
上海交通大学学报(医学版) ›› 2024, Vol. 44 ›› Issue (11): 1335-1346.doi: 10.3969/j.issn.1674-8115.2024.11.001
• 创新团队成果专栏 • 下一篇
收稿日期:2024-02-21
接受日期:2024-04-23
出版日期:2024-11-28
发布日期:2024-11-28
通讯作者:
郑 超,电子信箱:zhengchao@shsmu.edu.cn。作者简介:罗蓝鸽(1999—),女,硕士生;电子信箱:l.lange@sjtu.edu.cn。
基金资助:
LUO Lange( ), ZHENG Chao(
), ZHENG Chao( ), LEI Ming(
), LEI Ming( )
)
Received:2024-02-21
Accepted:2024-04-23
Online:2024-11-28
Published:2024-11-28
Contact:
ZHENG Chao, E-mail: zhengchao@shsmu.edu.cn.Supported by:摘要:
目的·研究癌-睾丸抗原(cancer-testis antigen,CTA)家族成员CT57对人肝癌细胞增殖、侵袭、迁移和裸鼠皮下成瘤的影响及可能的作用机制。方法·通过生物信息学方法分析CT57在多种肿瘤组织和正常组织中的表达差异及其对肝癌患者预后的影响;用慢病毒载体分别构建稳定敲低和过表达CT57的肝癌细胞系模型,并借助Western blotting验证CT57蛋白水平的变化;通过CCK-8细胞增殖实验、软琼脂克隆形成实验和细胞周期实验,检测CT57对肝癌细胞增殖与集落形成能力的影响;通过流式细胞术检测CT57对细胞周期的影响;通过划痕实验和Transwell实验检测CT57对肝癌细胞侵袭和迁移能力的影响;通过实时荧光定量PCR(quantitative real-time PCR,qRT-PCR)检测上皮间质转化(epithelial-mesenchymal transition,EMT)标志物的表达变化;通过在裸鼠皮下注射敲低CT57的肝癌细胞株(实验组)和对照细胞株(对照组)构建皮下成瘤模型,探究CT57在体内环境下的生物学功能。结果·对肿瘤基因组图谱计划(The Cancer Genome Atlas Program,TCGA)数据库中的多种肿瘤和相对应正常组织的生物信息学分析表明,CT57在包括肝癌在内的大部分肿瘤组织中高表达,并且其表达水平与肝癌患者预后相关。细胞增殖实验、软琼脂克隆形成实验和细胞周期实验证明,敲低CT57可抑制肝癌细胞增殖和集落形成,并导致细胞周期阻滞;划痕实验和Transwell实验证明,敲低CT57可抑制肝癌细胞侵袭和迁移,而过表达CT57则促进这些过程;qRT-PCR结果表明,过表达CT57可导致上皮细胞标志物E-钙黏蛋白(E-cadherin,ECAD)和闭合蛋白(occludin,OCLN)表达下调,间质细胞标志物波形蛋白(vimentin,VIM)、Twist相关蛋白1(twist family bHLH transcription factor 1,TWIST1)和基质金属肽酶2(matrix metallopeptidase 2,MMP2)表达上调;体内实验表明,敲低CT57可降低肝癌细胞在裸鼠中的成瘤率及肿瘤的体积和质量。结论·敲低肝癌细胞CT57导致细胞周期阻滞,从而抑制肝癌细胞增殖和裸鼠皮下成瘤;CT57可以促进肝癌细胞上皮间质转化,增强其侵袭和迁移能力。
中图分类号:
罗蓝鸽, 郑超, 雷鸣. 癌-睾丸抗原CT57促进肝癌细胞增殖、侵袭、迁移和上皮间质转化[J]. 上海交通大学学报(医学版), 2024, 44(11): 1335-1346.
LUO Lange, ZHENG Chao, LEI Ming. Promotive effect of cancer-testis antigen CT57 on proliferation, invasion, migration and epithelial-mesenchymal transition of liver cancer cells[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2024, 44(11): 1335-1346.
| Primer | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| GAPDH | AATGGGCAGCCGTTAGGAAA | GCCCAATACGACCAAATCAGAG |
| CT57 | GAACATCGTGAACTACCTACCG | CAAGGGTGTCTCCGTGATGAT |
