
上海交通大学学报(医学版) ›› 2023, Vol. 43 ›› Issue (1): 70-78.doi: 10.3969/j.issn.1674-8115.2023.01.009
收稿日期:2022-09-22
接受日期:2022-12-27
出版日期:2023-01-28
发布日期:2023-01-28
通讯作者:
章雅青,电子信箱:zhangyqf@163.com。作者简介:方 芳(1971—),女,主任护师,博士生;电子信箱:fang_fang0604@163.com。
基金资助:
FANG Fang1( ), TAI Rui1, YU Qian1, ZHANG Yaqing2(
), TAI Rui1, YU Qian1, ZHANG Yaqing2( )
)
Received:2022-09-22
Accepted:2022-12-27
Online:2023-01-28
Published:2023-01-28
Contact:
ZHANG Yaqing, E-mail: zhangyqf@163.com.Supported by:摘要:
目的·系统评价预康复对择期行胃肠道手术患者术后恢复的影响。方法·系统检索PubMed、EMbase、Cochrane Library、Web of Science、CINAHL、中国生物医学文献数据库(CBM)、中国知网、万方、维普数据库,检索时限均为建库至2022年1月31日。按照预先设定的标准筛选文献,对文献质量进行评价并提取资料,对纳入的随机对照试验采用RevMan 5.4进行meta分析。主要观察指标为术后总体并发症发生率和手术部位感染发生率,次要观察指标为住院天数、6 min步行试验(6-minute walk test,6MWT)、医院焦虑抑郁量表(Hospital Anxiety and Depression Scale,HADS)评分,以及死亡率和预康复执行的依从性。结果·共纳入16篇文献,其中英文15篇,中文1篇,共1 616例研究对象,纳入研究的总体质量较好。Meta分析结果显示,相较于对照组,实施了预康复的试验组患者术后总体并发症的发生率降低[比值比(odds ratio,OR)=0.57,95%置信区间(confidence interval,CI)0.35~0.94,P=0.030],手术部位感染发生率降低(OR=0.64,95%CI 0.46~0.90,P=0.009),住院天数缩短[均数差值(mean difference,MD)=-2.45,95%CI -3.17~-1.73,P=0.000]。2组患者术前6MWT水平的差异无统计学意义;试验组术前6MWT水平相较于基线的提升程度优于对照组(MD=24.19,95%CI 3.77~44.60,P=0.020)。2组患者术前HADS评分和术后死亡率差异均无统计学意义。结论·预康复有利于降低胃肠道择期手术患者术后总体并发症发生率,尤其是手术部位感染发生率,从而缩短住院天数,有利于患者康复。
中图分类号:
方芳, 台瑞, 余倩, 章雅青. 预康复对胃肠道择期手术患者术后恢复效果的系统评价[J]. 上海交通大学学报(医学版), 2023, 43(1): 70-78.
FANG Fang, TAI Rui, YU Qian, ZHANG Yaqing. Effect of prehabilitation on outcomes in patients undergoing elective gastrointestinal surgery: a systematic review[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2023, 43(1): 70-78.
