: 1595. |
| 3 | LANDER E S. The heroes of CRISPR[J]. Cell, 2016, 164(1/2): 18-28. |
| 4 | FRIEDLAENDER C. Ueber Die schizomyceten Bei der acuten fibrösen pneumonie[J]. Arch Für Pathol Anat Und Physiol Und Für Klinische Med, 1882, 87(2): 319-324. |
| 5 | BAGLEY S T. Habitat association of Klebsiella species[J]. Infect Control, 1985, 6(2): 52-58. |
| 6 | HOLT K E, WERTHEIM H, ZADOKS R N, et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health[J]. Proc Natl Acad Sci USA, 2015, 112(27): E3574-E3581. |
| 7 | WYRES K L, HOLT K E. Klebsiella pneumoniae population genomics and antimicrobial-resistant clones[J]. Trends Microbiol, 2016, 24(12): 944-956. |
| 8 | DIANCOURT L, PASSET V, VERHOEF J, et al. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates[J]. J Clin Microbiol, 2005, 43(8): 4178-4182. |
| 9 | PODSCHUN R, ULLMANN U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors[J]. Clin Microbiol Rev, 1998, 11(4): 589-603. |
| 10 | DELEO F R, CHEN L, PORCELLA S F, et al. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae[J]. Proc Natl Acad Sci USA, 2014, 111(13): 4988-4993. |
| 11 | CONLAN S, THOMAS P J, DEMING C, et al. Single-molecule sequencing to track plasmid diversity of hospital-associated carbapenemase-producing Enterobacteriaceae[J]. Sci Transl Med, 2014, 6(254): 254ra126. |
| 12 | CAI M F, PU B C, WANG Y, et al. A plasmid with conserved phage genes helps Klebsiella pneumoniae defend against the invasion of transferable DNA elements at the cost of reduced virulence[J]. Front Microbiol, 2022, 13: 827545. |
| 13 | IREDELL J, BROWN J, TAGG K. Antibiotic resistance in Enterobacteriaceae: mechanisms and clinical implications[J]. BMJ, 2016, 352: h6420. |
| 14 | RAMIREZ M S, TRAGLIA G M, LIN D L, et al. Plasmid-mediated antibiotic resistance and virulence in gram-negatives: the Klebsiella pneumoniae paradigm[J]. Microbiol Spectr, 2014, 2(5). DOI: 10.1128/microbiolspec.PLAS-0016-2013. |
| 15 | SEBGHATI T A, KORHONEN T K, HORNICK D B, et al. Characterization of the type 3 fimbrial adhesins of Klebsiella strains[J]. Infect Immun, 1998, 66(6): 2887-2894. |
| 16 | KOSKINEN K, PENTTINEN R, ÖRMÄLÄ-ODEGRIP A M, et al. Systematic comparison of epidemic and non-epidemic carbapenem resistant Klebsiella pneumoniae strains[J]. Front Cell Infect Microbiol, 2021, 11: 599924. |
| 17 | CANDAN E D, AKSÖZ N. Klebsiella pneumoniae: characteristics of carbapenem resistance and virulence factors[J]. Acta Biochim Pol, 2015, 62(4): 867-874. |
| 18 | MEDINI D, DONATI C, TETTELIN H, et al. The microbial pan-genome[J]. Curr Opin Genet Dev, 2005, 15(6): 589-594. |
| 19 | BIALEK-DAVENET S, CRISCUOLO A, AILLOUD F, et al. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups[J]. Emerg Infect Dis, 2014, 20(11): 1812-1820. |
| 20 | CHOBY J E, HOWARD-ANDERSON J, WEISS D S. Hypervirulent Klebsiella pneumoniae-clinical and molecular perspectives[J]. J Intern Med, 2020, 287(3): 283-300. |
| 21 | LAM M M C, WYRES K L, WICK R R, et al. Convergence of virulence and MDR in a single plasmid vector in MDR Klebsiella pneumoniae ST15[J]. J Antimicrob Chemother, 2019, 74(5): 1218-1222. |
| 22 | DIAS C, BORGES A, OLIVEIRA D, et al. Biofilms and antibiotic susceptibility of multidrug-resistant bacteria from wild animals[J]. PeerJ, 2018, 6: e4974. |