| ECAD | TCTTCAATCCCACCACGTACA | CTGGGGTATTGGGGGCATC |
| VIM | GACGCCATCAACACCGAGTT | CTTTGTCGTTGGTTAGCTGGT |
| OCLN | GGCACCTGCATACTCACCC | CTGGGAGAGCAACTCATCCTC |
| TWIST1 | GTCCGCAGTCTTACGAGGAG | GCTTGAGGGTCTGAATCTTGCT |
| MMP2 | TACAGGATCATTGGCTACACACC | GGTCACATCGCTCCAGACT |
| FOXM1 | CGTCGGCCACTGATTCTCAAA | GGCAGGGGATCTCTTAGGTTC |
| SNA | TCGGAAGCCTAACTACAGCGA | AGATGAGCATTGGCAGCGAG |
表1 qRT-PCR引物序列
Tab 1 Primer sequences for qRT-PCR
| Primer | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| GAPDH | AATGGGCAGCCGTTAGGAAA | GCCCAATACGACCAAATCAGAG |
| CT57 | GAACATCGTGAACTACCTACCG | CAAGGGTGTCTCCGTGATGAT |
| ECAD | TCTTCAATCCCACCACGTACA | CTGGGGTATTGGGGGCATC |
| VIM | GACGCCATCAACACCGAGTT | CTTTGTCGTTGGTTAGCTGGT |
| OCLN | GGCACCTGCATACTCACCC | CTGGGAGAGCAACTCATCCTC |
| TWIST1 | GTCCGCAGTCTTACGAGGAG | GCTTGAGGGTCTGAATCTTGCT |
| MMP2 | TACAGGATCATTGGCTACACACC | GGTCACATCGCTCCAGACT |
| FOXM1 | CGTCGGCCACTGATTCTCAAA | GGCAGGGGATCTCTTAGGTTC |
| SNA | TCGGAAGCCTAACTACAGCGA | AGATGAGCATTGGCAGCGAG |
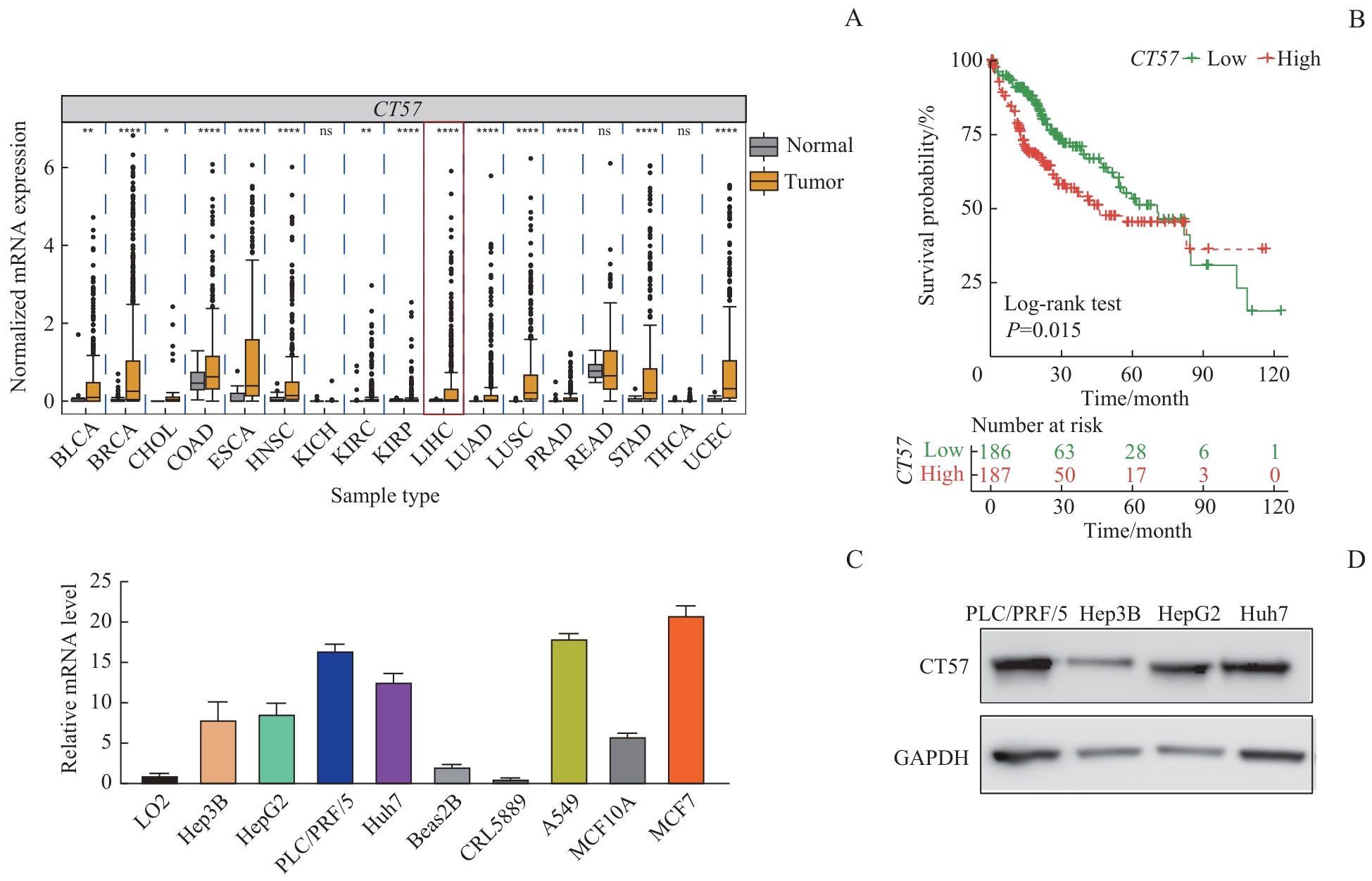
图1 CT57 在肝癌组织及细胞株中的表达情况以及与患者预后的关系Note: A. CT57 expression levels of different tumor types in the TCGA database. BLCA—bladder urothelial carcinoma; BRCA—breast invasive carcinoma; CHOL—cholangiocarcinoma; COAD—colon adenocarcinoma; ESCA—esophageal carcinoma; HNSC—head and neck squamous cell carcinoma; KICH—kidney chromophobe; KIRC—kidney renal clear cell carcinoma; KIRP—kidney renal papillary cell carcinoma; LIHC—liver hepatocellular carcinoma; LUAD—lung adenocarcinoma; LUSC—lung squamous cell carcinoma; PRAD—prostate adenocarcinoma; READ—rectum adenocarcinoma; STAD—stomach adenocarcinoma; THCA—thyroid carcinoma; UCEC—uterine corpus endometrial carcinoma. The expression of CT57 in the liver cancer is marked by the red box. B. Kaplan-Meier survival curves comparison between the liver cancer patients with high expression and low expression of CT57 in livers. C. The mRNA expression levels of CT57 in cell lines. D. Western blotting analysis of CT57 expression level in four human liver cancer cells.
