
上海交通大学学报(医学版) ›› 2024, Vol. 44 ›› Issue (9): 1115-1123.doi: 10.3969/j.issn.1674-8115.2024.09.006
收稿日期:2024-05-03
接受日期:2024-06-04
出版日期:2024-09-28
发布日期:2024-09-28
通讯作者:
张 磊,电子信箱:weiymzhl@126.com。作者简介:施灵玲(1997—),女,硕士;电子信箱:shill_07@126.com。
基金资助:
SHI Lingling( ), CHENG Yanyong(
), CHENG Yanyong( ), ZHANG Lei(
), ZHANG Lei( )
)
Received:2024-05-03
Accepted:2024-06-04
Online:2024-09-28
Published:2024-09-28
Contact:
ZHANG Lei, E-mail: weiymzhl@126.com.Supported by:摘要:
目的·探索多次七氟烷处理对原代少突胶质细胞增殖和分化的影响。方法·提取出生当日大鼠皮层的少突胶质前体细胞(oligodendrocyte precursor cell,OPC)并进行体外培养。细胞分为对照组和七氟烷处理组。为了模拟临床使用七氟烷的情况,将七氟烷组细胞使用3%七氟烷连续处理3 d,每日1次,每次处理2 h。OPC分化成熟为少突胶质细胞后,使用免疫荧光染色和蛋白质印迹法(Western blotting)检测髓鞘碱性蛋白(myelin basic protein,MBP)和髓鞘关联糖蛋白(myelin-associated glycoprotein,MAG)的表达情况。采用细胞增殖实验(BrdU、Ki67染色)、细胞存活率实验(CCK8)检测七氟烷对OPC增殖能力和少突胶质细胞存活率的影响。采用Western blotting检测半胱氨酸蛋白酶-3(caspase-3)的蛋白含量。使用慢病毒转染技术,在OPC内过表达YTH N6-甲基腺苷RNA结合蛋白1(YTH N6-methyladenosine RNA binding protein F1,YTHDF1),后采用CCK8检测细胞增殖和存活情况。结果·免疫荧光结果提示,反复暴露于七氟烷会导致表达成熟髓鞘表面标志物(MBP、MAG)的原代少突胶质细胞数量减少;Western blotting结果表明,多次七氟烷处理导致原代OPC中caspase-3表达上调;CCK8结果表明,随着七氟烷处理次数的增加,原代OPC的存活率下降;然而,BrdU、Ki67染色结果显示,原代OPC在七氟烷处理后增殖能力增强。此外,过表达YTHDF1可以部分改善多次七氟烷处理而导致的原代OPC存活率下降(均P<0.05)。结论·多次七氟烷处理损伤原代少突胶质细胞的成髓鞘能力和存活率,表现为部分原代OPC凋亡;同时七氟烷处理代偿性提高了存活的原代OPC的增殖能力。
中图分类号:
施灵玲, 程燕咏, 张磊. 七氟烷对原代少突胶质细胞增殖和分化的影响[J]. 上海交通大学学报(医学版), 2024, 44(9): 1115-1123.
SHI Lingling, CHENG Yanyong, ZHANG Lei. Effects of sevoflurane exposure on proliferation and differentiation of primary oligodendrocytes[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2024, 44(9): 1115-1123.