| 23 | TAN T Y, ONG M, CHENG Y, et al. Hypermucoviscosity, rmpA, and aerobactin are associated with community-acquired Klebsiella pneumoniae bacteremic isolates causing liver abscess in Singapore[J]. J Microbiol Immunol Infect, 2019, 52(1): 30-34. |
| 24 | SCHUBERT S, PICARD B, GOURIOU S, et al. Yersinia high-pathogenicity island contributes to virulence in Escherichia coli causing extraintestinal infections[J]. Infect Immun, 2002, 70(9): 5335-5337. |
| 25 | RUSSO T A, OLSON R, MACDONALD U, et al. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae[J]. Infect Immun, 2014, 82(6): 2356-2367. |
| 26 | NASSIF X, FOURNIER J M, ARONDEL J, et al. Mucoid phenotype of Klebsiella pneumoniae is a plasmid-encoded virulence factor[J]. Infect Immun, 1989, 57(2): 546-552. |
| 27 | GU D X, DONG N, ZHENG Z W, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study[J]. Lancet Infect Dis, 2018, 18(1): 37-46. |
| 28 | PEIRANO G, CHEN L, KREISWIRTH B N, et al. Emerging antimicrobial-resistant high-risk Klebsiella pneumoniae clones ST307 and ST147[J]. Antimicrob Agents Chemother, 2020, 64(10): e01148-e01120. |
| 29 | GU D X, HUANG Y L, MA J H, et al. Detection of colistin resistance gene mcr-1 in hypervirulent Klebsiella pneumoniae and Escherichia coli isolates from an infant with diarrhea in China[J]. Antimicrob Agents Chemother, 2016, 60(8): 5099-5100. |
| 30 | LAM M M C, WYRES K L, DUCHÊNE S, et al. Population genomics of hypervirulent Klebsiella pneumoniae clonal-group 23 reveals early emergence and rapid global dissemination[J]. Nat Commun, 2018, 9(1): 2703. |
| 31 | XIE Y Z, TIAN L J, LI G, et al. Emergence of the third-generation cephalosporin-resistant hypervirulent Klebsiella pneumoniae due to the acquisition of a self-transferable blaDHA-1-carrying plasmid by an ST23 strain[J]. Virulence, 2018, 9(1): 838-844. |
| 32 | TURTON J F, PAYNE Z, COWARD A, et al. Virulence genes in isolates of Klebsiella pneumoniae from the UK during 2016, including among carbapenemase gene-positive hypervirulent K1-ST23 and 'non-hypervirulent' types ST147, ST15 and ST383[J]. J Med Microbiol, 2018, 67(1): 118-128. |
| 33 | QIN J H, WU N N, BAO J, et al. Heterogeneous Klebsiella pneumoniae co-infections complicate personalized bacteriophage therapy[J]. Front Cell Infect Microbiol, 2021, 10: 608402. |
| 34 | LABRIE S J, SAMSON J E, MOINEAU S. Bacteriophage resistance mechanisms[J]. Nat Rev Microbiol, 2010, 8(5): 317-327. |
| 35 | HILLE F, RICHTER H, WONG S P, et al. The biology of CRISPR-cas: backward and forward[J]. Cell, 2018, 172(6): 1239-1259. |
| 36 | LOUWEN R, STAALS R H J, ENDTZ H P, et al. The role of CRISPR-Cas systems in virulence of pathogenic bacteria[J]. Microbiol Mol Biol Rev, 2014, 78(1): 74-88. |
| 37 | ALMENDROS C, MOJICA F J M, DÍEZ-VILLASEÑOR C, et al. CRISPR-Cas functional module exchange in Escherichia coli[J]. mBio, 2014, 5(1): e00767-e00713. |
| 38 | BIKARD D, HATOUM-ASLAN A, MUCIDA D, et al. CRISPR interference can prevent natural transformation and virulence acquisition during in vivo bacterial infection[J]. Cell Host Microbe, 2012, 12(2): 177-186. |