Fig 1 Expression of CT57 in liver cancer tissues and cell lines and its relationship with patients′ prognosis
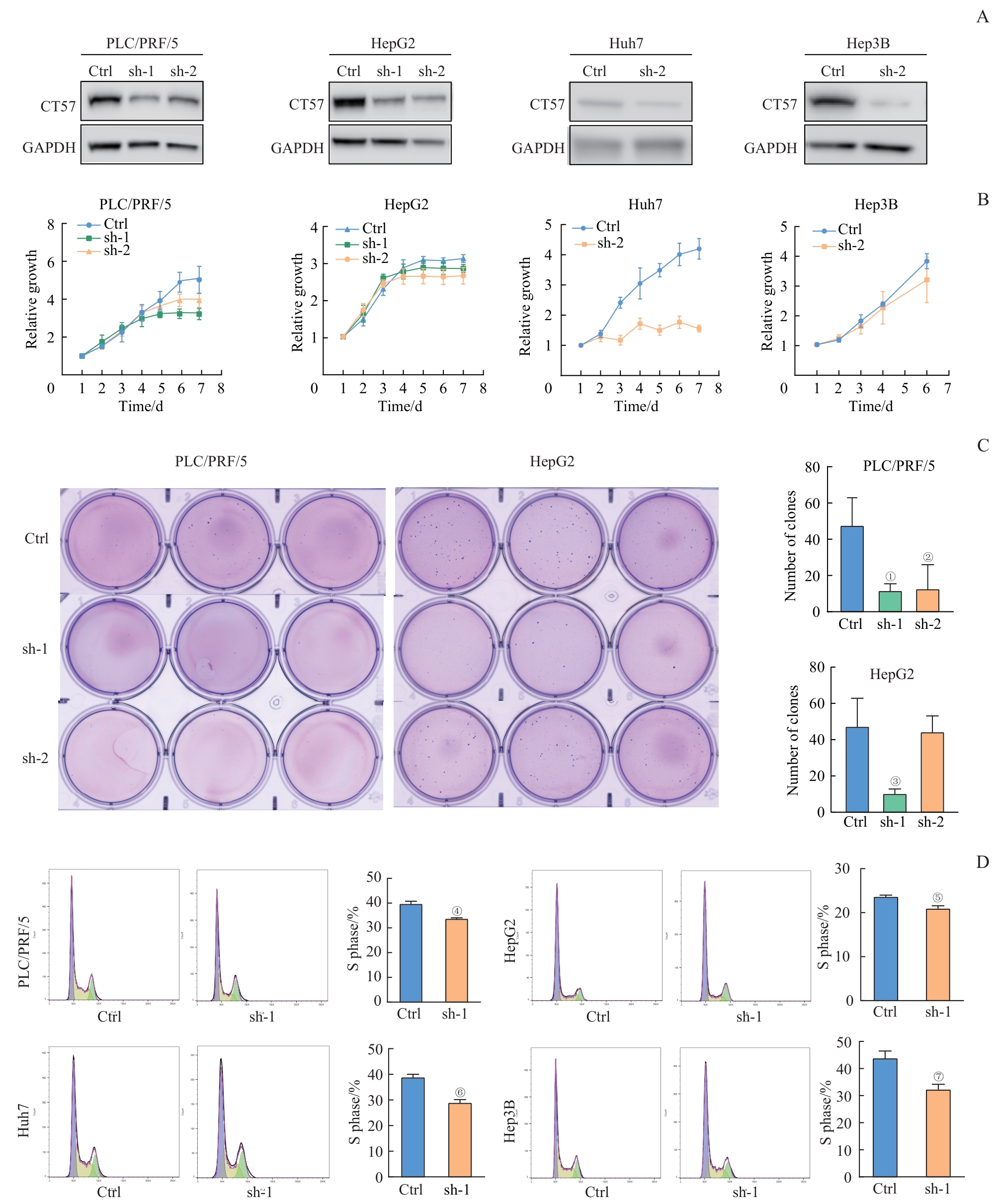
图2 敲低 CT57 对肝癌细胞增殖能力及细胞周期的影响Note: A. CT57 knockdown (sh-1 and sh-2) efficiency detected by Western blotting analysis. B. CCK?8 proliferation assay. C. Soft agar colony formation assay. D. Flow cytometry for analyzing cell cycle. ①P=0.016, ②P=0.039, ③P=0.015, ④P=0.014, ⑤P=0.026, ⑥P=0.012, ⑦P=0.003, compared with the corresponding control (Ctrl) group.
Fig 2 Effect of CT57 knockdown on proliferation and cell cycle in liver cancer cells