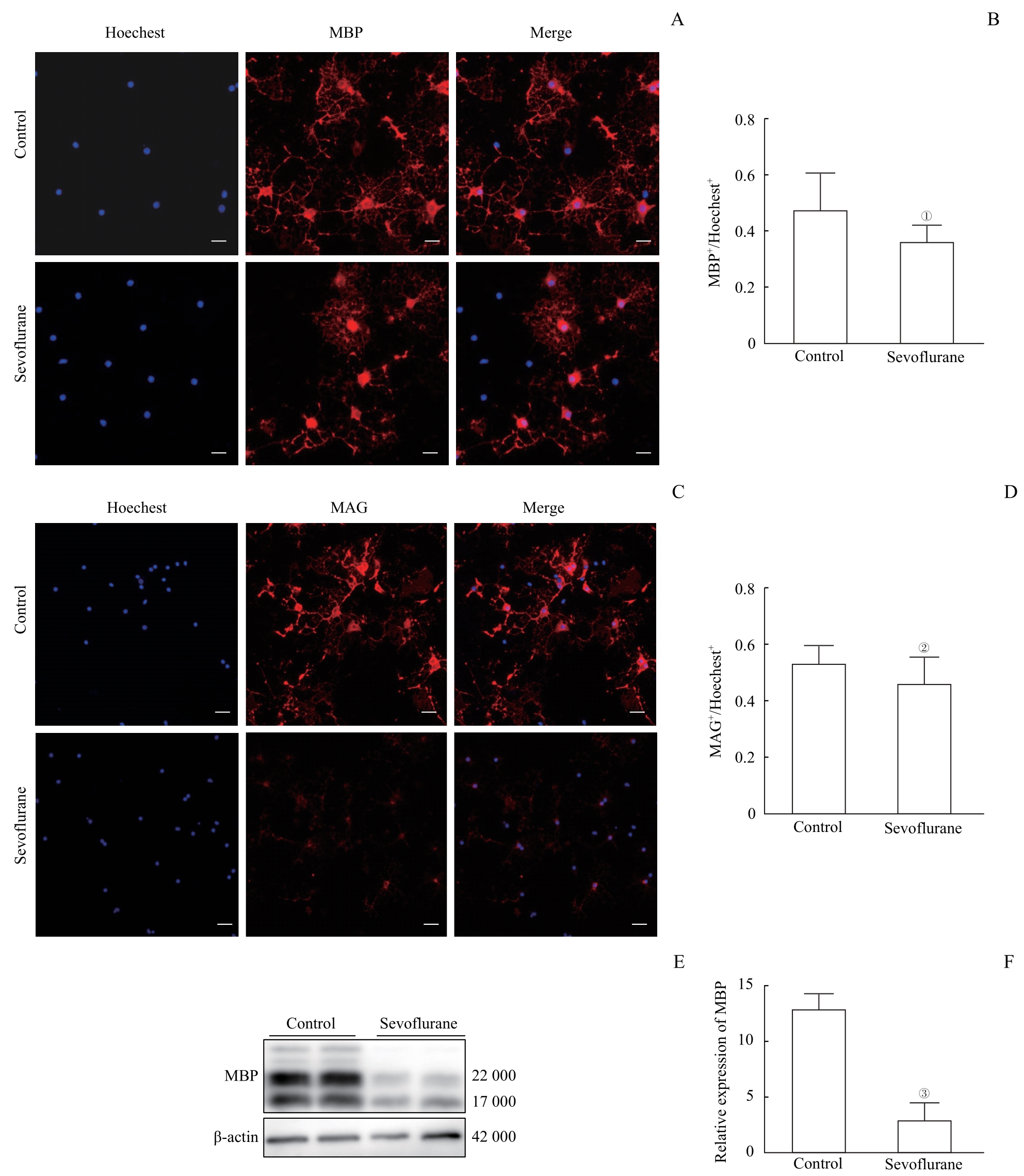
图1 多次七氟烷处理对少突胶质细胞成髓鞘能力的影响Note: A. Immunofluorescence staining showed the expression of MBP in oligodendrocytes. The scale bar is 100 μm. B. The number of Hoechest-positive cells normalized the number of MBP-positive cells. Multiple sevoflurane exposures significantly reduced the number of MBP-expressing cells. C. Immunofluorescence staining showed the expression of MAG in oligodendrocytes. The scale bar is 100 μm. D. The number of MAG-positive cells was normalized by the number of Hoechest-positive cells. Multiple sevoflurane exposures significantly reduced the number of MAG-expressing cells. E. Detection of MBP expression levels in oligodendrocytes by Western blotting. F. Western blotting results showed that multiple sevoflurane exposures significantly reduced the expression of MBP protein levels. ①P=0.030, ②P=0.026, ③P=0.001, compared with the control group.