| Study | Population | Sample size (I/C) | Intervention method | Control method | Follow-up | Outcome |
|---|---|---|---|---|---|---|
| LÓPEZ-RODRÍGUEZ-ARIAS, 2021[ | Adults, elective surgery for colon or rectal neoplasm | 10/10 | A 30-d trimodal prehabilitation program with recommendations on physical exercise, nutritional supplementation, and relaxation exercises to be performed at home before surgery and the first 30 d after hospital discharge | Standard care | 12 weeks postoper-ative | ①②⑥ |
| BARBERAN-GARCIA, 2018[ | Adults, elective major abdominal surgery with high risk | 62/63 | A personalized prehabilitation program based on their health conditions and social circumstances which encompassed 3 major steps: motivational interview to assess the patients′ adherence profile and to codesign the characteristics of the physical activity program with the patient; personalized program to promote daily physical activity; supervised high-intensity endurance exercise training program | Standard care | Before discharge | ①②③ ④⑤⑥ |
| BOUSQUET-DION, 2018[ | Adults, elective surgery for colon or rectal neoplasm | 37/26 | A 4-week multi-modal home-based exercise program with once-weekly supervision consisted of exercise intervention, nutritional intervention and anxiety-reduction strategies | Standard care | 8 weeks postoper-ative | ①②③ ④⑥⑦ |
| OMMUNDSEN, 2018[ | >65 years, elective colorectal cancer surgery and fulfilling predefined criteria for frailty | 53/63 | A 3-week pragmatic tailored intervention based on the results of the geriatric assessment | Standard care | 3 months postoper-ative | ①②⑤ ⑥ |
| BURDEN, 2017[ | Adults, elective colorectal cancer surgery with preoperative weight loss>1 kg per 3‒6 months | 55/45 | Oral supplementation (10.1 KJ, 0.096 g of protein per mL) at a dose of 250 mL daily for a minimum of 5 d before the operation | Bottled water | 30 days postoper-ative | ①②⑥ ⑦ |
| GILLIS, 2016[ | Adults, elective surgery for colon or rectal neoplasm | 22/21 | A whey protein supplement was provided in a quantity that matched the patient′s need according to the estimated deficit in dietary protein intake for approximately 4 weeks leading up to the surgery | Placebo | 4 weeks postoper-ative | ③⑥ |
| GILLIS, 2014[ | Adults, elective surgery for colon or rectal neoplasm | 38/39 | A 4-week trimodal prehabilitation program at home included exercise intervention, nutrition intervention, and coping strategies to reduce anxiety | Standard care | 8 weeks postoper-ative | ①③④ ⑥ |
| FUJITANI, 2012[ | ≤80 years, elective total gastrectomy, body weight loss of 10 percent or less within 6 months before entry | 127/117 | Preoperative oral supplementation of 1 000 mL/d in the form of an immunonutrient-enriched enteral feed added to normal diet for 5 d consecutively before the surgery | Normal diet | Before discharge | ①②⑥ |