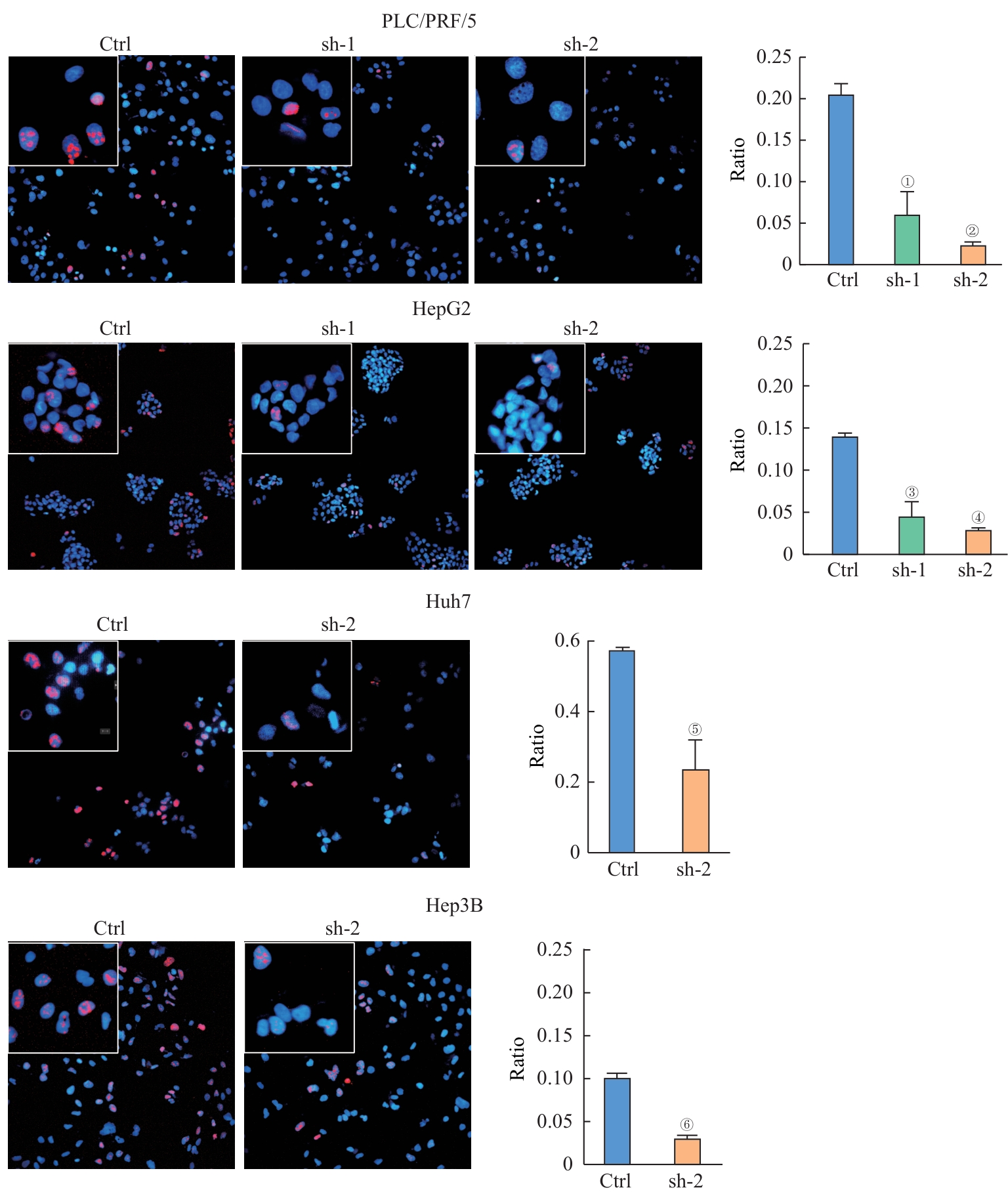
图3 敲低 CT57 后肝癌细胞Ki67阳性细胞比例的变化Note:Ki67 immunostaining of indicated cells (×200 in big boxes; ×800 in small boxes). ①P=0.020, ②P=0.002, ③P=0.015, ④P=0.000, ⑤P=0.027, ⑥P=0.003, compared with the corresponding control (Ctrl) group.
Fig 3 Changes in the proportion of Ki67-positive cells in liver cancer cells after knocking down CT57
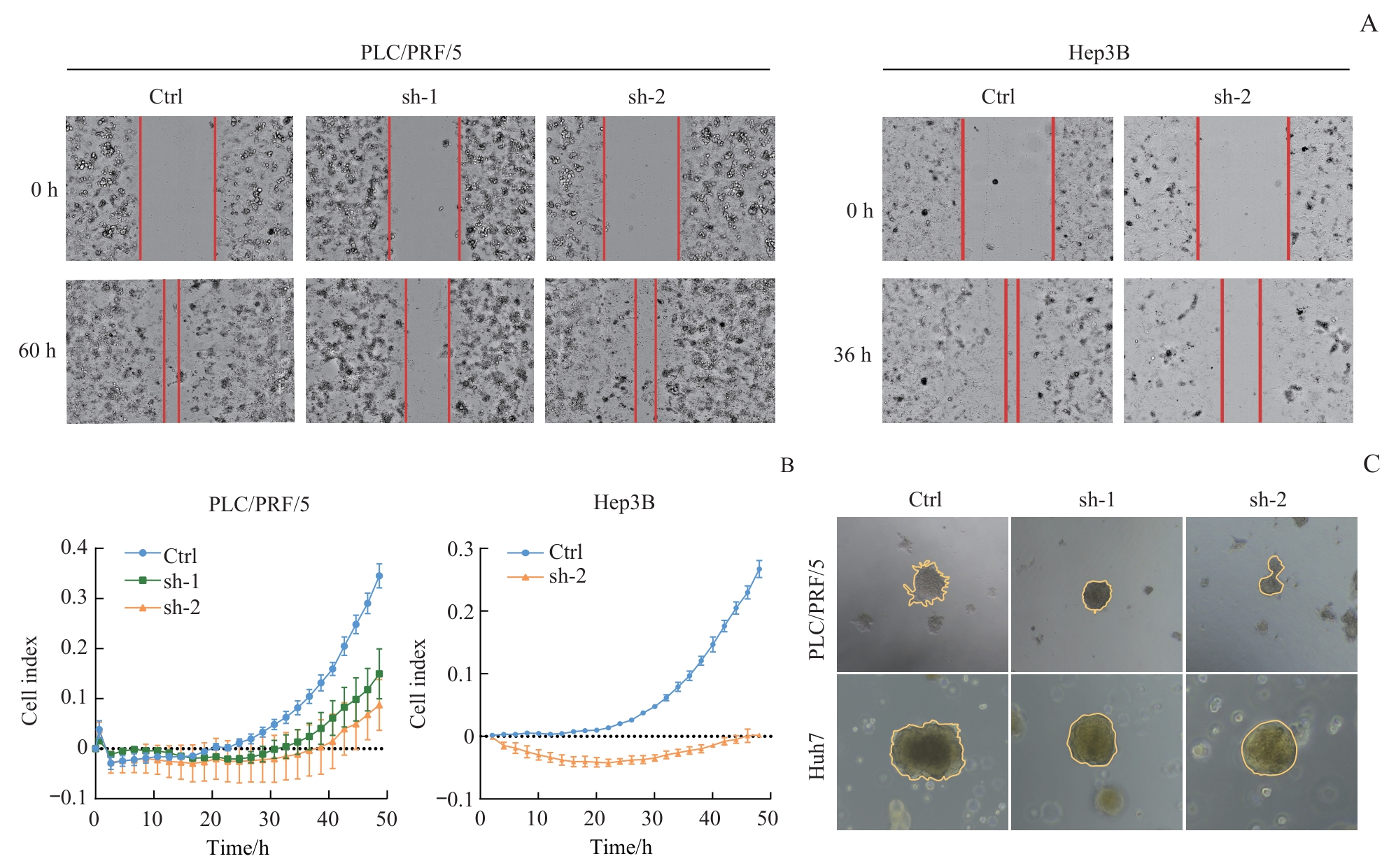
图4 敲低 CT57 对肝癌细胞侵袭和迁移能力的影响Note: A. Wound healing assay (×200) of indicated cells. B. RTCA coupled Transwell migration/invasion assay of indicated cells. C. 3D culture of indicated cells (×200).