| 39 | GRISSA I, VERGNAUD G, POURCEL C. The CRISPRdb database and tools to display CRISPRs and to generate dictionaries of spacers and repeats[J]. BMC Bioinformatics, 2007, 8: 172. |
| 40 | HOOTON S P T, CONNERTON I F. Campylobacter jejuni acquire new host-derived CRISPR spacers when in association with bacteriophages harboring a CRISPR-like Cas4 protein[J]. Front Microbiol, 2015, 5: 744. |
| 41 | LIN T L, PAN Y J, HSIEH P F, et al. Imipenem represses CRISPR-Cas interference of DNA acquisition through H-NS stimulation in Klebsiella pneumoniae[J]. Sci Rep, 2016, 6: 31644. |
| 42 | ZHOU Y, TANG Y, FU P, et al. The type I-E CRISPR-Cas system influences the acquisition of blaKPC-IncF plasmid in Klebsiella pneumonia[J]. Emerg Microbes Infect, 2020, 9(1): 1011-1022. |
| 43 | ISHINO Y, SHINAGAWA H, MAKINO K, et al. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product[J]. J Bacteriol, 1987, 169(12): 5429-5433. |
| 44 | SHMAKOV S A, SITNIK V, MAKAROVA K S, et al. The CRISPR spacer space is dominated by sequences from species-specific mobilomes[J]. mBio, 2017, 8(5): e01397-e01317. |
| 45 | DATSENKO K A, POUGACH K, TIKHONOV A, et al. Molecular memory of prior infections activates the CRISPR/Cas adaptive bacterial immunity system[J]. Nat Commun, 2012, 3: 945. |
| 46 | MAKAROVA K S, WOLF Y I, KOONIN E V. The basic building blocks and evolution of CRISPR-CAS systems[J]. Biochem Soc Trans, 2013, 41(6): 1392-1400. |
| 47 | SHMAKOV S, SAVITSKAYA E, SEMENOVA E, et al. Pervasive generation of oppositely oriented spacers during CRISPR adaptation[J]. Nucleic Acids Res, 2014, 42(9): 5907-5916. |
| 48 | DÍEZ-VILLASEÑOR C, GUZMÁN N M, ALMENDROS C, et al. CRISPR-spacer integration reporter plasmids reveal distinct genuine acquisition specificities among CRISPR-Cas I-E variants of Escherichia coli[J]. RNA Biol, 2013, 10(5): 792-802. |
| 49 | KOONIN E V, MAKAROVA K S, ZHANG F. Diversity, classification and evolution of CRISPR-Cas systems[J]. Curr Opin Microbiol, 2017, 37: 67-78. |
| 50 | MAKAROVA K S, WOLF Y I, ALKHNBASHI O S, et al. An updated evolutionary classification of CRISPR-Cas systems[J]. Nat Rev Microbiol, 2015, 13(11): 722-736. |
| 51 | YOSEF I, GOREN M G, QIMRON U. Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli[J]. Nucleic Acids Res, 2012, 40(12): 5569-5576. |
| 52 | DELTCHEVA E, CHYLINSKI K, SHARMA C M, et al. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase Ⅲ[J]. Nature, 2011, 471(7340): 602-607. |
| 53 | HAURWITZ R E, JINEK M, WIEDENHEFT B, et al. Sequence- and structure-specific RNA processing by a CRISPR endonuclease[J]. Science, 2010, 329(5997): 1355-1358. |
| 54 | BROUNS S J J, JORE M M, LUNDGREN M, et al. Small CRISPR RNAs guide antiviral defense in prokaryotes[J]. Science, 2008, 321(5891): 960-964. |
| 55 | GARNEAU J E, DUPUIS M È, VILLION M, et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA[J]. Nature, 2010, 468(7320): 67-71. |
| 56 | HALE C R, ZHAO P, OLSON S, et al. RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex[J]. Cell, 2009, 139(5): 945-956. |
| 57 | WESTRA E R, VAN ERP P B G, KÜNNE T, et al. CRISPR immunity relies on the consecutive binding and degradation of negatively supercoiled invader DNA by Cascade and Cas3[J]. Mol Cell, 2012, 46(5): 595-605. |