Fig 1 Effects of multiple sevoflurane exposures on the myelinating ability of oligodendrocytes

图2 多次七氟烷处理通过诱导凋亡对OPC的杀伤作用Note: A/B. CCK8 results suggested that sevoflurane had a killing effect on OPCs. A. 1, 2, and 3 treatments with sevoflurane all had a killing effect on OPCs. As the number of sevoflurane exposures increased, the survival rate of OPCs decreased. B. The survival rate of OPCs decreased significantly on day 1, 2, and 3 after sevoflurane exposure, but there was no significant difference between each detection day. C. Detection of caspase-3 expression levels in OPCs by Western blotting. D. Western blotting showed that multiple sevoflurane exposures increased the expression of caspase-3 protein levels. ①P=0.000, compared with the group with 0 sevoflurane exposure; ②P=0.020, compared with the group with 1 sevoflurane exposure; ③P=0.011, ④P=0.030, ⑤P=0.046, compared with the group without sevoflurane exposure; ⑥P=0.012, compared with the control group.
Fig 2 Toxic effects of multiple sevoflurane exposure on OPCs by inducing apoptosis
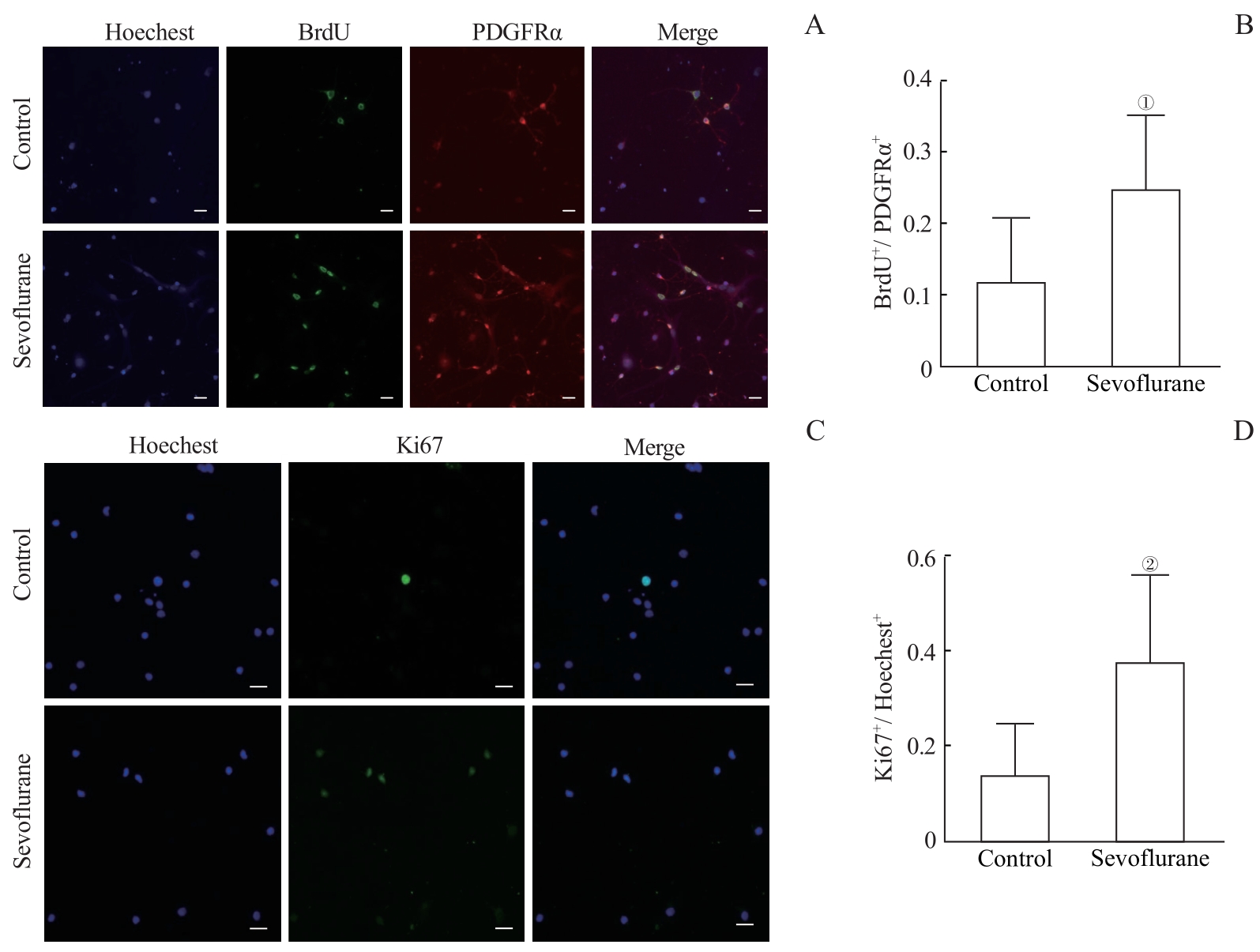
图3 多次七氟烷处理对OPC增殖能力的影响Note: A. Immunofluorescence staining showed the expression of BrdU in OPCs. The scale bar is 100 μm. B. The number of BrdU-positive cells was normalized to the number of PDGFRα positive cells. Multiple sevoflurane exposures increased the proliferation level of PDGFRα-positive OPCs. C. Immunofluorescence staining showed the expression of Ki67 in OPCs. The scale bar is 100 μm. D. The number of Ki67-positive cells was normalized to the number of Hoechest-positive cells. Multiple sevoflurane exposures increased the proliferation level of PDGFRα-positive OPCs. ①P=0.008, ②P=0.007, compared with the control group.
