
上海交通大学学报(医学版) ›› 2023, Vol. 43 ›› Issue (8): 997-1007.doi: 10.3969/j.issn.1674-8115.2023.08.007
收稿日期:2023-03-16
接受日期:2023-08-01
出版日期:2023-08-28
发布日期:2023-08-28
通讯作者:
刘玉敏,电子信箱:ymliu@sjtu.edu.cn。作者简介:高 羽(1990—),女,助理实验师,硕士;电子信箱:shirlygao@sjtu.edu.cn。
基金资助:
GAO Yu( ), YIN Shan, PANG Yue, LIANG Wenyi, LIU Yumin(
), YIN Shan, PANG Yue, LIANG Wenyi, LIU Yumin( )
)
Received:2023-03-16
Accepted:2023-08-01
Online:2023-08-28
Published:2023-08-28
Contact:
LIU Yumin, E-mail: ymliu@sjtu.edu.cn.Supported by:摘要:
目的·观察大黄对大鼠肠道菌群与机体间的平衡关系的影响。方法·以Wistar大鼠为研究模型,将其随机分为4组(每组n=8),分别以大黄提取液0.1 g/kg(低剂量大黄组)、2.5 g/kg(中剂量大黄组)、4.5 g/kg(高剂量大黄组)及等量生理盐水(对照组)进行灌胃,连续给药5 d。每日观察大鼠粪便含水量的变化情况。采用气相色谱-飞行时间质谱(gas chromatography/time of flight mass spectrometry, GC/TOFMS)技术对第5日大鼠体内血清、结肠组织、粪便的代谢物进行检测,并采用主成分分析(principal component analysis,PCA)和偏最小二乘判别分析(partial least squares discrimination analysis,PLS-DA)方法分析不同剂量组与对照组间代谢物的差异,进一步采用t检验获得差异具有统计学意义的代谢物。结果·大黄给药后,大黄组大鼠粪便含水量随时间及剂量的增加逐渐增大。给药第5日,与对照组相比,不同剂量大黄组大鼠体内血清、结肠组织和粪便中分别检测到28、18和20种差异代谢物的水平有显著变化(P<0.05),且有17种血清代谢物、2种结肠组织代谢物、10种粪便代谢物的水平变化呈现剂量效应。其中,部分神经递质类物质、吲哚类物质、胆酸类物质等肠道菌群-宿主共代谢物给药后发生了显著变化。粪便中儿茶酚、吲哚-3-乙酸的水平明显升高,而苯丙氨酸、4-氨基丁酸、左旋多巴、吲哚-3-丙酸的水平明显降低;在高剂量大黄组结肠组织中脱氧胆酸明显升高;与对照组相比,不同剂量组中血清苯丙氨酸、酪氨酸、色氨酸的水平明显升高。此外,富马酸(能量代谢相关的有机酸类物质)在大黄给药后的粪便中明显下调,而在结肠组织和血清中明显上调。随着给药剂量增大,谷氨酸(氨基酸类物质之一)水平在血清中明显递增,但在粪便中逐渐递减。除了6-磷酸葡萄糖酸外,果糖、丙酮酸、乳酸、葡萄糖-1-磷酸、D-甘油-1-磷酸等糖代谢物和二十二酸、13-二十二碳烯酸、单硬脂酸甘油酯、胆固醇等脂代谢物的水平在血清中升高,而结肠组织中D-甘油-1-磷酸和粪便中乳酸、葡萄糖-1-磷酸、亚麻酸明显降低。结论·大黄通过肠道菌群-宿主共代谢作用影响了脑-肠轴、胆汁酸代谢,进一步作用于机体的能量代谢、氨基酸代谢、糖代谢和脂代谢。
中图分类号:
高羽, 殷姗, 庞玥, 梁文懿, 刘玉敏. 大黄对大鼠体内肠道菌群-宿主共代谢作用的影响[J]. 上海交通大学学报(医学版), 2023, 43(8): 997-1007.
GAO Yu, YIN Shan, PANG Yue, LIANG Wenyi, LIU Yumin. Effect of rhubarb on gut microbiota-host co-metabolism in rats[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2023, 43(8): 997-1007.