Fig 4 Effect of CT57 knockdown on invasion and migration ability of liver cancer cells
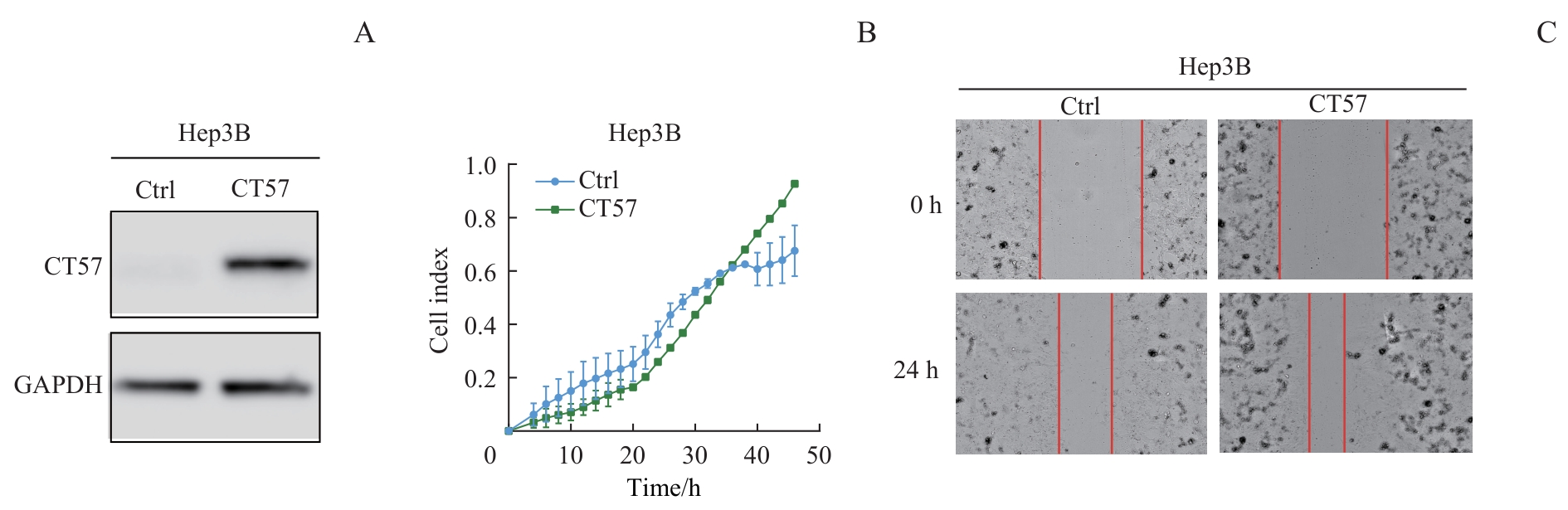
图5 CT57过表达对肝癌细胞侵袭和迁移能力的影响Note: A. Western blotting analysis of CT57 overexpression in Hep3B cells. B. RTCA coupled Transwell migration/invasion assay of Hep3B cells. C. Wound healing assay (×200) of Hep3B cells.
Fig 5 Effect of CT57 overexpression on invasion and migration ability of liver cancer cells
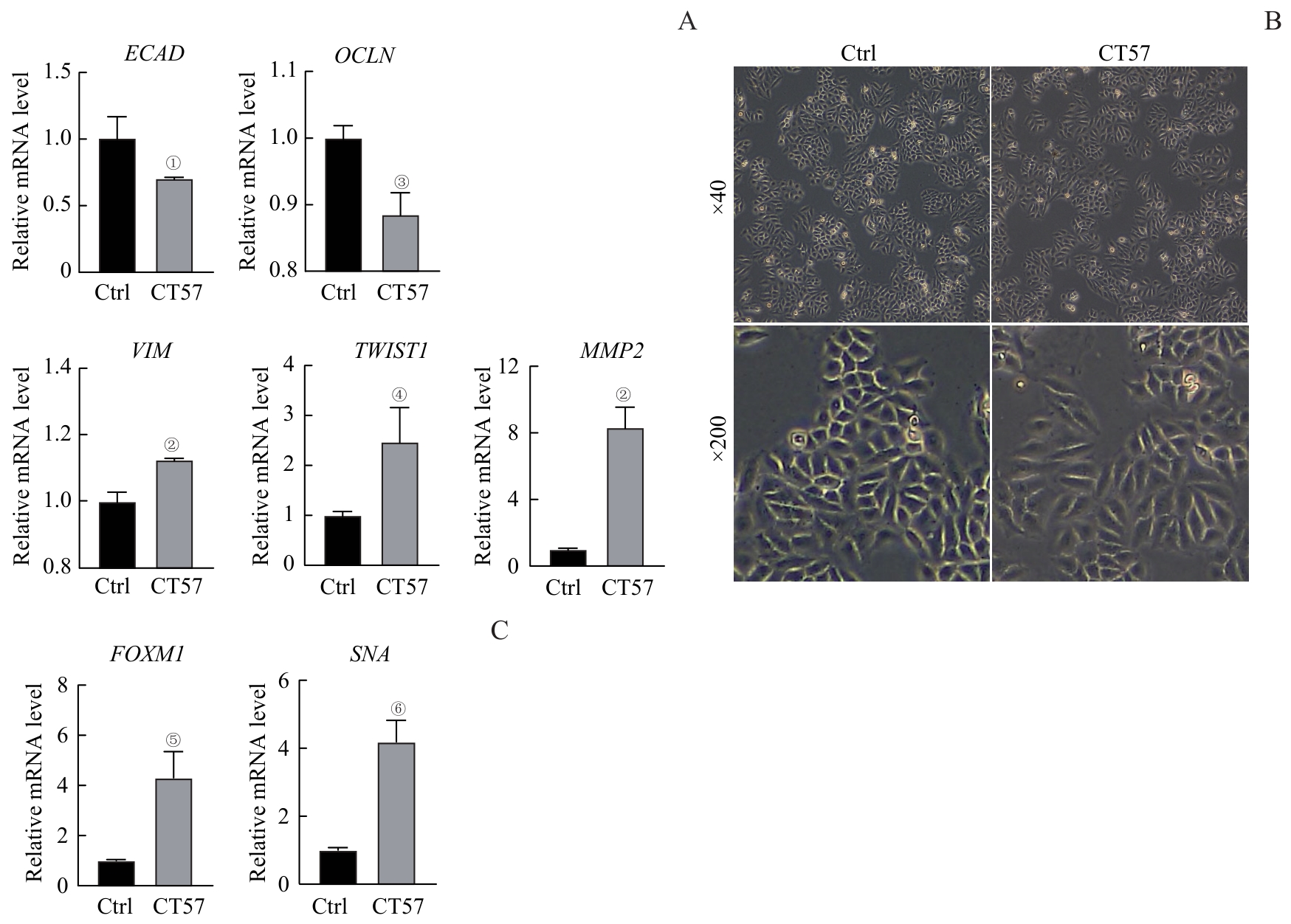
图6 CT57过表达对肝癌细胞EMT的影响Note: A. The mRNA expression of EMT markers after CT57 overexpression in Hep3B cells detected by qRT-PCR. B. The cell