Fig 3 Effects of multiple sevoflurane exposure on the proliferation of OPCs
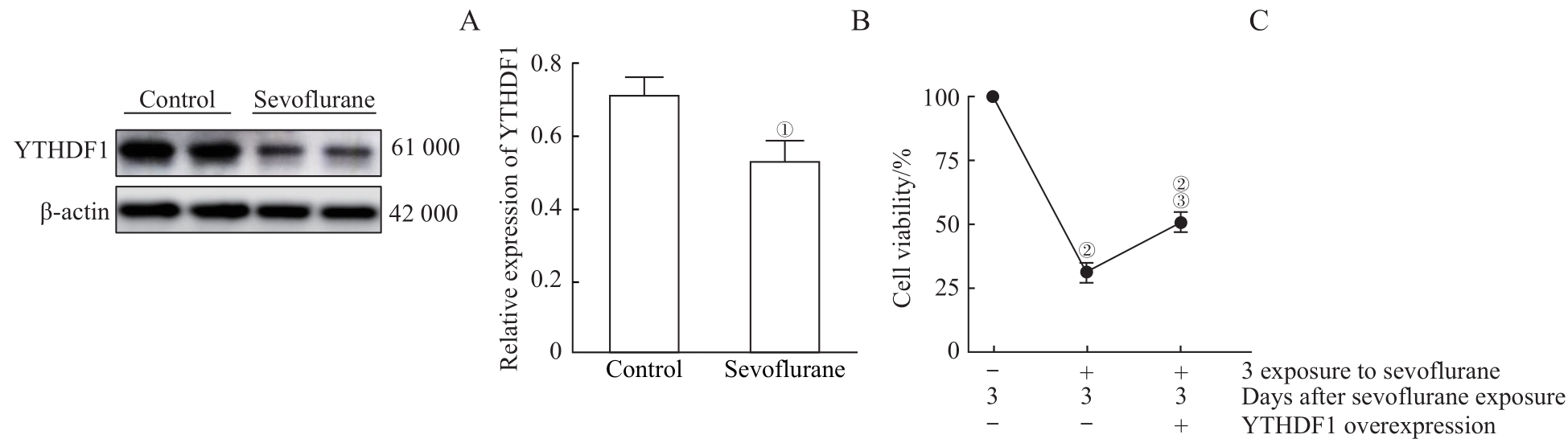
图4 过表达YTHDF1对多次七氟烷处理引起OPC损伤的修复作用Note: A. Detection of YTHDF1 expression levels in OPCs by Western blotting. B. Western blotting results showed that multiple sevoflurane exposures reduced the expression level of YTHDF1 protein. C. CCK8 experiments indicated that sevoflurane exposures killed OPCs and significantly reduced cell survival rate. However, cells overexpressing YTHDF1 had a partial protective effect on the killing effect of sevoflurane. ①P=0.011, compared with the control group; ②P=0.000, compared with the group without sevoflurane exposure and with control virus infection; ③P=0.007, compared with the group with 3 sevoflurane exposures and control virus infection.