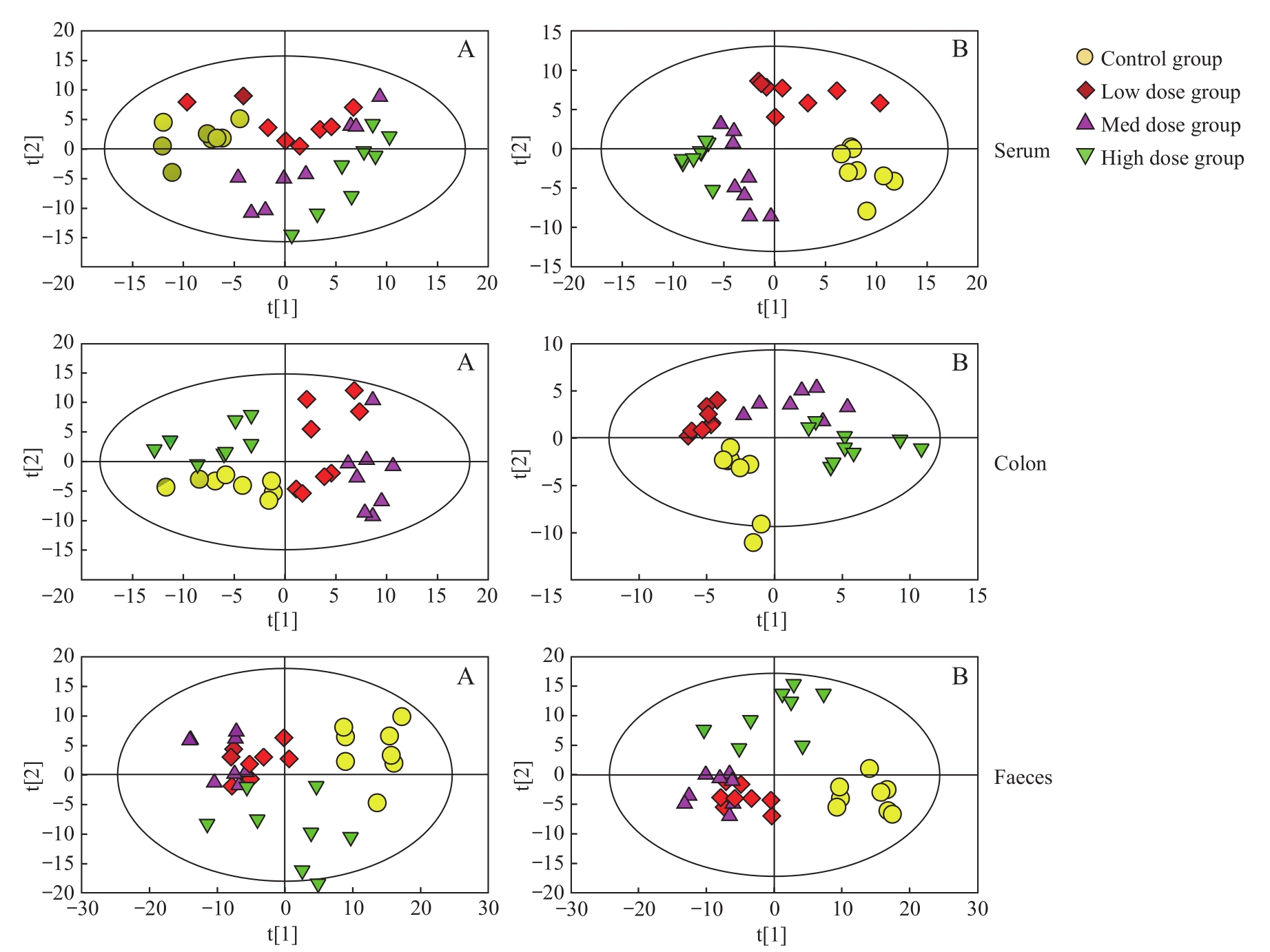
图3 各组大鼠的 PCA (A) 和 PLS-DA (B) 得分图Note: t[1] is the score value of each sample on principal component 1. t[2] is the score value of each sample on principal component 2.
Fig 3 PCA (A) and PLS-DA (B) score plot of each group
| Class | Significant metabolite | Related bacteria [ | Fc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum | Colon | Fecal | |||||||||
| Fc1 | Fc2 | Fc3 | Fc1 | Fc2 | Fc3 | Fc1 | Fc2 | Fc3 | |||
| Metabolites of gut microbial-proteins, peptides and amino acids/gut microbial-host co-metabolism | Catechol | Lactobacillus[+ -] Clostridium[] | 1.09 | -1.06 | 1.42① | ||||||
| Phenylpropionic acid | 1.01 | 1.75① | 1.48① | 5.27① | 8.35① | 5.44① | |||||
| 2-hydroxyphenylpropionic acid | -2.08① | -2.33① | -2.33① | ||||||||
| L-DOPA | 1.10 | -1.44① | -1.30① | ||||||||
| Phenylalanine | 1.12 | 1.20① | 1.23① | -1.28 | -1.43① | -1.49① | |||||
| Tyrosine | 1.41① | 1.53① | 1.58① | ||||||||
| 4-aminobutyric acid | Clostridium[] | -1.30 | -1.79① | -1.35 | |||||||
| Ornithine | 1.21① | 1.31① | 2.03① | -2.08① | -1.85① | -2.00① | |||||
| Spermidine | 1.58① | 2.17① | 2.30① | ||||||||
| Tryptophan | Escherichia coli[+ -] Bifidobacterium[+] | 1.07 | 1.41① | 1.46① | |||||||
| Indole-3-acetic acid | 1.12 | 2.06① | 1.92① | ||||||||
| Indole-3-propionic acid | 1.00 | -1.10 | -2.00① | ||||||||
| Indole-3-lactic acid | 1.08 | 1.36① | 1.43① | ||||||||
| Kynurenic acid | 1.29① | 1.44① | -7.14① | ||||||||
| Xanthuric acid | -1.09 | -1.25① | -1.47① | ||||||||
| Nicotinic acid | -1.11 | -1.11 | 2.85① | -1.12 | -1.25① | -1.28① | |||||
| Deoxycholic acid | Lactobacillus[+ -] Bifidobacterium[+] Bacteroides[+] Escherichia coli[+ -] Clostridium[] | -1.03 | 1.04 | 2.88① | |||||||
| Cholesterol | 1.75① | 1.84① | 2.46① | -1.85 | -4.17① | -3.85① | |||||
| Isoleucine | 1.18 | 1.18 | 1.22① | -1.26 | -1.28① | -1.39① | |||||
| Leucine | 1.15 | 1.19 | 1.24① | -1.17 | -1.25① | -1.26① | |||||