| 58 | DEVEAU H, BARRANGOU R, GARNEAU J E, et al. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus[J]. J Bacteriol, 2008, 190(4): 1390-1400. |
| 59 | MARRAFFINI L A, SONTHEIMER E J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA[J]. Science, 2008, 322(5909): 1843-1845. |
| 60 | MOJICA F J M, DÍEZ-VILLASEÑOR C, GARCÍA-MARTÍNEZ J, et al. Short motif sequences determine the targets of the prokaryotic CRISPR defence system[J]. Microbiology (Reading), 2009, 155(Pt 3): 733-740. |
| 61 | SWARTS D C, MOSTERD C, VAN PASSEL M W J, et al. CRISPR interference directs strand specific spacer acquisition[J]. PLoS One, 2012, 7(4): e35888. |
| 62 | BURSTEIN D, SUN C L, BROWN C T, et al. Major bacterial lineages are essentially devoid of CRISPR-Cas viral defence systems[J]. Nat Commun, 2016, 7: 10613. |
| 63 | MULEPATI S, BAILEY S. Structural and biochemical analysis of nuclease domain of clustered regularly interspaced short palindromic repeat (CRISPR)-associated protein 3 (Cas3)[J]. J Biol Chem, 2011, 286(36): 31896-31903. |
| 64 | MAKAROVA K S, HAFT D H, BARRANGOU R, et al. Evolution and classification of the CRISPR-Cas systems[J]. Nat Rev Microbiol, 2011, 9(6): 467-477. |
| 65 | CADY K C, O'TOOLE G A. Non-identity-mediated CRISPR-bacteriophage interaction mediated via the csy and Cas3 proteins[J]. J Bacteriol, 2011, 193(14): 3433-3445. |
| 66 | GESNER E M, SCHELLENBERG M J, GARSIDE E L, et al. Recognition and maturation of effector RNAs in a CRISPR interference pathway[J]. Nat Struct Mol Biol, 2011, 18(6): 688-692. |
| 67 | HOCHSTRASSER M L, TAYLOR D W, BHAT P, et al. CasA mediates Cas3-catalyzed target degradation during CRISPR RNA-guided interference[J]. Proc Natl Acad Sci USA, 2014, 111(18): 6618-6623. |
| 68 | SASHITAL D G, WIEDENHEFT B, DOUDNA J A. Mechanism of foreign DNA selection in a bacterial adaptive immune system[J]. Mol Cell, 2012, 46(5): 606-615. |
| 69 | CHARPENTIER E, RICHTER H, VAN DER OOST J, et al. Biogenesis pathways of RNA guides in archaeal and bacterial CRISPR-Cas adaptive immunity[J]. FEMS Microbiol Rev, 2015, 39(3): 428-441. |
| 70 | SHEN J T, LV L, WANG X D, et al. Comparative analysis of CRISPR-Cas systems in Klebsiella genomes[J]. J Basic Microbiol, 2017, 57(4): 325-336. |
| 71 | MAKAROVA K S, WOLF Y I, IRANZO J, et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants[J]. Nat Rev Microbiol, 2020, 18(2): 67-83. |
| 72 | RUSSEL J, PINILLA-REDONDO R, MAYO-MUÑOZ D, et al. CRISPRCasTyper: automated identification, annotation, and classification of CRISPR-cas loci[J]. CRISPR J, 2020, 3(6): 462-469. |
| 73 | ZHANG F, ZHAO S J, REN C Y, et al. CRISPRminer is a knowledge base for exploring CRISPR-Cas systems in microbe and phage interactions[J]. Commun Biol, 2018, 1: 180. |
| 74 | DÍEZ-VILLASEÑOR C, ALMENDROS C, GARCÍA-MARTÍNEZ J, et al. Diversity of CRISPR loci in Escherichia coli[J]. Microbiology (Reading), 2010, 156(5): 1351-1361. |
| 75 | HAYES R P, XIAO Y B, DING F, et al. Structural basis for promiscuous PAM recognition in type I-E Cascade from E. coli[J]. Nature, 2016, 530(7591): 499-503. |
| 76 | PINILLA-REDONDO R, MAYO-MUÑOZ D, RUSSEL J, et al. Type IV CRISPR-Cas systems are highly diverse and involved in competition between plasmids[J]. Nucleic Acids Res, 2020, 48(4): 2000-2012. |