morphology after overexpression of CT57 in Hep3B cells. C. FOXM1 and SNA expression after CT57 overexpression in Hep3B cells detected by qRT-PCR. ①P=0.031, ②P=0.002, ③P=0.006, ④P=0.021, ⑤P=0.035, ⑥P=0.012, compared with the corresponding control (Ctrl) group.
Fig 6 Effect of CT57 overexpression on EMT in liver cancer cells
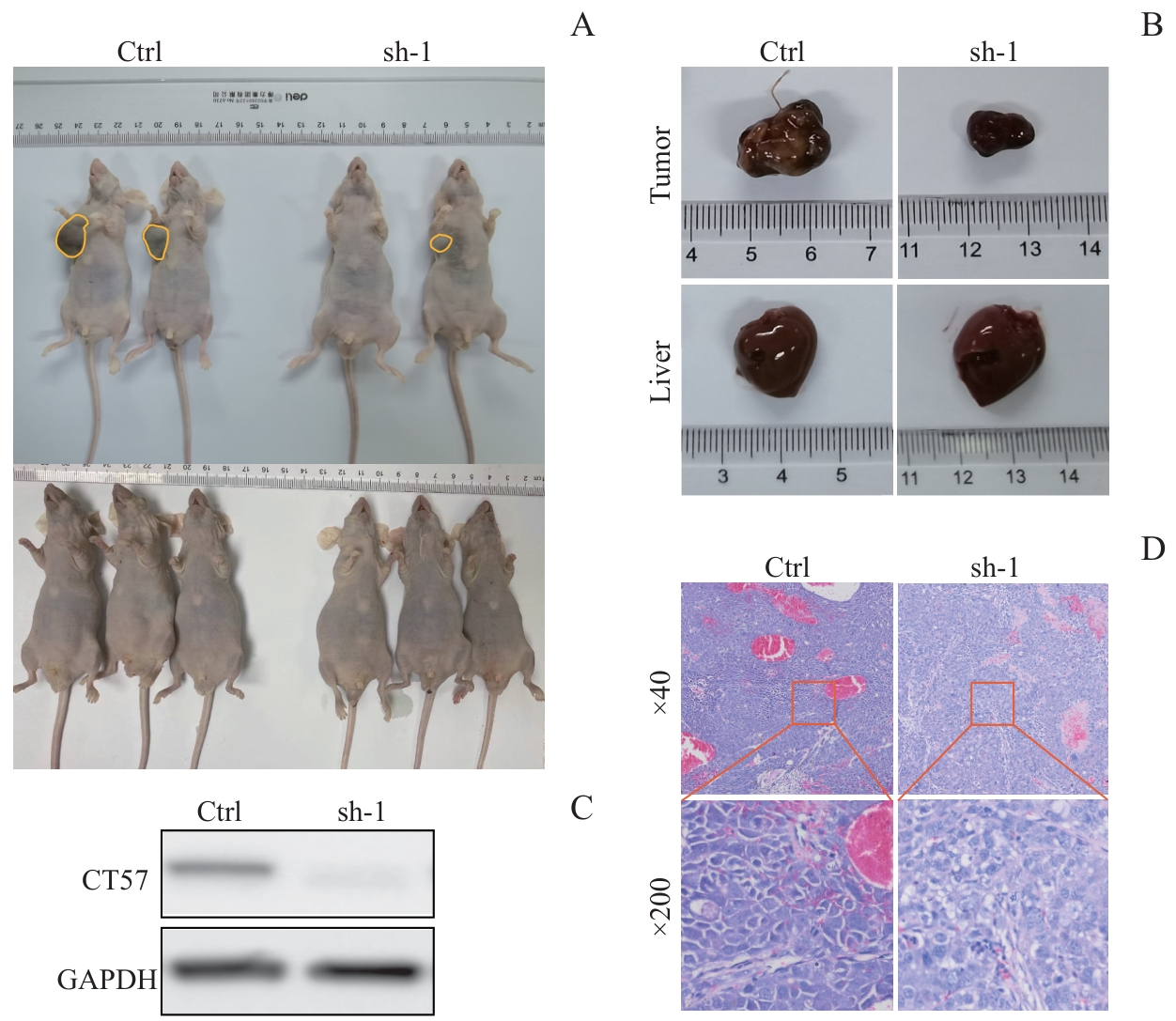
图7 敲低 CT57 对肝癌细胞在裸鼠皮下成瘤的影响Note: A. The tumorigenic ability of Hep3B cells after knockdown of CT57 in nude mice. B. Tumor and liver size in nude mice. C. Western blotting analysis of CT57 knockdown efficiency in the tumors. D. H-E staining of tumor tissues.