| BURDEN, 2011[ | Adults, elective surgery for colon or rectal neoplasm | 54/62 | Oral supplementary drink of 400 mL daily, and dietary advice for 10 d before the operation | Dietary advice only | Before discharge | ①②⑥ |
| SMEDLEY, 2004[ | Adults, elective moderate to severe lower gastrointestinal surgery | 48/50 | Oral nutritional supplement in small, frequent quantities between meals for 7 d before the operation, the volume consumed was recorded | Normal diet | Before discharge | ①②⑥ ⑦ |
| GIANOTTI, 2002[ | Adults, major elective surgery for malignancy of the gastrointestinal tract | 102/102 | Oral nutritional supplement (1 000 mL/d) of a formula enriched with arginine, ω-3 fatty acids, and RNA for 5 d before the operation | Standard care | 30 d after discharge | ①②⑤ ⑥ |
| BRAGA, 2002[ | Adults, elective surgery for gastroin-testinal neoplasm, body weight loss of 10% or more within 6 months before entry | 50/50 | Oral nutritional supplement (1 000 mL/d) added to normal diet for 7 d consecutively before the surgery | Normal diet | 30 d after discharge | ①②⑤ ⑥ |
| KABATA, 2015[ | Adults, elective, radical gastroin-testinal and abdominal cancer surgery | 54/48 | Oral nutritional supplement (400 mL/d) added to normal diet for 14 d consecutively before the surgery | Normal diet | 30 d postoper-ative | ①⑤⑥ |
| BARKER, 2013[ | Adults, elective upper and lower gastrointestinal surgery | 46/49 | Three packs of 237 mL of oral nutritional supplement per day for 5 d before the surgery | Standard care | 30 d postoper-ative | ①②⑤ ⑥ |
| BERKEL, 2022[ | ≥60 years, elective surgery for colon or rectal neoplasm | 28/29 | A personalized 3-week (3 sessions per week, 9 sessions in total) supervised exercise program | Standard care | 90 d postoper-ative | ①②⑥ |
| HUANG, 2014[ | Adults, elective, radical gastroin-testinal, cancer surgery | 41/41 | Oral nutritional supplement (25 mL/kg) for 7 d before the surgery | Normal diet | Before discharge | ①②⑥ |
表1 纳入研究的基本信息
Tab 1 Basic information of the selected studies
| Study | Population | Sample size (I/C) | Intervention method | Control method | Follow-up | Outcome |
|---|---|---|---|---|---|---|
| LÓPEZ-RODRÍGUEZ-ARIAS, 2021[ | Adults, elective surgery for colon or rectal neoplasm | 10/10 | A 30-d trimodal prehabilitation program with recommendations on physical exercise, nutritional supplementation, and relaxation exercises to be performed at home before surgery and the first 30 d after hospital discharge | Standard care | 12 weeks postoper-ative | ①②⑥ |