| Valine | 1.21 | 1.19 | 1.26① | -1.21 | -1.58① | -1.67① | |||||
| Isovaleric acid | 1.28 | 1.02 | -2.94① | ||||||||
| Caproic acid | 1.04 | 4.37① | 4.30① | ||||||||
| TCA cycle | Fumaric acid | Bifidobacterium[+] Lactobacillus[+ -] | 2.29① | 2.84① | 3.83① | 1.73① | 1.28 | 1.25① | -1.85① | -1.79① | -1.75① |
| Malic acid | 1.60① | 2.28① | 3.49① | 1.71① | -1.06① | 1.00 | -1.64① | -2.17① | -2.04① | ||
| α-ketoglutaric acid | 1.33 | 1.34 | 1.60① | ||||||||
| Citric acid | 1.48① | 1.41① | 1.74① | ||||||||
| Isocitrate | 1.53 | 1.64① | 1.77① | ||||||||
| TCA cycle-amino acid | Glutamic acid | 1.27① | 1.31① | 1.43① | -1.23 | -1.61① | -1.72① | ||||
| Glutamine | 2.15① | 2.37① | 3.08① | ||||||||
| Aspartic acid | 1.31① | 1.46① | 1.60① | ||||||||
| Asparagine | 1.17 | 1.59① | 1.40① | ||||||||
| TCA cycle-glycometabolism | Pyruvate | Bifidobacterium[+] Bacteroides[+] Lactobacillus[+ -] | 1.09 | 1.37 | 1.67① | -1.03 | 1.47 | 2.48① | |||
| Lactic acid | 1.16 | 1.46① | 1.46① | 1.55① | -1.07 | -1.05 | -3.52① | -2.68① | -1.05① | ||
| Fructose | 1.08 | 1.23① | 1.41① | 1.46① | 2.20① | 3.41① | |||||
| 6-phosphogluconic acid | -1.09① | -1.10① | -1.33① | 1.34① | 1.55① | -10.00① | |||||
| D-glycero-1-phosphate | 1.45 | 1.95① | 2.03① | -1.35① | -1.37 | -1.69① | |||||
| Glucose-1-phosphate | 1.05 | 0.85 | 1.42① | 1.31 | 1.22 | -2.63① | -1.25① | -1.29① | -1.31① | ||
| β-glycerophosphate | -1.33① | 1.00 | -1.56① | ||||||||
| Ethanolamine phosphate | 1.21 | -1.67 | -1.96① | ||||||||
| Phosphoenolpyruvate | 1.54① | 1.32 | -1.56① | ||||||||
| TCA cycle-lipid metabolism | Linolenic acid | Bifidobacterium[+] Lactobacillus[+-] | 1.04 | -1.53① | -1.52① | ||||||
| Docosanoic acid | 1.32 | 3.29① | 1.92① | ||||||||
| 13-docosenoic acid | 1.43 | 1.80① | 2.27① | ||||||||
| 1-monostearoylglycerol | -1.30 | 1.01 | 1.53① | ||||||||
表1 对照组和实验组大鼠体内的差异性代谢物