Fig 7 Effect of CT57 knockdown on subcutaneous tumor formation of liver cancer cells in nude mice
| 1 | LLOVET J M, KELLEY R K, VILLANUEVA A, et al. Hepatocellular carcinoma[J]. Nat Rev Dis Primers, 2021, 7(1): 6. |
| 2 | TANG A, HALLOUCH O, CHERNYAK V, et al. Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis[J]. Abdom Radiol, 2018, 43(1): 13-25. |
| 3 | CHENG A L, KANG Y K, CHEN Z D, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase Ⅲ randomised, double-blind, placebo-controlled trial[J]. Lancet Oncol, 2009, 10(1): 25-34. |
| 4 | KIRKIN A F, DZHANDZHUGAZYAN K N, ZEUTHEN J. Cancer/testis antigens: structural and immunobiological properties[J]. Cancer Invest, 2002, 20(2): 222-236. |
| 5 | ALMEIDA L G, SAKABE N J, DEOLIVEIRA A R, et al. CTdatabase: a knowledge-base of high-throughput and curated data on cancer-testis antigens[J]. Nucleic Acids Res, 2009, 37(Database issue): D816-D819. |
| 6 | SIMPSON A J, CABALLERO O L, JUNGBLUTH A, et al. Cancer/testis antigens, gametogenesis and cancer[J]. Nat Rev Cancer, 2005, 5(8): 615-625. |
| 7 | CHENG W, LI H L, XI S Y, et al. Growth differentiation factor 1-induced tumour plasticity provides a therapeutic window for immunotherapy in hepatocellular carcinoma[J]. Nat Commun, 2021, 12(1): 7142. |
| 8 | KIM M C, BORCHERDING N, AHMED K K, et al. CD177 modulates the function and homeostasis of tumor-infiltrating regulatory T cells[J]. Nat Commun, 2021, 12(1): 5764. |
| 9 | DONG X Y, YANG X A, WANG Y D, et al. Zinc-finger protein ZNF165 is a novel cancer-testis antigen capable of eliciting antibody response in hepatocellular carcinoma patients[J]. Br J Cancer, 2004, 91(8): 1566-1570. |
| 10 | CHEN Y T, SCANLAN M J, VENDITTI C A, et al. Identification of cancer/testis-antigen genes by massively parallel signature sequencing[J]. Proc Natl Acad Sci U S A, 2005, 102(22): 7940-7945. |
| 11 | FAN S X, YAN S, YANG Y, et al. Actin-like protein 8 promotes the progression of triple-negative breast cancer via activating PI3K/AKT/mTOR pathway[J]. Onco Targets Ther, 2021, 14: 2463-2473. |
| 12 | WANG L F, XING X L, TIAN H, et al. Actin-like protein 8, a member of cancer/testis antigens, supports the aggressive development of oral squamous cell carcinoma cells via activating cell cycle signaling[J]. Tissue Cell, 2022, 75: 101708. |
| 13 | MA S W, WANG X W, ZHANG Z R, et al. Actin-like protein 8 promotes cell proliferation, colony-formation, proangiogenesis, migration and invasion in lung adenocarcinoma cells[J]. Thorac Cancer, 2020, 11(3): 526-536. |
| 14 | YANG P, QIAO Y N, MENG M, et al. Cancer/testis antigens as biomarker and target for the diagnosis, prognosis, and therapy of lung cancer[J]. Front Oncol, 2022, 12: 864159. |
| 15 | FREITAS M, MALHEIROS S, STÁVALE J N, et al. Expression of cancer/testis antigens is correlated with improved survival in glioblastoma[J]. Oncotarget, 2013, 4(4): 636-646. |
| 16 | LI B, ZHU J, MENG L. High expression of ACTL8 is poor prognosis and accelerates cell progression in head and neck squamous cell carcinoma[J]. Mol Med Rep, 2019, 19(2): 877-884. |
| 17 | MA S W, QIANG G L, SHAO W P, et al. Knockdown of actin-like 8 inhibits cell proliferation by regulating FOXM1, STMN1, PLK1, and BIRC5 in lung adenocarcinoma A549 cells[J]. Transl Cancer Res, 2019, 8(5): 1975-1984. |
| 18 | HAN Q, SUN M L, LIU W S, et al. Upregulated expression of ACTL8 contributes to invasion and metastasis and indicates poor prognosis in colorectal cancer[J]. Onco Targets Ther, 2019, 12: 1749-1763. |