Fig 4 Overexpression of YTHDF1 rescued the damage of OPCs caused by multiple sevoflurane exposures
| 1 | WARNER D O, ZACCARIELLO M J, KATUSIC S K, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the Mayo Anesthesia Safety in Kids (MASK) study[J]. Anesthesiology, 2018, 129(1): 89-105. |
| 2 | WALKDEN G J, GILL H, DAVIES N M, et al. Early childhood general anesthesia and neurodevelopmental outcomes in the Avon longitudinal study of parents and children birth cohort[J]. Anesthesiology, 2020, 133(5): 1007-1020. |
| 3 | RAPER J, DE BIASIO J C, MURPHY K L, et al. Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy[J]. Br J Anaesth, 2018, 120(4): 761-767. |
| 4 | ROSADO-MENDEZ I M, NOGUCHI K K, CASTAÑEDA-MARTINEZ L, et al. Quantitative ultrasound and apoptotic death in the neonatal primate brain[J]. Neurobiol Dis, 2019, 127: 554-562. |
| 5 | DAI C L, LI H C, HU X, et al. Neonatal exposure to anesthesia leads to cognitive deficits in old age: prevention with intranasal administration of insulin in mice[J]. Neurotox Res, 2020, 38(2): 299-311. |
| 6 | XIE L H, LIU Y, HU Y H, et al. Neonatal sevoflurane exposure induces impulsive behavioral deficit through disrupting excitatory neurons in the medial prefrontal cortex in mice[J]. Transl Psychiatry, 2020, 10(1): 202. |
| 7 | GUO S B, LIU L D, WANG C, et al. Repeated exposure to sevoflurane impairs the learning and memory of older male rats[J]. Life Sci, 2018, 192: 75-83. |
| 8 | JIANG J, LV X, WU X Y, et al. Downregulation of circulating insulin-like growth factor 1 contributes to memory impairment in aged mice after sevoflurane anesthesia[J]. Behav Pharmacol, 2017, 28(2 and 3-Spec Issue): 238-243. |
| 9 | SHEN Y S, ZHOU T, LIU X B, et al. Sevoflurane-induced miR-211-5p promotes neuronal apoptosis by inhibiting Efemp2[J]. ASN Neuro, 2021, 13: 17590914211035036. |
| 10 | TIAN Y, CHEN K Y, LIU L D, et al. Sevoflurane exacerbates cognitive impairment induced by Aβ1-40 in rats through initiating neurotoxicity, neuroinflammation, and neuronal apoptosis in rat hippocampus[J]. Mediators Inflamm, 2018, 2018: 3802324. |
| 11 | ZHENG S Q, AN L X, CHENG X, et al. Sevoflurane causes neuronal apoptosis and adaptability changes of neonatal rats[J]. Acta Anaesthesiol Scand, 2013, 57(9): 1167-1174. |
| 12 | ZHANG L, XUE Z Y, LIU Q D, et al. Disrupted folate metabolism with anesthesia leads to myelination deficits mediated by epigenetic regulation of ERMN[J]. EBioMedicine, 2019, 43: 473-486. |
| 13 | SHI L L, MIAO Z J, CHENG Y Y, et al. Folic acid ameliorated sevoflurane exposure-induced decrease in differentiation capacity of oligodendrocyte precursor cells[J]. Anesthesiol Perioper Sci, 2024, 2(2): 13. |
| 14 | LIU B, XIN W, TAN J R, et al. Myelin sheath structure and regeneration in peripheral nerve injury repair[J]. Proc Natl Acad Sci U S A, 2019, 116(44): 22347-22352. |