| BARBERAN-GARCIA, 2018[ | Adults, elective major abdominal surgery with high risk | 62/63 | A personalized prehabilitation program based on their health conditions and social circumstances which encompassed 3 major steps: motivational interview to assess the patients′ adherence profile and to codesign the characteristics of the physical activity program with the patient; personalized program to promote daily physical activity; supervised high-intensity endurance exercise training program | Standard care | Before discharge | ①②③ ④⑤⑥ |
| BOUSQUET-DION, 2018[ | Adults, elective surgery for colon or rectal neoplasm | 37/26 | A 4-week multi-modal home-based exercise program with once-weekly supervision consisted of exercise intervention, nutritional intervention and anxiety-reduction strategies | Standard care | 8 weeks postoper-ative | ①②③ ④⑥⑦ |
| OMMUNDSEN, 2018[ | >65 years, elective colorectal cancer surgery and fulfilling predefined criteria for frailty | 53/63 | A 3-week pragmatic tailored intervention based on the results of the geriatric assessment | Standard care | 3 months postoper-ative | ①②⑤ ⑥ |
| BURDEN, 2017[ | Adults, elective colorectal cancer surgery with preoperative weight loss>1 kg per 3‒6 months | 55/45 | Oral supplementation (10.1 KJ, 0.096 g of protein per mL) at a dose of 250 mL daily for a minimum of 5 d before the operation | Bottled water | 30 days postoper-ative | ①②⑥ ⑦ |
| GILLIS, 2016[ | Adults, elective surgery for colon or rectal neoplasm | 22/21 | A whey protein supplement was provided in a quantity that matched the patient′s need according to the estimated deficit in dietary protein intake for approximately 4 weeks leading up to the surgery | Placebo | 4 weeks postoper-ative | ③⑥ |
| GILLIS, 2014[ | Adults, elective surgery for colon or rectal neoplasm | 38/39 | A 4-week trimodal prehabilitation program at home included exercise intervention, nutrition intervention, and coping strategies to reduce anxiety | Standard care | 8 weeks postoper-ative | ①③④ ⑥ |
| FUJITANI, 2012[ | ≤80 years, elective total gastrectomy, body weight loss of 10 percent or less within 6 months before entry | 127/117 | Preoperative oral supplementation of 1 000 mL/d in the form of an immunonutrient-enriched enteral feed added to normal diet for 5 d consecutively before the surgery | Normal diet | Before discharge | ①②⑥ |
| BURDEN, 2011[ | Adults, elective surgery for colon or rectal neoplasm | 54/62 | Oral supplementary drink of 400 mL daily, and dietary advice for 10 d before the operation | Dietary advice only | Before discharge | ①②⑥ |
| SMEDLEY, 2004[ | Adults, elective moderate to severe lower gastrointestinal surgery | 48/50 | Oral nutritional supplement in small, frequent quantities between meals for 7 d before the operation, the volume consumed was recorded | Normal diet | Before discharge | ①②⑥ ⑦ |
| GIANOTTI, 2002[ | Adults, major elective surgery for malignancy of the gastrointestinal tract | 102/102 | Oral nutritional supplement (1 000 mL/d) of a formula enriched with arginine, ω-3 fatty acids, and RNA for 5 d before the operation | Standard care | 30 d after discharge | ①②⑤ ⑥ |