| 19 | HUBER B E, THORGEIRSSON S S. Analysis of c-myc expression in a human hepatoma cell line[J]. Cancer Res, 1987, 47(13): 3414-3420. |
| 20 | CHEN Y P, CHAN A T C, LE Q T, et al. Nasopharyngeal carcinoma[J]. Lancet, 2019, 394(10192): 64-80. |
| 21 | HANAHAN D. Hallmarks of cancer: new dimensions[J]. Cancer Discov, 2022, 12(1): 31-46. |
| 22 | SERRANO-GOMEZ S J, MAZIVEYI M, ALAHARI S K. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications[J]. Mol Cancer, 2016, 15: 18. |
| 23 | SMITH B N, BHOWMICK N A. Role of EMT in metastasis and therapy resistance[J]. J Clin Med, 2016, 5(2): 17. |
| 24 | YE H, KELLY T F, SAMADANI U, et al. Hepatocyte nuclear factor 3/fork head homolog 11 is expressed in proliferating epithelial and mesenchymal cells of embryonic and adult tissues[J]. Mol Cell Biol, 1997, 17(3): 1626-1641. |
| 25 | KORVER W, ROOSE J, HEINEN K, et al. The human TRIDENT/HFH-11/FKHL16 gene: structure, localization, and promoter characterization[J]. Genomics, 1997, 46(3): 435-442. |
| 26 | MENG F D, WEI J C, QU K, et al. FoxM1 overexpression promotes epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma[J]. World J Gastroenterol, 2015, 21(1): 196-213. |
| 27 | YU M, TANG Z, MENG F D, et al. Elevated expression of FoxM1 promotes the tumor cell proliferation in hepatocellular carcinoma[J]. Tumour Biol, 2016, 37(1): 1289-1297. |
| 28 | BALLI D, USTIYAN V, ZHANG Y F, et al. Foxm1 transcription factor is required for lung fibrosis and epithelial-to-mesenchymal transition[J]. EMBO J, 2013, 32(2): 231-244. |
| 29 | WANG C R, WANG X J, SU Z J, et al. MiR-25 promotes hepatocellular carcinoma cell growth, migration and invasion by inhibiting RhoGDI1[J]. Oncotarget, 2015, 6(34): 36231-36244. |
| 30 | ZHANG N, WEI P, GONG A H, et al. FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis[J]. Cancer Cell, 2011, 20(4): 427-442. |
| [1] | 朱子俊, 钱逸斐, 李倩玉, 李松玲, 覃雯莉, 刘艳丰. 后期促进复合体亚基10调控PI3K-AKT-mTOR通路促进肝细胞癌进展的研究[J]. 上海交通大学学报(医学版), 2025, 45(9): 1171-1182. |
| [2] | 杨娜, 刘俊丽, 白静, 杨思怡, 韩继明, 张华华. HENMT1通过激活PI3K-AKT-mTOR信号通路促进胃癌的增殖与迁移[J]. 上海交通大学学报(医学版), 2025, 45(6): 717-726. |
| [3] | 许沐馨, 刘贤, 蒋立姗, 孙青. Nd:YAP激光生物刺激通过WNT/β-catenin信号通路促进人牙周韧带细胞增殖和成骨分化[J]. 上海交通大学学报(医学版), 2025, 45(5): 562-569. |
| [4] | 陈怡楠, 郑旸, 曾汉林, 雷鸣. Fas相关死亡结构域蛋白促进头颈部鳞状细胞癌细胞增殖能力的机制研究[J]. 上海交通大学学报(医学版), 2025, 45(4): 404-414. |
| [5] | 张先洲, 杜凤麟, 吴雷, 任逸喆, 赵明娜, 娄加陶. OGT通过ERK信号通路促进非小细胞肺癌增殖的机制研究[J]. 上海交通大学学报(医学版), 2025, 45(10): 1288-1297. |
| [6] | 李想, 魏鸣, 吴文曦, 罗小琴, 姚彪, 伍思宇. 芦丁对骨肉瘤生长和转移的体内外抑制作用[J]. 上海交通大学学报(医学版), 2025, 45(1): 20-28. |
| [7] | 孙晨玮, 海汪溪, 屈骞, 席云. [18F]F-FMISO和[18F]F-FLT PET/CT双核素显像预测胰腺癌耐药性的体内研究[J]. 上海交通大学学报(医学版), 2025, 45(1): 60-68. |
| [8] | 杨涵, 雷浩, 徐彼得, 吴淏, 马寻君, 皇艳波, 毛远青, 张经纬, 王金武. 透视立体影像分析技术在假体无菌性松动检测中的应用研究[J]. 上海交通大学学报(医学版), 2024, 44(9): 1061-1068. |
| [9] | 施灵玲, 程燕咏, 张磊. 七氟烷对原代少突胶质细胞增殖和分化的影响[J]. 上海交通大学学报(医学版), 2024, 44(9): 1115-1123. |
| [10] | 钱立恒, 温凯玲, 廖颖娜, 李书鑫, 聂惠贞. 分选链接蛋白1抑制结直肠癌细胞增殖和迁移的作用和机制研究[J]. 上海交通大学学报(医学版), 2024, 44(9): 1124-1135. |
| [11] | 韩依杉, 徐梓淇, 陶梦玉, 范广建, 余波. PRMT6促进乳腺癌细胞的增殖和迁移[J]. 上海交通大学学报(医学版), 2024, 44(8): 999-1010. |
| [12] | 薛煜, 张海龙, 雷鸣. 癌-睾丸抗原SPANXB在肝癌中的表达及其影响肝癌进展的机制研究[J]. 上海交通大学学报(医学版), 2024, 44(7): 801-813. |
| [13] | 杨舒雯, 陈娟, 杨琴, 雷鸣, 黄晨辉. 利用秀丽隐杆线虫模型研究CT14在发育中的功能[J]. 上海交通大学学报(医学版), 2024, 44(7): 871-882. |
| [14] | 谭露, 沈少明, 何平. 低氧诱导的长链非编码RNA 68在肝癌中的功能及其作用机制[J]. 上海交通大学学报(医学版), 2024, 44(6): 702-712. |
| [15] | 蔡人杰, 徐明. KHSRP通过ANK3调节前列腺癌细胞对雄激素的反应性[J]. 上海交通大学学报(医学版), 2024, 44(4): 417-426. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||