| 15 | READHEAD C, POPKO B, TAKAHASHI N, et al. Expression of a myelin basic protein gene in transgenic shiverer mice: correction of the dysmyelinating phenotype[J]. Cell, 1987, 48(4): 703-712. |
| 16 | WU Z Y, XUE H, GAO Q S, et al. Effects of early postnatal sevoflurane exposure on oligodendrocyte maturation and myelination in cerebral white matter of the rat[J]. Biomed Pharmacother, 2020, 131: 110733. |
| 17 | ZHAO D, ZHANG M L, YANG L L, et al. GPR68 improves nerve damage and myelination in an immature rat model induced by sevoflurane anesthesia by activating cAMP/CREB to mediate BDNF[J]. ACS Chem Neurosci, 2022, 13(3): 423-431. |
| 18 | LIANG L R, ZENG T, ZHAO Y Y, et al. Melatonin pretreatment alleviates the long-term synaptic toxicity and dysmyelination induced by neonatal sevoflurane exposure via MT1 receptor-mediated Wnt signaling modulation[J]. J Pineal Res, 2021, 71(4): e12771. |
| 19 | ZUO Y, LI B W, XIE J H, et al. Sevoflurane anesthesia during pregnancy in mice induces cognitive impairment in the offspring by causing iron deficiency and inhibiting myelinogenesis[J]. Neurochem Int, 2020, 135: 104693. |
| 20 | IKONOMIDOU C, KIRVASSILIS G, SWINEY B S, et al. Mild hypothermia ameliorates anesthesia toxicity in the neonatal macaque brain[J]. Neurobiol Dis, 2019, 130: 104489. |
| 21 | ZHANG Z H, LIU H Q, JIA S S, et al. Spatial and temporal alterations of developing oligodendrocytes induced by repeated sevoflurane exposure in neonatal mice[J]. Biochem Biophys Res Commun, 2023, 640: 12-20. |
| 22 | SONG S Y, PENG K, MENG X W, et al. Single-nucleus atlas of sevoflurane-induced hippocampal cell type- and sex-specific effects during development in mice[J]. Anesthesiology, 2023, 138(5): 477-495. |
| 23 | ZHANG L, CHENG Y Y, XUE Z Y, et al. Sevoflurane impairs m6A-mediated mRNA translation and leads to fine motor and cognitive deficits[J]. Cell Biol Toxicol, 2022, 38(2): 347-369. |
| 24 | ALMEIDA R G, LYONS D A. On myelinated axon plasticity and neuronal circuit formation and function[J]. J Neurosci, 2017, 37(42): 10023-10034. |
| 25 | LI C, XIAO L, LIU X Y, et al. A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination[J]. Glia, 2013, 61(5): 732-749. |
| 26 | MAURER U, CHARVET C, WAGMAN A S, et al. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1[J]. Mol Cell, 2006, 21(6): 749-760. |
| 27 | YANG F, ZHANG Y F, TANG Z Y, et al. Hemin treatment protects neonatal rats from sevoflurane-induced neurotoxicity via the phosphoinositide 3-kinase/Akt pathway[J]. Life Sci, 2020, 242: 117151. |
| 28 | GREEN D R, LLAMBI F. Cell death signaling[J]. Cold Spring Harb Perspect Biol, 2015, 7(12): a006080. |
| 29 | LIU X Y, JI J, ZHAO G Q. General anesthesia affecting on developing brain: evidence from animal to clinical research[J]. J Anesth, 2020, 34(5): 765-772. |