Tab 1 Significant metabolites in rats of the control group and the rhubarb administration groups
| Class | Significant metabolite | Related bacteria [ | Fc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum | Colon | Fecal | |||||||||
| Fc1 | Fc2 | Fc3 | Fc1 | Fc2 | Fc3 | Fc1 | Fc2 | Fc3 | |||
| Metabolites of gut microbial-proteins, peptides and amino acids/gut microbial-host co-metabolism | Catechol | Lactobacillus[+ -] Clostridium[] | 1.09 | -1.06 | 1.42① | ||||||
| Phenylpropionic acid | 1.01 | 1.75① | 1.48① | 5.27① | 8.35① | 5.44① | |||||
| 2-hydroxyphenylpropionic acid | -2.08① | -2.33① | -2.33① | ||||||||
| L-DOPA | 1.10 | -1.44① | -1.30① | ||||||||
| Phenylalanine | 1.12 | 1.20① | 1.23① | -1.28 | -1.43① | -1.49① | |||||
| Tyrosine | 1.41① | 1.53① | 1.58① | ||||||||
| 4-aminobutyric acid | Clostridium[] | -1.30 | -1.79① | -1.35 | |||||||
| Ornithine | 1.21① | 1.31① | 2.03① | -2.08① | -1.85① | -2.00① | |||||
| Spermidine | 1.58① | 2.17① | 2.30① | ||||||||
| Tryptophan | Escherichia coli[+ -] Bifidobacterium[+] | 1.07 | 1.41① | 1.46① | |||||||
| Indole-3-acetic acid | 1.12 | 2.06① | 1.92① | ||||||||
| Indole-3-propionic acid | 1.00 | -1.10 | -2.00① | ||||||||
| Indole-3-lactic acid | 1.08 | 1.36① | 1.43① | ||||||||
| Kynurenic acid | 1.29① | 1.44① | -7.14① | ||||||||
| Xanthuric acid | -1.09 | -1.25① | -1.47① | ||||||||
| Nicotinic acid | -1.11 | -1.11 | 2.85① | -1.12 | -1.25① | -1.28① | |||||
| Deoxycholic acid | Lactobacillus[+ -] Bifidobacterium[+] Bacteroides[+] Escherichia coli[+ -] Clostridium[] | -1.03 | 1.04 | 2.88① | |||||||
| Cholesterol | 1.75① | 1.84① | 2.46① | -1.85 | -4.17① | -3.85① | |||||
| Isoleucine | 1.18 | 1.18 | 1.22① | -1.26 | -1.28① | -1.39① | |||||
| Leucine | 1.15 | 1.19 | 1.24① | -1.17 | -1.25① | -1.26① | |||||
| Valine | 1.21 | 1.19 | 1.26① | -1.21 | -1.58① | -1.67① | |||||
| Isovaleric acid | 1.28 | 1.02 | -2.94① | ||||||||
| Caproic acid | 1.04 | 4.37① | 4.30① | ||||||||