| 77 | STERN A, KEREN L, WURTZEL O, et al. Self-targeting by CRISPR: gene regulation or autoimmunity? [J]. Trends Genet, 2010, 26(8): 335-340. |
| 78 | GOLDBERG G W, JIANG W Y, BIKARD D, et al. Conditional tolerance of temperate phages via transcription-dependent CRISPR-Cas targeting[J]. Nature, 2014, 514(7524): 633-637. |
| 79 | WESTRA E R, BUCKLING A, FINERAN P C. CRISPR-Cas systems: beyond adaptive immunity[J]. Nat Rev Microbiol, 2014, 12(5): 317-326. |
| 80 | LABRIE S J, SAMSON J E, MOINEAU S. Bacteriophage resistance mechanisms[J]. Nat Rev Microbiol, 2010, 8(5): 317-327. |
| 81 | SAMSON J E, MAGADÁN A H, SABRI M, et al. Revenge of the phages: defeating bacterial defences[J]. Nat Rev Microbiol, 2013, 11(10): 675-687. |
| 82 | JACKSON S A, BIRKHOLZ N, MALONE L M, et al. Imprecise spacer acquisition generates CRISPR-cas immune diversity through primed adaptation[J]. Cell Host Microbe, 2019, 25(2): 250-260.e4. |
| 83 | BONDY-DENOMY J, DAVIDSON A R. When a virus is not a parasite: the beneficial effects of prophages on bacterial fitness[J]. J Microbiol, 2014, 52(3): 235-242. |
| 84 | FEINER R, ARGOV T, RABINOVICH L, et al. A new perspective on lysogeny: prophages as active regulatory switches of bacteria[J]. Nat Rev Microbiol, 2015, 13(10): 641-650. |
| 85 | BONDY-DENOMY J, PAWLUK A, MAXWELL K L, et al. Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system[J]. Nature, 2013, 493(7432): 429-432. |
| 86 | PAWLUK A, BONDY-DENOMY J, CHEUNG V H W, et al. A new group of phage anti-CRISPR genes inhibits the type I-E CRISPR-Cas system of Pseudomonas aeruginosa[J]. mBio, 2014, 5(2): e00896. |
| 87 | LI Y P, BONDY-DENOMY J. Anti-CRISPRs go viral: the infection biology of CRISPR-Cas inhibitors[J]. Cell Host Microbe, 2021, 29(5): 704-714. |
| 88 | JIA N, PATEL D J. Structure-based functional mechanisms and biotechnology applications of anti-CRISPR proteins[J]. Nat Rev Mol Cell Biol, 2021, 22(8): 563-579. |
| 89 | DAVIDSON A R, LU W T, STANLEY S Y, et al. Anti-CRISPRs: protein inhibitors of CRISPR-cas systems[J]. Annu Rev Biochem, 2020, 89: 309-332. |
| 90 | SHIVRAM H, CRESS B F, KNOTT G J, et al. Controlling and enhancing CRISPR systems[J]. Nat Chem Biol, 2021, 17(1): 10-19. |
| 91 | SHEHREEN S, CHYOU T Y, FINERAN P C, et al. Genome-wide correlation analysis suggests different roles of CRISPR-Cas systems in the acquisition of antibiotic resistance genes in diverse species[J]. Philos Trans R Soc Lond B Biol Sci, 2019, 374(1772): 20180384. |
| 92 | WIEGAND T, KARAMBELKAR S, BONDY-DENOMY J, et al. Structures and strategies of anti-CRISPR-mediated immune suppression[J]. Annu Rev Microbiol, 2020, 74: 21-37. |
| 93 | PAWLUK A, SHAH M, MEJDANI M, et al. Disabling a type I-E CRISPR-cas nuclease with a bacteriophage-encoded anti-CRISPR protein[J]. mBio, 2017, 8(6): e01751-e01717. |
| 94 | ZHANG H, LI Z, DACZKOWSKI C M, et al. Structural basis for the inhibition of CRISPR-Cas12a by anti-CRISPR proteins[J]. Cell Host Microbe, 2019, 25(6): 815-826.e4. |
| 95 | KNOTT G J, THORNTON B W, LOBBA M J, et al. Broad-spectrum enzymatic inhibition of CRISPR-Cas12a[J]. Nat Struct Mol Biol, 2019, 26(4): 315-321. |