| BRAGA, 2002[ | Adults, elective surgery for gastroin-testinal neoplasm, body weight loss of 10% or more within 6 months before entry | 50/50 | Oral nutritional supplement (1 000 mL/d) added to normal diet for 7 d consecutively before the surgery | Normal diet | 30 d after discharge | ①②⑤ ⑥ |
| KABATA, 2015[ | Adults, elective, radical gastroin-testinal and abdominal cancer surgery | 54/48 | Oral nutritional supplement (400 mL/d) added to normal diet for 14 d consecutively before the surgery | Normal diet | 30 d postoper-ative | ①⑤⑥ |
| BARKER, 2013[ | Adults, elective upper and lower gastrointestinal surgery | 46/49 | Three packs of 237 mL of oral nutritional supplement per day for 5 d before the surgery | Standard care | 30 d postoper-ative | ①②⑤ ⑥ |
| BERKEL, 2022[ | ≥60 years, elective surgery for colon or rectal neoplasm | 28/29 | A personalized 3-week (3 sessions per week, 9 sessions in total) supervised exercise program | Standard care | 90 d postoper-ative | ①②⑥ |
| HUANG, 2014[ | Adults, elective, radical gastroin-testinal, cancer surgery | 41/41 | Oral nutritional supplement (25 mL/kg) for 7 d before the surgery | Normal diet | Before discharge | ①②⑥ |
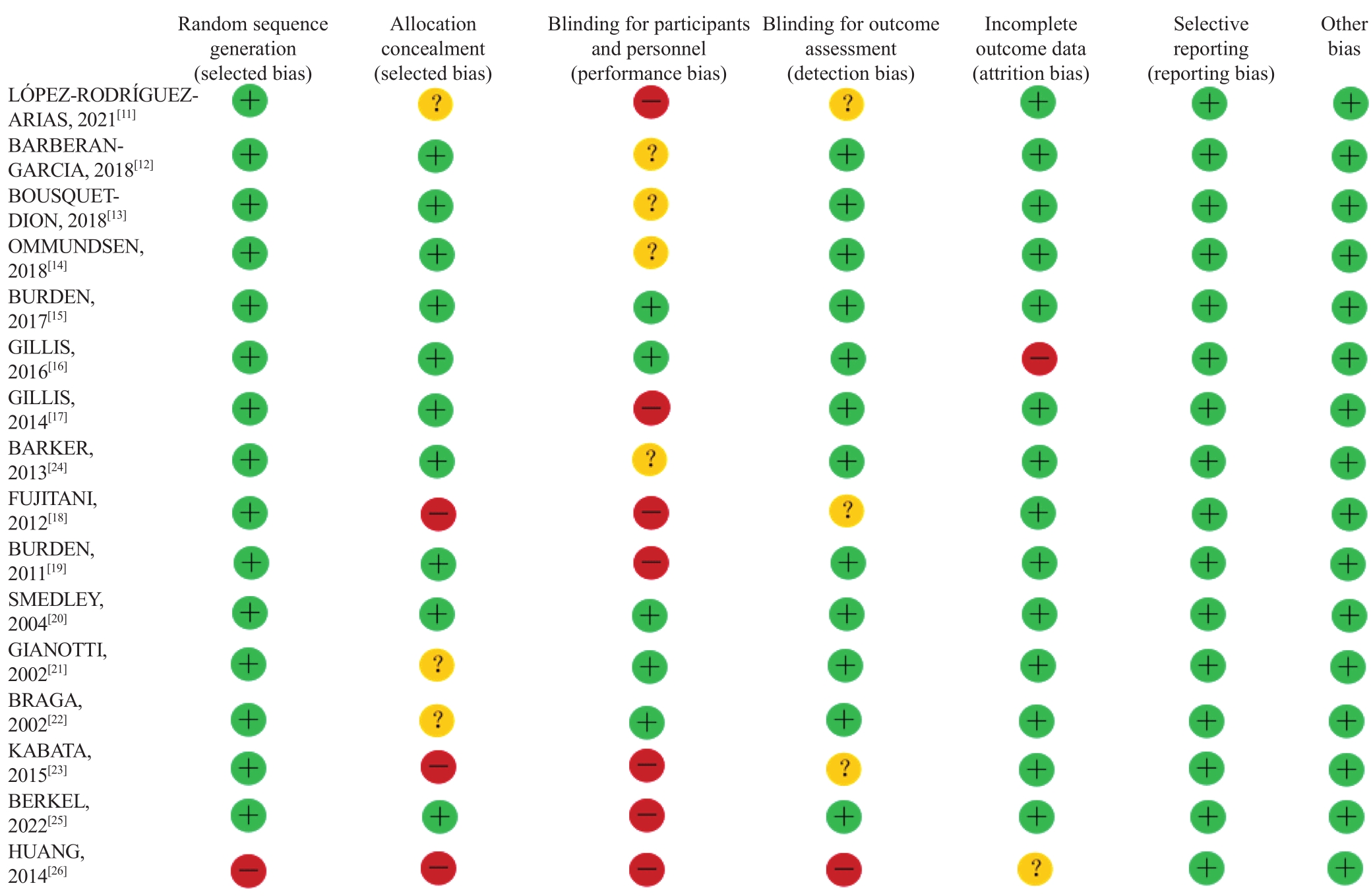
图2 纳入文献的风险偏倚
Fig 2 Risk bias of the included studiesNote: “+”—up to the standard; “-”—failure to meet the standard; “?”—not mentioned or described clearly in the literature.
| 1 | MCDERMOTT F D, HEENEY A, KELLY M E, et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks[J]. Br J Surg, 2015, 102(5): 462-479. |
| 2 | GOVAERT J A, FIOCCO M, VAN DIJK W A, et al. Costs of complications after colorectal cancer surgery in the Netherlands: building the business case for hospitals[J]. Eur J Surg Oncol, 2015, 41(8): 1059-1067. |
| 3 | KIRCHHOFF P, CLAVIEN P A, HAHNLOSER D. Complications in colorectal surgery: risk factors and preventive strategies[J]. Patient Saf Surg, 2010, 4(1): 5. |
| 4 | GUSTAFSSON U O, SCOTT M J, HUBNER M, et al. Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS®) society recommendations: 2018[J]. World J Surg, 2019, 43(3): 659-695. |
| 5 | CARLI F, BESSISSOW A, AWASTHI R, et al. Prehabilitation: finally utilizing frailty screening data[J]. Eur J Surg Oncol, 2020, 46(3): 321-325. |
| 6 | LI C, CARLI F, LEE L, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study[J]. Surg Endosc, 2013, 27(4): 1072-1082. |
| 7 | CARLI F, BOUSQUET-DION G, AWASTHI R, et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial[J]. JAMA Surg, 2020, 155(3): 233-242. |
| 8 | LE ROY B, SLIM K. Is prehabilitation limited to preoperative exercise?[J]. Surgery, 2017, 162(1): 192. |