| 30 | SONG Q, MA Y L, SONG J Q, et al. Sevoflurane induces neurotoxicity in young mice through FAS/FASL signaling[J]. Genet Mol Res, 2015, 14(4): 18059-18068. |
| 31 | WERTZ I E, KUSAM S, LAM C, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7[J]. Nature, 2011, 471(7336): 110-114. |
| 32 | YON J H, DANIEL-JOHNSON J, CARTER L B, et al. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways[J]. Neuroscience, 2005, 135(3): 815-827. |
| 33 | CHEN X H, ZHOU X, LU D H, et al. Aberrantly expressed long noncoding RNAs are involved in sevoflurane-induced developing hippocampal neuronal apoptosis: a microarray related study[J]. Metab Brain Dis, 2016, 31(5): 1031-1040. |
| 34 | LI N N, ZHU R L, ZENG S, et al. The role of depolarizing activation of Na+-Ca2+ exchanger by oligodendrocyte progenitor cells in the effect of sevoflurane on myelination[J]. Life Sci, 2022, 308: 120951. |
| 35 | NEUDECKER V, PEREZ-ZOGHBI J F, MIRANDA-DOMÍNGUEZ O, et al. Early-in-life isoflurane exposure alters resting-state functional connectivity in juvenile non-human primates[J]. Br J Anaesth, 2023, 131(6): 1030-1042. |
| 36 | YOUNG J T, VLASOVA R M, HOWELL B R, et al. General anaesthesia during infancy reduces white matter micro-organisation in developing rhesus monkeys[J]. Br J Anaesth, 2021, 126(4): 845-853. |
| [1] | 朱子俊, 钱逸斐, 李倩玉, 李松玲, 覃雯莉, 刘艳丰. 后期促进复合体亚基10调控PI3K-AKT-mTOR通路促进肝细胞癌进展的研究[J]. 上海交通大学学报(医学版), 2025, 45(9): 1171-1182. |
| [2] | 许沐馨, 刘贤, 蒋立姗, 孙青. Nd:YAP激光生物刺激通过WNT/β-catenin信号通路促进人牙周韧带细胞增殖和成骨分化[J]. 上海交通大学学报(医学版), 2025, 45(5): 562-569. |
| [3] | 陈怡楠, 郑旸, 曾汉林, 雷鸣. Fas相关死亡结构域蛋白促进头颈部鳞状细胞癌细胞增殖能力的机制研究[J]. 上海交通大学学报(医学版), 2025, 45(4): 404-414. |
| [4] | 张先洲, 杜凤麟, 吴雷, 任逸喆, 赵明娜, 娄加陶. OGT通过ERK信号通路促进非小细胞肺癌增殖的机制研究[J]. 上海交通大学学报(医学版), 2025, 45(10): 1288-1297. |
| [5] | 李想, 魏鸣, 吴文曦, 罗小琴, 姚彪, 伍思宇. 芦丁对骨肉瘤生长和转移的体内外抑制作用[J]. 上海交通大学学报(医学版), 2025, 45(1): 20-28. |
| [6] | 孙晨玮, 海汪溪, 屈骞, 席云. [18F]F-FMISO和[18F]F-FLT PET/CT双核素显像预测胰腺癌耐药性的体内研究[J]. 上海交通大学学报(医学版), 2025, 45(1): 60-68. |
| [7] | 钱立恒, 温凯玲, 廖颖娜, 李书鑫, 聂惠贞. 分选链接蛋白1抑制结直肠癌细胞增殖和迁移的作用和机制研究[J]. 上海交通大学学报(医学版), 2024, 44(9): 1124-1135. |
| [8] | 谭露, 沈少明, 何平. 低氧诱导的长链非编码RNA 68在肝癌中的功能及其作用机制[J]. 上海交通大学学报(医学版), 2024, 44(6): 702-712. |
| [9] | 张勇, 李伟宏, 程志鹏, 王斌, 王思珩, 王毓斌. 受体相互作用蛋白激酶1调节癌症进展和免疫反应的研究现状[J]. 上海交通大学学报(医学版), 2024, 44(6): 788-794. |
| [10] | 蔡人杰, 徐明. KHSRP通过ANK3调节前列腺癌细胞对雄激素的反应性[J]. 上海交通大学学报(医学版), 2024, 44(4): 417-426. |
| [11] | 安俊伊, 陈必颖, 陈循睿, 尹姗姗, 边洲亮, 刘峰. SFXN3在头颈部鳞状细胞癌中的表达及其对细胞增殖的影响[J]. 上海交通大学学报(医学版), 2024, 44(4): 427-434. |
| [12] | 李虎虓, 李笑甜, 赵旭日, 张桓瑜, 周薇, 宋忠臣. 牙龈素提取物对口腔鳞癌细胞HN6生物学特性的影响[J]. 上海交通大学学报(医学版), 2024, 44(2): 161-168. |
| [13] | 罗蓝鸽, 郑超, 雷鸣. 癌-睾丸抗原CT57促进肝癌细胞增殖、侵袭、迁移和上皮间质转化[J]. 上海交通大学学报(医学版), 2024, 44(11): 1335-1346. |
| [14] | 孔汝心, 周亚群, 魏婷宜, 雷鸣. 癌-睾丸抗原CT63在慢性髓系白血病中的作用及其机制[J]. 上海交通大学学报(医学版), 2024, 44(11): 1347-1358. |
| [15] | 高珂星, 廖春华, 李昇泽, 马双羽, 黄雷. 黏蛋白1调控肿瘤细胞恶性特征的功能位点分析[J]. 上海交通大学学报(医学版), 2024, 44(11): 1370-1382. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||