| 96 | BONDY-DENOMY J, GARCIA B, STRUM S, et al. Multiple mechanisms for CRISPR-Cas inhibition by anti-CRISPR proteins[J]. Nature, 2015, 526(7571): 136-139. |
| 97 | ROLLINS M F, CHOWDHURY S, CARTER J, et al. Structure reveals a mechanism of CRISPR-RNA-guided nuclease recruitment and anti-CRISPR viral mimicry[J]. Mol Cell, 2019, 74(1): 132-142.e5. |
| 98 | WANG X F, YAO D Q, XU J G, et al. Structural basis of Cas3 inhibition by the bacteriophage protein AcrF3[J]. Nat Struct Mol Biol, 2016, 23(9): 868-870. |
| 99 | WANG J Y, MA J, CHENG Z, et al. A CRISPR evolutionary arms race: structural insights into viral anti-CRISPR/Cas responses[J]. Cell Res, 2016, 26(10): 1165-1168. |
| 100 | ATHUKORALAGE J S, MCMAHON S A, ZHANG C Y, et al. An anti-CRISPR viral ring nuclease subverts type Ⅲ CRISPR immunity[J]. Nature, 2020, 577(7791): 572-575. |
| 101 | MARINO N D, ZHANG J Y, BORGES A L, et al. Discovery of widespread type I and type V CRISPR-Cas inhibitors[J]. Science, 2018, 362(6411): 240-242. |
| 102 | PAWLUK A, AMRANI N, ZHANG Y, et al. Naturally occurring off-switches for CRISPR-Cas9[J]. Cell, 2016, 167(7): 1829-1838.e9. |
| 103 | BISWAS A, GAGNON J N, BROUNS S J J, et al. CRISPRTarget [J]. RNA Biology, 2013, 10(5): 817-27. |
| 104 | PINILLA-REDONDO R, SHEHREEN S, MARINO ND, et al. Discovery of multiple anti-CRISPRs highlights anti-defense gene clustering in mobile genetic elements[J]. Nat Commun, 2020, 11(1): 5652. |
| 105 | WYRES K L, WICK R R, JUDD L M, et al. Distinct evolutionary dynamics of horizontal gene transfer in drug resistant and virulent clones of Klebsiella pneumoniae[J]. PLoS Genet, 2019, 15(4): e1008114. |
| 106 | WATTERS K E, FELLMANN C, BAI H B, et al. Systematic discovery of natural CRISPR-Cas12a inhibitors[J]. Science, 2018, 362(6411): 236-239. |
| 107 | BARRANGOU R, HORVATH P. CRISPR: new horizons in phage resistance and strain identification[J]. Annu Rev Food Sci Technol, 2012, 3: 143-162. |
| 108 | TAN D M, ZHANG Y Y, CHENG M J, et al. Characterization of Klebsiella pneumoniae ST11 isolates and their interactions with lytic phages[J]. Viruses, 2019, 11(11): 1080. |
| [1] | 蒋婕, 张泓, 伦赫远, 潘芬, 于方圆, 何平. 儿童肺炎克雷伯菌感染分子流行病学特征[J]. 上海交通大学学报(医学版), 2025, 45(8): 1027-1034. |
| [2] | 李莉莎, 李建辉, 何斌, 吴楠楠, 朱同玉, 郭晓奎, 陈峥宏. 噬菌体治疗泛耐药肺炎克雷伯菌肺部感染的临床应用及效果初探[J]. 上海交通大学学报(医学版), 2021, 41(9): 1272-1276. |
| [3] | 管红艳,刘婧娴,刘 瑛. 分离自血培养肺炎克雷伯菌的毒力基因及患者临床特征分析[J]. 上海交通大学学报(医学版), 2020, 40(2): 235-. |
| [4] | 吕 霖,石 鑫,郭晓奎,秦金红. 耐药肺炎克雷伯菌噬菌体JD902的分离及生物学特性和安全性研究[J]. 上海交通大学学报(医学版), 2019, 39(12): 1389-. |
| [5] | 吴广喜,石学银,何斌. 肺炎克雷伯菌疫苗的研制进展[J]. 上海交通大学学报(医学版), 2018, 38(4): 458-. |
| [6] | 刘洋,郑丹丹,韩逸超,史玮炀,戴尔宽,李敏,郑冰 . 多重耐药肺炎克雷伯菌感染的危险因素及治疗方案比较[J]. 上海交通大学学报(医学版), 2017, 37(7): 973-. |
| [7] | 支烨,罗婷婷,王睿,冯希佳,刘珂,石臣坤,王璐璐,胡付品,何平 . 泛耐药肺炎克雷伯菌噬菌体SH-Kp152234的生物学特性和基因组学研究[J]. 上海交通大学学报(医学版), 2017, 37(3): 273-. |
| [8] | 田李均,王晓丽,肖淑珍,孙景勇,刘嘉琳,瞿洪平. 医院内高黏液性肺炎克雷伯菌的流行分布、毒力基因及临床特征分析[J]. 上海交通大学学报(医学版), 2017, 37(1): 43-. |
| [9] | 刘婧娴,俞静,李媛睿,等. 肺炎克雷伯菌对碳青霉烯类抗生素的耐药机制研究[J]. 上海交通大学学报(医学版), 2016, 36(1): 93-. |
| [10] | 刘婧娴,俞静,刘瑛. ST571型产NDM-1肺炎克雷伯菌检出及分子流行病学研究[J]. 上海交通大学学报(医学版), 2015, 35(3): 402-. |
| [11] | 黄洁雯, 陈志俊, 沈隽霏, 等. 壳聚糖季铵盐对肺炎克雷伯菌的体外抑菌作用[J]. , 2012, 32(11): 1421-. |
| [12] | 李 俐, 蒋燕群. 大肠埃希菌和肺炎克雷伯菌外膜蛋白与耐药机制的研究进展[J]. , 2010, 30(11): 1433-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||