| 9 | CLAVIEN P A, BARKUN J, DE OLIVEIRA M L, et al. The Clavien-Dindo classification of surgical complications: five-year experience[J]. Ann Surg, 2009, 250(2): 187-196. |
| 10 | 胡雁, 郝玉芳. 循证护理学[M]. 2版. 北京: 人民卫生出版社, 2018. |
| HU Y, HAO Y F. Evidence-based nursing[M]. 2nd ed. Beijing: People′s Medical Publishing House, 2018. | |
| 11 | LÓPEZ-RODRÍGUEZ-ARIAS F, SÁNCHEZ-GUILLÉN L, ARANAZ-OSTÁRIZ V, et al. Effect of home-based prehabilitation in an enhanced recovery after surgery program for patients undergoing colorectal cancer surgery during the COVID-19 pandemic[J]. Support Care Cancer, 2021, 29(12): 7785-7791. |
| 12 | BARBERAN-GARCIA A, UBRÉ M, ROCA J, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial[J]. Ann Surg, 2018, 267(1): 50-56. |
| 13 | BOUSQUET-DION G, AWASTHI R, LOISELLE S È, et al. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: a randomized control trial[J]. Acta Oncol, 2018, 57(6): 849-859. |
| 14 | OMMUNDSEN N, WYLLER T B, NESBAKKEN A, et al. Preoperative geriatric assessment and tailored interventions in frail older patients with colorectal cancer: a randomized controlled trial[J]. Colorectal Dis, 2018, 20(1): 16-25. |
| 15 | BURDEN S T, GIBSON D J, LAL S, et al. Pre-operative oral nutritional supplementation with dietary advice versus dietary advice alone in weight-losing patients with colorectal cancer: single-blind randomized controlled trial[J]. J Cachexia Sarcopenia Muscle, 2017, 8(3): 437-446. |
| 16 | GILLIS C, LOISELLE S E, FIORE J F J R, et al. Prehabilitation with whey protein supplementation on perioperative functional exercise capacity in patients undergoing colorectal resection for cancer: a pilot double-blinded randomized placebo-controlled trial[J]. J Acad Nutr Diet, 2016, 116(5): 802-812. |
| 17 | GILLIS C, LI C, LEE L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer[J]. Anesthesiology, 2014, 121(5): 937-947. |
| 18 | FUJITANI K, TSUJINAKA T, FUJITA J, et al. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer[J]. Br J Surg, 2012, 99(5): 621-629. |
| 19 | BURDEN S T, HILL J, SHAFFER J L, et al. An unblinded randomised controlled trial of preoperative oral supplements in colorectal cancer patients[J]. J Hum Nutr Diet, 2011, 24(5): 441-448. |
| 20 | SMEDLEY F, BOWLING T, JAMES M, et al. Randomized clinical trial of the effects of preoperative and postoperative oral nutritional supplements on clinical course and cost of care[J]. Br J Surg, 2004, 91(8): 983-990. |
| 21 | GIANOTTI L, BRAGA M, NESPOLI L, et al. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer[J]. Gastroenterology, 2002, 122(7): 1763-1770. |
| 22 | BRAGA M, GIANOTTI L, NESPOLI L, et al. Nutritional approach in malnourished surgical patients: a prospective randomized study[J]. Arch Surg, 2002, 137(2): 174-180. |