| TCA cycle | Fumaric acid | Bifidobacterium[+] Lactobacillus[+ -] | 2.29① | 2.84① | 3.83① | 1.73① | 1.28 | 1.25① | -1.85① | -1.79① | -1.75① |
| Malic acid | 1.60① | 2.28① | 3.49① | 1.71① | -1.06① | 1.00 | -1.64① | -2.17① | -2.04① | ||
| α-ketoglutaric acid | 1.33 | 1.34 | 1.60① | ||||||||
| Citric acid | 1.48① | 1.41① | 1.74① | ||||||||
| Isocitrate | 1.53 | 1.64① | 1.77① | ||||||||
| TCA cycle-amino acid | Glutamic acid | 1.27① | 1.31① | 1.43① | -1.23 | -1.61① | -1.72① | ||||
| Glutamine | 2.15① | 2.37① | 3.08① | ||||||||
| Aspartic acid | 1.31① | 1.46① | 1.60① | ||||||||
| Asparagine | 1.17 | 1.59① | 1.40① | ||||||||
| TCA cycle-glycometabolism | Pyruvate | Bifidobacterium[+] Bacteroides[+] Lactobacillus[+ -] | 1.09 | 1.37 | 1.67① | -1.03 | 1.47 | 2.48① | |||
| Lactic acid | 1.16 | 1.46① | 1.46① | 1.55① | -1.07 | -1.05 | -3.52① | -2.68① | -1.05① | ||
| Fructose | 1.08 | 1.23① | 1.41① | 1.46① | 2.20① | 3.41① | |||||
| 6-phosphogluconic acid | -1.09① | -1.10① | -1.33① | 1.34① | 1.55① | -10.00① | |||||
| D-glycero-1-phosphate | 1.45 | 1.95① | 2.03① | -1.35① | -1.37 | -1.69① | |||||
| Glucose-1-phosphate | 1.05 | 0.85 | 1.42① | 1.31 | 1.22 | -2.63① | -1.25① | -1.29① | -1.31① | ||
| β-glycerophosphate | -1.33① | 1.00 | -1.56① | ||||||||
| Ethanolamine phosphate | 1.21 | -1.67 | -1.96① | ||||||||
| Phosphoenolpyruvate | 1.54① | 1.32 | -1.56① | ||||||||
| TCA cycle-lipid metabolism | Linolenic acid | Bifidobacterium[+] Lactobacillus[+-] | 1.04 | -1.53① | -1.52① | ||||||
| Docosanoic acid | 1.32 | 3.29① | 1.92① | ||||||||
| 13-docosenoic acid | 1.43 | 1.80① | 2.27① | ||||||||
| 1-monostearoylglycerol | -1.30 | 1.01 | 1.53① | ||||||||
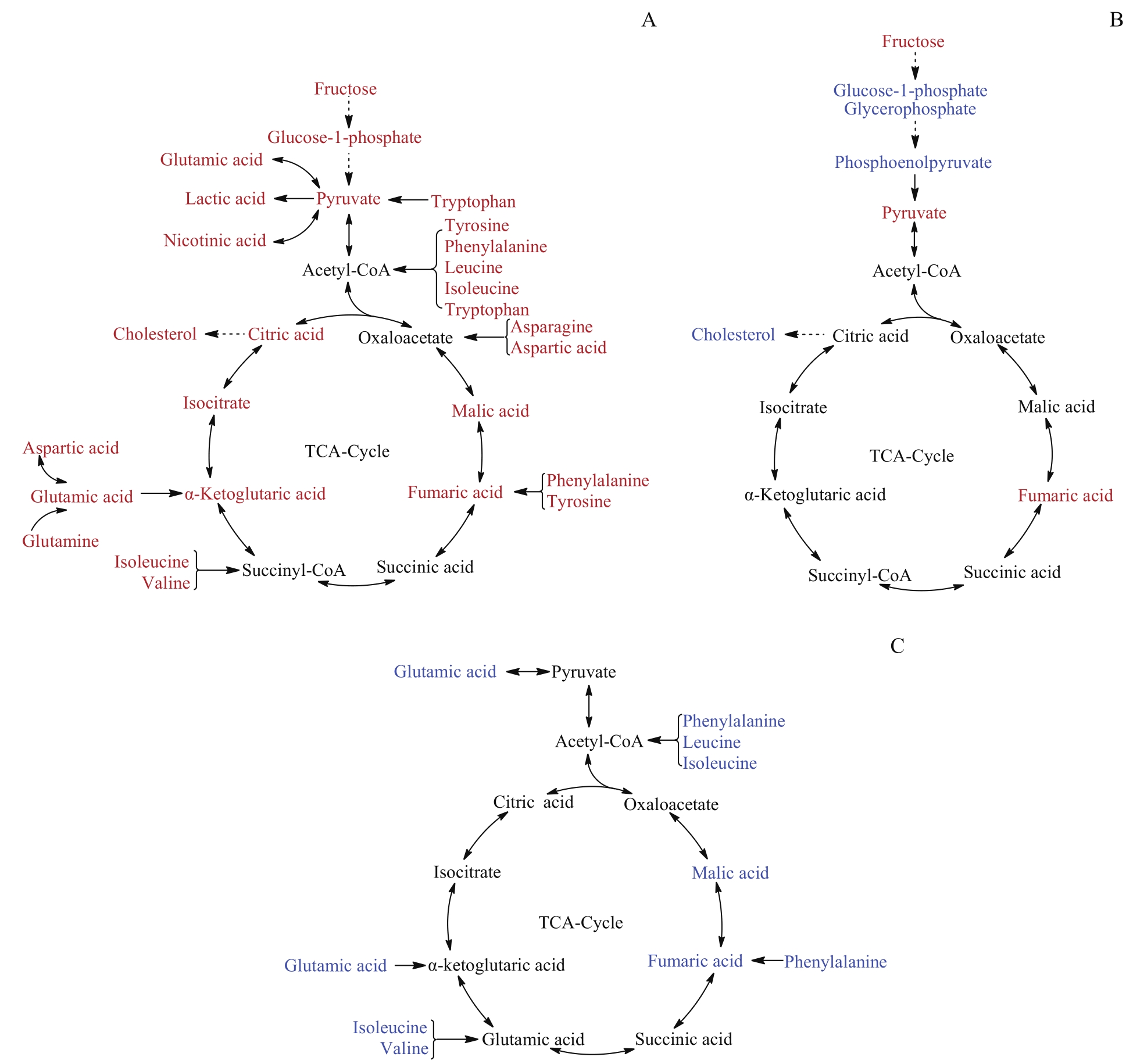
图4 代谢通路分析示意图Note: A. Serum.B. Colon. C. Fecal. Red indicates a relatively higher concentration present in the rhubarb-dosed group compared to the control group, whereas blue means a relatively lower concentration.
