
上海交通大学学报(医学版) ›› 2025, Vol. 45 ›› Issue (9): 1149-1160.doi: 10.3969/j.issn.1674-8115.2025.09.007
收稿日期:2024-12-13
接受日期:2025-04-08
出版日期:2025-09-28
发布日期:2025-09-30
通讯作者:
黄 陈,主任医师,博士;电子信箱:richard-hc@hotmail.com。基金资助:
PAERHATI Nadina, ZHANG Pengshan, XU Yitian, CHEN Yunqi, HUANG Chen( )
)
Received:2024-12-13
Accepted:2025-04-08
Online:2025-09-28
Published:2025-09-30
Contact:
HUANG Chen, E-mail: richard-hc@hotmail.com.Supported by:摘要:
目的·构建去泛素化酶圆柱瘤蛋白(cylindromatosis,CYLD)截短体质粒,初步分析CYLD对胃癌细胞增殖的影响。方法·收集癌症基因组图谱数据库(The Cancer Genome Atlas,TCGA)、基因型-组织表达数据库(Genotype-Tissue Expression,GTEx)及Kaplan-Meier Plotter数据库中CYLD在胃癌组织与正常组织中表达数据,以及与胃癌患者总生存期的相关性数据;免疫组织化学和Western blotting法检测胃癌组织与癌旁组织中CYLD的表达情况;Western blotting法和荧光实时定量PCR检测正常胃黏膜上皮细胞与胃癌细胞系中CYLD蛋白和mRNA表达情况。根据CYLD基因序列及结构特点,设计引物,构建其截短体真核表达质粒,琼脂糖凝胶电泳和Western blotting法检测及鉴定其表达情况,免疫荧光(immunofluorescence,IF)观察定位。在AGS细胞中敲低CYLD并且在敲低后分别回补CYLD野生型、酶失活突变型及截短体,细胞计数试剂盒-8(cell counting kit-8,CCK-8)和平板克隆实验检测CYLD野生型、酶失活突变型及截短体对细胞增殖的影响。免疫共沉淀(co-immunoprecipitation,Co-IP)、去泛素化实验、Western blotting法检测CYLD野生型、酶失活突变型及截短体与钙/钙调蛋白依赖性蛋白激酶Ⅱα亚基(calcium/calmodulin dependent protein kinase Ⅱα,CAMK2A)的相互结合能力、CAMK2A去泛素化修饰水平及STAT3、p-STAT3蛋白表达情况。结果·胃正常组织中CYLD含量显著高于胃癌组织,正常胃黏膜上皮细胞中CYLD含量显著高于胃癌细胞系,高表达CYLD的胃癌患者预后较好。人CYLD截短体质粒构建成功,CYLD野生型、酶失活突变型以及3个截短体主要定位在细胞质的细胞中。敲低CYLD,AGS细胞增殖能力得到显著增强。而在敲低后分别回补CYLD野生型、酶失活突变型及截短体的细胞中,敲低CYLD后过表达CYLD野生型以及CAP3和USP区段能够显著抑制胃癌细胞的增殖。此外,CYLD能够与蛋白激酶CAMK2A相互结合并介导CAMK2A的K63去泛素化修饰,并且抑制CAMK2A对STAT3蛋白的磷酸化修饰。结论·成功构建人CYLD截短体真核表达质粒,CYLD野生型以及CAP3和USP区段显著抑制胃癌细胞增殖能力。
中图分类号:
那迪娜·帕尔哈提, 张鹏善, 徐亦天, 陈赟琪, 黄陈. 人去泛素化酶圆柱瘤蛋白截短体质粒的构建及其对胃癌细胞表型的调控研究[J]. 上海交通大学学报(医学版), 2025, 45(9): 1149-1160.
PAERHATI Nadina, ZHANG Pengshan, XU Yitian, CHEN Yunqi, HUANG Chen. Construction of a truncated cylindromatosis tumor suppressor deubiquitinase plasmid and its regulation of the phenotypes of gastric cancer cells[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2025, 45(9): 1149-1160.