| 23 | KABATA P, JASTRZĘBSKI T, KĄKOL M, et al. Preoperative nutritional support in cancer patients with no clinical signs of malnutrition: prospective randomized controlled trial[J]. Support Care Cancer, 2015, 23(2): 365-370. |
| 24 | BARKER L A, GRAY C, WILSON L, et al. Preoperative immunonutrition and its effect on postoperative outcomes in well-nourished and malnourished gastrointestinal surgery patients: a randomised controlled trial[J]. Eur J Clin Nutr, 2013, 67(8): 802-807. |
| 25 | BERKEL A E M, BONGERS B C, KOTTE H, et al. Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of a randomized clinical trial[J]. Ann Surg, 2022, 275(2): e299-e306. |
| 26 | 黄正接, 陈百胜, 尤俊, 等. 胃肠道恶性肿瘤术前肠内免疫营养支持的临床意义[J]. 四川大学学报(医学版), 2014, 45(1): 167-170. |
| HUANG Z J, CHEN B S, YOU J, et al. The clinical significance of preoperative enteral immune nutrition in patients with malignant gastrointestinal tumors[J]. Journal of Sichuan University (Medical Sciences), 2014, 45(1): 167-170. | |
| 27 | 周岩冰. 胃肠肿瘤患者的术前预康复[J]. 中华胃肠外科杂志, 2021, 24(2): 122-127. |
| ZHOU Y B. Prehabilitation for gastrointestinal cancer patients[J]. Chinese Journal of Gastrointestinal Surgery, 2021, 24(2): 122-127. | |
| 28 | ADIAMAH A, SKOŘEPA P, WEIMANN A, et al. The impact of preoperative immune modulating nutrition on outcomes in patients undergoing surgery for gastrointestinal cancer: a systematic review and meta-analysis[J]. Ann Surg, 2019, 270(2): 247-256. |
| 29 | REECE L, DRAGICEVICH H, LEWIS C, et al. Preoperative nutrition status and postoperative outcomes in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy[J]. Ann Surg Oncol, 2019, 26(8): 2622-2630. |
| 30 | 吴秀文, 张旭飞, 阳怡羽, 等. 2018—2020年中国结直肠术后手术部位感染现状研究[J]. 中华胃肠外科杂志, 2022, 25(9): 804-811. |
| WU X W, ZHANG X F, YANG Y Y, et al. Surgical site infection after colorectal surgery in China from 2018 to 2020[J]. Chinese Journal of Gastrointestinal Surgery, 2022, 25(9): 804-811. | |
| 31 | 匡荣康, 王成龙, 强光辉, 等. 营养状态对结直肠癌病人手术部位感染的影响及相关因素分析[J]. 肠外与肠内营养, 2021, 28(2): 84-87. |
| KUANG R K, WANG C L, QIANG G H, et al. Analysis of the influencing factors for SSI in colorectal cancer patients with different nutrition disorder[J]. Parenteral & Enteral Nutrition, 2021, 28(2): 84-87. | |
| 32 | 张芳芳, 胡雅静, 黄华勇, 等. 基于CT诊断的肌少症对结直肠癌患者发生手术部位感染的影响因素分析[J]. 中国全科医学, 2022, 25(29): 3658-3663. |
| ZHANG F F, HU Y J, HUANG H Y, et al. Association of CT-assessed sarcopenia with postoperative surgical site infections in patients with colorectal cancer[J]. Chinese General Practice, 2022, 25(29): 3658-3663. | |
| 33 | GILLIS C, BUHLER K, BRESEE L, et al. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta-analysis[J]. Gastroenterology, 2018, 155(2): 391-410.e4. |
| 34 | YANG J, ZHANG T, FENG D, et al. A new diagnostic index for sarcopenia and its association with short-term postoperative complications in patients undergoing surgery for colorectal cancer[J]. Colorectal Dis, 2019, 21(5): 538-547. |