Fig 4 Schematic diagram of metabolic pathway analysis
| 1 | 国家药典委员会. 中华人民共和国药典:一部(2020年版)[M]. 北京: 中国医药科技出版社, 2020: 24-25. |
| National Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China: A(2020)[M]. Beijing: China Medical Science and Technology Press, 2020: 24-25. | |
| 2 | 金丽霞, 金丽军, 栾仲秋, 等. 大黄的化学成分和药理研究进展[J]. 中医药信息, 2020, 37(1): 121-126. |
| JIN L X, JIN L J, LUAN Z Q, et al. Research progress on chemical constituents and pharmacology of Rhubarb[J]. Information on Traditional Chinese Medicine, 2020,37(1): 121-126. | |
| 3 | PENG Y, WU C F, YANG J Y, et al. Gut microbial diversity in rat model induced by rhubarb[J]. Exp Anim, 2014, 63(4): 415-422. |
| 4 | 王荣荣, 曹志尉, 孟静. 大黄牡丹汤保留灌肠联合血液净化治疗重症急性胰腺炎的临床疗效及对患者肠黏膜屏障功能和炎症因子的影响[J]. 中国中医急症, 2018, 27(9): 1618-1620. |
| WANG R R, CAO Z W, MENG J. Clinical effect of Rhubarb Peony Decoction retention enema combined with blood purification in the treatment of severe acute pancreatitis and its effect on intestinal mucosal barrier function and inflammatory factors[J]. Journal of Emergency in Traditional Chinese Medicine, 2018,27(9): 1618-1620. | |
| 5 | 张开弦, 姚秋阳, 吴发明, 等. 大黄属药用植物化学成分及药理作用研究进展[J]. 中国新药杂志, 2022, 31(6) :555-566. |
| ZHANG K X, YAO Q Y, WU F M, et al. Resrarch progress on chemical constituents and pharmacological effects of medicinal plants in genus Rheum[J]. Chinese Journal of New Drugs, 2022, 31(6): 555-566. | |
| 6 | 张丽恒, 乙引. 大黄素对大肠杆菌的体外抗菌活性及其抗菌机理研究[J]. 黑龙江畜牧兽医, 2021, 622(10): 127-130, 134. |
| ZHANG L H, YI Y. Study on the in vitro antibacterial activity of emodin against Escherichia coli and its antibacterial mechanism[J]. Heilongjiang Animal Science and Veterinary Medicine, 2021, 622(10): 127-130, 134. | |
| 7 | 聂银利, 段学清, 陈瑞, 等. 大黄对大鼠肠道菌群的影响[J]. 实用中医药杂志, 2021, 37(4): 529-535. |
| NIE Y L, DUAN X Q, CHEN R, et al. Effect of Rhubarb on the intestinal flora of rat[J]. Journal of Practical Traditional Chinese Medicine, 2021, 37(4): 529-535. | |
| 8 | 符子艺, 魏成功, 刘小虹, 等. 从大黄对肠道微生态的影响探讨肺肠相关理论[J]. 亚太传统医药, 2014, 10(8): 44-46. |
| FU Z Y, WEI C G, LIU X H, et al. To explore lung-intestinal theory from the effect of Rhubarb on intestinal microecology[J]. Asia-Pacific Traditional Medicine, 2014, 10(8): 44-46. | |
| 9 | 孙元莹, 李志军, 王今达. 从 "肺与大肠相表里" 论治多脏器功能障碍综合征[J]. 时珍国医国药, 2007, 18(5): 1220-1221. |
| SUN Y Y, LI Z J, WANG J D. Treatment of multiple organ dysfunction syndrome from the "lung and large intestine"[J]. Lishizhen Medicine and Materia Medica Research, 2007, 18(5): 1220-1221. | |
| 10 | 韦忠红, 赵杨, 李晓曼, 等. 大黄蒽醌类成分影响肠道微生物的组成平衡损伤结肠黏膜屏障促进结肠癌发展[J]. 中国药理学与毒理学杂志, 2021, 35(10): 750-751. |
| WEI Z H, ZHAO Y, LI X M, et al. The anthraquinones of Rhubarb affect the composition balance of intestinal microorganisms, damage the mucosal barrier of colon and promote the development of colon cancer [J].Chinese Journal of Pharmacology and Toxicology, 2021,35(10): 750-751. | |
| 11 | 乔进, 赵彦, 陈霞, 等. 基于PI3K/Akt/FoxO1通路探讨大黄酸对2型糖尿病大鼠肾损伤的作用[J]. 中成药, 2023, 45(2): 609-613. |
| QIAO J, ZHAO Y, CHEN X, et al. Effect of rhein on kidney injury in type 2 diabetic rats based on PI3K/Akt/FoxO1 pathway[J]. Chinese Traditional Patent Medicine, 2023,45(2): 609-613. | |