| 35 | MALIETZIS G, JOHNS N, AL-HASSI H O, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer[J]. Ann Surg, 2016, 263(2): 320-325. |
| 36 | MALIETZIS G, CURRIE A C, JOHNS N, et al. Skeletal muscle changes after elective colorectal cancer resection: a longitudinal study[J]. Ann Surg Oncol, 2016, 23(8): 2539-2547. |
| 37 | PECORELLI N, FIORE J F JR, GILLIS C, et al. The six-minute walk test as a measure of postoperative recovery after colorectal resection: further examination of its measurement properties[J]. Surg Endosc, 2016, 30(6): 2199-2206. |
| 38 | MOUG S J, BARRY S J E, MAGUIRE S, et al. Does prehabilitation modify muscle mass in patients with rectal cancer undergoing neoadjuvant therapy? A subanalysis from the REx randomised controlled trial[J]. Tech Coloproctol, 2020, 24(9): 959-964. |
| 39 | XU L, PAN Q, LIN R. Prevalence rate and influencing factors of preoperative anxiety and depression in gastric cancer patients in China: preliminary study[J]. J Int Med Res, 2016, 44(2): 377-388. |
| 40 | VAN ROOIJEN S, CARLI F, DALTON S, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation[J]. BMC Cancer, 2019, 19(1): 98. |
| [1] | 赵建磊, 赵婧琦, 刘唱, 黄靖竣, 金升元. 水动力清创治疗烧伤的效果:随机对照试验的系统评价[J]. 上海交通大学学报(医学版), 2025, 45(5): 614-623. |
| [2] | 孙晨寅, 吴百川, 张慧凤, 方贻儒, 彭代辉. 体动记录仪评估抑郁症昼夜节律:一项系统综述和meta分析[J]. 上海交通大学学报(医学版), 2024, 44(5): 606-616. |
| [3] | 台瑞, 孙菊芳, 林英, 章雅青, 黄陈, 方芳. 院前多模式预康复对胃肠道恶性肿瘤患者术前功能及术后恢复效果的影响[J]. 上海交通大学学报(医学版), 2024, 44(10): 1229-1234. |
| [4] | 杨越, 何开举, 宗家豪, 杨自逸, 吴向嵩, 龚伟. 细胞游离DNA在胆道癌诊断中的价值:一项meta分析[J]. 上海交通大学学报(医学版), 2023, 43(9): 1175-1185. |
| [5] | 陈惠, 朱唯一, 姚屹瑾. 调整左旋甲状腺素治疗剂量对甲状腺功能减退孕妇母婴结局影响的meta分析[J]. 上海交通大学学报(医学版), 2023, 43(7): 906-915. |
| [6] | 马卓然, 袁安彩, 蒋惠如, 陈潇雨, 张薇, 卜军. 脂质蓄积指数与中国成年人高血压关系的meta分析[J]. 上海交通大学学报(医学版), 2023, 43(4): 466-473. |
| [7] | 张媛媛, 吴安琪, 吴捷, 朱雅琪, 李梦瑶, 闫德修, 章雅青, 侯黎莉. 中青年癌症生存者重返工作干预方案的系统评价[J]. 上海交通大学学报(医学版), 2023, 43(3): 333-341. |
| [8] | 梁妍景, 黄楚贤, 李红艳, 侯黎莉. 维生素E对放疗或化疗导致的口腔黏膜炎有效性的meta分析[J]. 上海交通大学学报(医学版), 2023, 43(2): 208-214. |
| [9] | 杨玲, 侯黎莉, 赵燕, 陈卫宏, 张金凤, 毛艳. 口腔癌患者张口受限患病率的meta分析[J]. 上海交通大学学报(医学版), 2023, 43(1): 61-69. |
| [10] | 陈卫宏, 侯黎莉, 杨玲, 毛艳, 张金凤. 冷冻疗法预防头颈癌患者放射性口腔黏膜炎的meta分析[J]. 上海交通大学学报(医学版), 2022, 42(5): 635-645. |
| [11] | 谭颖超, 杨珺玥, 王莉娜. 白细胞介素-1B-511C/T基因多态性与冠状动脉粥样硬化性心脏病关联的meta分析[J]. 上海交通大学学报(医学版), 2022, 42(2): 197-204. |
| [12] | 钱德伟, 周任, 关礼春, 张航, 虞敏. 吸入一氧化氮气体对外科手术后肾功能损伤和出血影响的meta分析[J]. 上海交通大学学报(医学版), 2022, 42(1): 95-100. |
| [13] | 王毅, 程诚, 沈红艳, 高红艳, 戴悦宁, 易正辉. 经颅磁刺激对阿尔茨海默病患者认知功能及伴痴呆的行为精神症状疗效的meta分析[J]. 上海交通大学学报(医学版), 2021, 41(7): 931-941. |
| [14] | 刘洁, 谢新民, 支雪梅, 陆静毅. 睡眠时间与糖尿病性视网膜病变风险关系的meta分析[J]. 上海交通大学学报(医学版), 2021, 41(11): 1502-1508. |
| [15] | 陈智灵, 罗晨, 赵康佳, 沈玲, 胡三莲. 2种镇痛方式在结直肠癌术后应用效果的meta分析[J]. 上海交通大学学报(医学版), 2021, 41(10): 1344-1350. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||