| 12 | QIU Y P, CAI G X, SU M M, et al. Serum metabolite profiling of human colorectal cancer using GC-TOFMS and UPLC-QTOFMS[J]. J Proteome Res, 2009, 8(10): 4844-4850. |
| 13 | PAN L, QIU Y P, CHEN T L, et al. An optimized procedure for metabonomic analysis of rat liver tissue using gas chromatography/time-of-flight mass spectrometry[J]. J Pharm Biomed Anal, 2010, 52(4): 589-596. |
| 14 | YIN S, GUO P, HAI D F, et al. Optimization of GC/TOF MS analysis conditions for assessing host-gut microbiota metabolic interactions: Chinese rhubarb alters fecal aromatic amino acids and phenol metabolism[J]. Anal Chim Acta, 2017, 995: 21-33. |
| 15 | NICHOLSON J K, HOLMES E, KINROSS J, et al. Host-gut microbiota metabolic interactions[J]. Science, 2012, 336(6086): 1262-1267. |
| 16 | 宋洋, 乐佳蕴, 王小翠, 等. 大黄调节肠道菌群干预急性胰腺炎的研究进展[J]. 中国中医急症, 2022, 31(8): 1307-1309. |
| SONG Y, LE J Y, WANG X C, et al. Research progress of Rhubarb regulating intestinal flora in the intervention of acute pancreatitis[J]. Journal of Emergency in Traditional Chinese Medicine, 2022,31(8): 1307-1309. | |
| 17 | 张孟之, 陈雨佳, 王宗陵, 等. 基于对肠道菌群调节探讨大黄醒脑开窍机制[J]. 辽宁中医杂志, 2019, 46(6): 1196-1198. |
| ZHANG M Z, CHEN Y J, WANG Z L, et al. Study on mechanism of resuscitation of Rhubarb based on regulation of intestinal flora[J]. Liaoning Journal of Traditional Chinese Medicine, 2019,46(6): 1196-1198. | |
| 18 | 王玉, 杨雪, 夏鹏飞, 等. 大黄化学成分、药理作用研究进展及质量标志物的预测分析[J]. 中草药, 2019, 50(19): 4821-4837. |
| WANG Y, YANG X, XIA P F, et al. Research progress on chemical composition and pharmacological effects of Rhei Radix et Rhizoma and predictive analysis on quality markers[J]. Chinese Traditional and Herbal Drugs, 2019, 50(19): 4821-4837. | |
| 19 | GAO X, PUJOS-GUILLOT E, SÉBÉDIO J L. Development of a quantitative metabolomic approach to study clinical human fecal water metabolome based on trimethylsilylation derivatization and GC/MS analysis[J]. Anal Chem, 2010, 82(15): 6447-6456. |
| 20 | RANHOTRA H S, FLANNIGAN K L, BRAVE M, et al. Xenobiotic receptor-mediated regulation of intestinal barrier function and innate immunity[J]. Nucl Receptor Res, 2016, 3: 101199. |
| 21 | KORECKA A, DONA A, LAHIRI S, et al. Bidirectional communication between the Aryl hydrocarbon Receptor (AhR) and the microbiome tunes host metabolism[J]. NPJ Biofilms Microbiomes, 2016, 2: 16014. |
| 22 | 王亦君, 冯舒涵, 程锦堂, 等. 大黄蒽醌类化学成分和药理作用研究进展[J]. 中国实验方剂学杂志, 2018, 24(13): 227-234. |
| WANG Y J, FENG S H, CHENG J T, et al. Research progress on chemical constituents and pharmacological action of anthraquinone in Rhei Radix et Rhizoma[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2018, 24(13): 227-234. | |
| 23 | VAN DER LEEK A P, YANISHEVSKY Y, KOZYRSKYJ A L. The kynurenine pathway as a novel link between allergy and the gut microbiome[J]. Front Immunol, 2017, 8: 1374. |
| 24 | 李吉平, 陈雪, 刘建华, 等. 双歧杆菌生物特性及其功能研究进展[J]. 中国奶牛, 2020, 362(6): 57-61. |
| LI J P, CHEN X, LIU J H, et al. Advances in biological characteristics and functions of bifidobacterium[J]. China Dairy Cattle, 2020, 362(6): 57-61. | |
| 25 | 孙蒋, 罗静雯, 姚文杰, 等. 大黄素对急性肾损伤大鼠肠道菌群的调节作用[J]. 中国中药杂志, 2019, 44(4): 758-764. |
| SUN J, LUO J W, YAO W J, et al. Effect of emodin on gun microbiota of rats with acute kidney failure[J]. China Journal of Chinese Materia Medica, 2019, 44(4): 758-764. | |
| 26 | 郑彦懿, 周联,罗霞. 大黄牡丹汤及其主要活性成分对三种肠道细菌的影响[C]. 北京: 第十届全国免疫学学术大会, 2015. |
| ZHENG Y Y, ZHOU L, LUO X. Effects of Rhubarb Peony Decoction and its main active components on three intestinal bacteria[C]. Beijing: Tenth National Conference on Immunology, 2015. | |
| 27 | 皮宇, 高侃, 朱伟云. 动物宿主—肠道微生物代谢轴研究进展[J]. 微生物学报, 2017, 57(2): 161-169. |
| PI Y, GAO K, ZHU W Y. Advances in host-microbe metabolic axis[J]. Acta Microbiologica Sinica, 2017, 57(2): 161-169. | |
| 28 | 司惠丽. 大黄对实热证大鼠能量代谢影响的研究[D]. 济南: 山东中医药大学, . |
| SI H L. Study on the influence of Rhubarb on excessive heat syndrome rats model[D]. Jinan: Shandong Traditional Chinese Medicine University, 2012. | |
| 29 | CHERRINGTON C A, HINTON M, MEAD G C, et al. Organic acids: chemistry, antibacterial activity and practical applications[J]. Adv Microb Physiol, 1991, 32: 87-108. |
| 30 | 黄慧, 熊雁, 唐艺加, 等. 大黄素对心肌梗死后心力衰竭大鼠心肌保护机制研究[J]. 中国临床解剖学杂志, 2023, 41(1): 64-71. |
| HUANG H, XIONG Y, TANG Y J, et al. The protective effects of emodin on myocardial energy metabolism in rats with heart failure after myocardial in farction[J]. Chinese Journal of Clinical Anatomy, 2023,41(1): 64-71. |
| [1] | 杨乐, 周怡, 王钶韵, 赖娅莉. 大黄素改善阿尔茨海默病认知障碍、内质网应激和神经炎症的研究[J]. 上海交通大学学报(医学版), 2025, 45(6): 727-734. |
| [2] | 陈深册, 陈依明, 王凡, 张梦珂, 杨惟杰, 吕洞宾, 洪武. 饮食干预治疗抑郁相关症状的研究进展[J]. 上海交通大学学报(医学版), 2024, 44(8): 1050-1055. |
| [3] | 夏西茜, 丁珂珂, 张慧恒, 彭旭飞, 孙昳旻, 唐雅珺, 汤晓芳. 肠道菌群介导胆汁酸影响炎症性肠病的研究进展[J]. 上海交通大学学报(医学版), 2024, 44(7): 839-846. |
| [4] | 杜亚格, 卢言慧, 安宇, 宋颖, 郑婕. 肠道菌群在糖尿病认知功能障碍中的作用机制及靶向干预的研究进展[J]. 上海交通大学学报(医学版), 2024, 44(4): 494-500. |
| [5] | 马锦倩, 范翩翩, 郑涛, 张琳, 陈远志, 申剑, 欧阳凤秀. 孕妇肠道、阴道菌群和新生儿胎粪、胎皮脂菌群的相关性研究[J]. 上海交通大学学报(医学版), 2024, 44(1): 50-63. |
| [6] | 李郡如, 欧阳彦, 谢静远. 肠道菌群在IgA肾病发病与治疗中的作用研究进展[J]. 上海交通大学学报(医学版), 2023, 43(8): 1044-1048. |
| [7] | 温亚锦, 何雯, 韩晓, 张晓波. 不同严重程度支气管哮喘儿童肠道菌群差异的探索性分析[J]. 上海交通大学学报(医学版), 2023, 43(6): 655-664. |
| [8] | 王洁仪, 郑丹, 郑晓皎, 贾伟, 赵爱华. 茶褐素生物学活性及其作用机制的研究进展[J]. 上海交通大学学报(医学版), 2023, 43(6): 768-774. |
| [9] | 刘芊若, 方子晨, 吴宇涵, 钟羡欣, 国沐禾, 贾洁. 肠道菌群及其代谢产物与妊娠期糖尿病相关性的研究进展[J]. 上海交通大学学报(医学版), 2023, 43(5): 641-647. |
| [10] | 王婕, 吴慧, 卢凌鹏, 杨科峰, 祝捷, 周恒益, 姚蝶, 高雅, 冯宇婷, 刘玉红, 贾洁. 妊娠期糖尿病女性肠道菌群的变化特征及其与血糖、血脂和膳食的相关性[J]. 上海交通大学学报(医学版), 2022, 42(9): 1336-1346. |
| [11] | 卢雨, 王昊, 巴乾. 肠道菌群在肝癌发生发展及治疗中的作用研究进展[J]. 上海交通大学学报(医学版), 2022, 42(7): 939-944. |
| [12] | 蒋怡, 江平, 张明明, 房静远. 嗜黏蛋白阿克曼菌在肠道相关疾病中作用的研究进展[J]. 上海交通大学学报(医学版), 2022, 42(10): 1490-1497. |
| [13] | 袁瑞雪, 傅迎美, 禹顺英. 辅助性T细胞17和调节性T细胞在抑郁症中的作用机制研究进展[J]. 上海交通大学学报(医学版), 2021, 41(10): 1384-1388. |
| [14] | 周 铖1,孙鹏飞2,尹继瑶3,王阳阳1,肖海娟4, 5. 粪菌移植治疗炎症性肠病的研究进展[J]. 上海交通大学学报(医学版), 2020, 40(2): 267-. |
| [15] | 储维薇,徐洁颖,李尚,翟君钰,杜艳芝. 脱氢表雄酮诱导的多囊卵巢综合征模型大鼠的肠道菌群研究[J]. 上海交通大学学报(医学版), 2019, 39(9): 975-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||