| Primer name | Primer sequence(5'→3') |
|---|---|
| Flag-CYLD-Del2-320aa | F:CGCCATGTTTATGTCAAGAGGTGTTGGGGACAAAGG |
| R:CTTGACATAAACATGGCGATCGCGGCGG | |
| Flag-CYLD-Del321-592aa | F:CCAAACTTGCCGGCATCCAGGGTCATTACAATTCTTGTTACTTAGAC |
| R:GGATGCCGGCAAGTTTGGGAGGCCTCCTTTCC | |
| Flag-CYLD-Del593-956aa | F:GGAAGAAGAAAACGCGTACGCGGCCGCTC |
| R:CGTACGCGTTTTCTTCTTCCCAATCATTATCTCCAAGCC |
表1 人 CYLD 基因截短体PCR引物序列
Tab 1 PCR primer sequences for truncated variants of the human CYLD gene
| Primer name | Primer sequence(5'→3') |
|---|---|
| Flag-CYLD-Del2-320aa | F:CGCCATGTTTATGTCAAGAGGTGTTGGGGACAAAGG |
| R:CTTGACATAAACATGGCGATCGCGGCGG | |
| Flag-CYLD-Del321-592aa | F:CCAAACTTGCCGGCATCCAGGGTCATTACAATTCTTGTTACTTAGAC |
| R:GGATGCCGGCAAGTTTGGGAGGCCTCCTTTCC | |
| Flag-CYLD-Del593-956aa | F:GGAAGAAGAAAACGCGTACGCGGCCGCTC |
| R:CGTACGCGTTTTCTTCTTCCCAATCATTATCTCCAAGCC |
| Gene | Primer sequence (5'→3') |
|---|---|
| CYLD | F:GGTAATCCGTTGGATCGGTCAG |
| R:AGTGCCTCTGAAGGTTCCATCC | |
| β-actin | F:CACCATTGGCAATGAGCGGTTC |
| R:AGGTCTTTGCGGATGTCCACGT |
表2 实时荧光定量引物信息
Tab 2 Primer sequences for qPCR
| Gene | Primer sequence (5'→3') |
|---|---|
| CYLD | F:GGTAATCCGTTGGATCGGTCAG |
| R:AGTGCCTCTGAAGGTTCCATCC | |
| β-actin | F:CACCATTGGCAATGAGCGGTTC |
| R:AGGTCTTTGCGGATGTCCACGT |
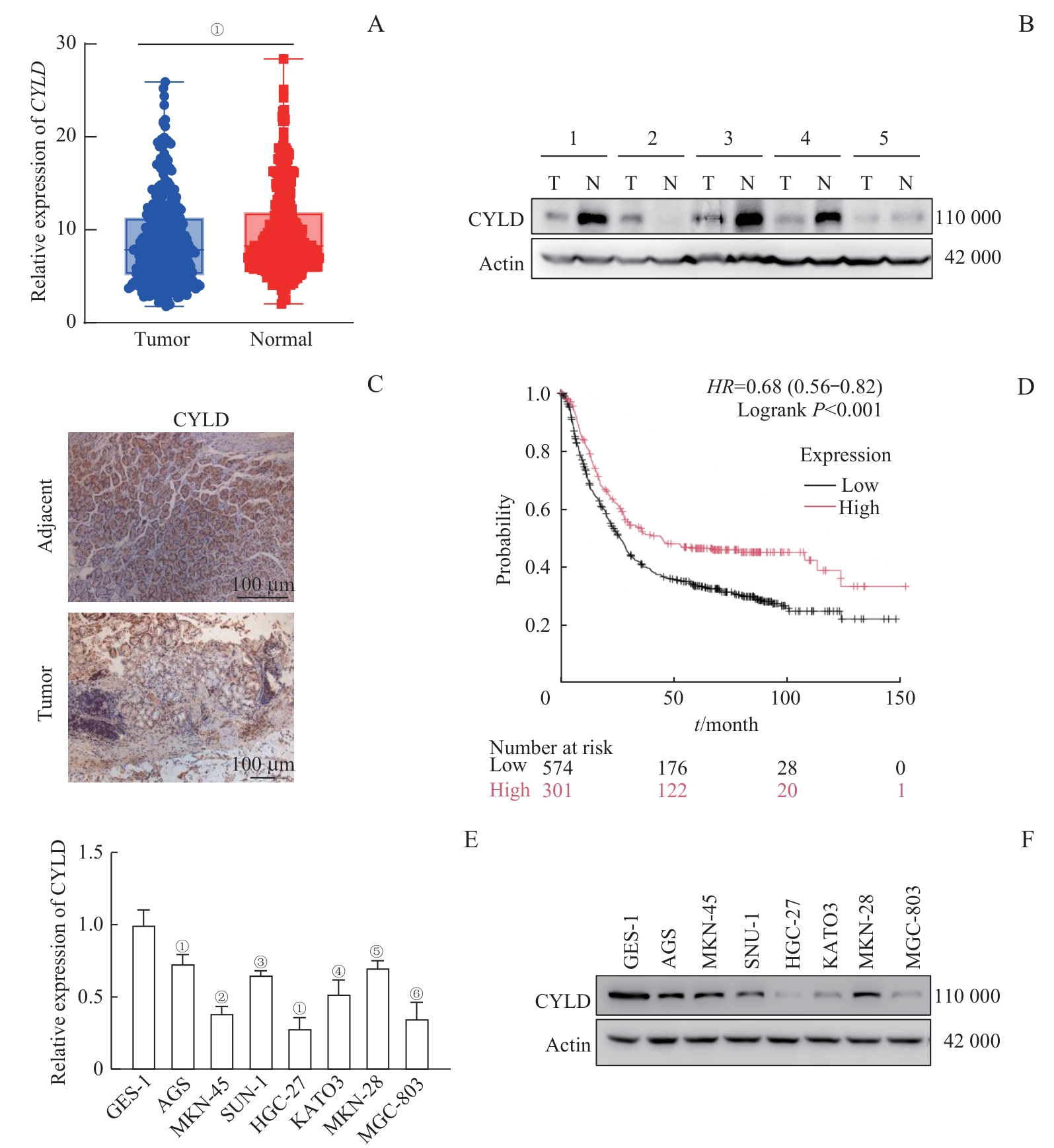
图1 去泛素化酶 CYLD 在胃癌组织及细胞系中的表达及其与胃癌患者预后的相关性分析Note: A. Relative expression of CYLD mRNA in STAD (stomach adenocarcinoma, gastric adenocarcinoma) and normal tissue samples from the TCGA and GTEx databases. ①P=0.002. B. CYLD protein expression levels in tumor and adjacent normal tissue samples from 5 gastric cancer patients were detected by Western blotting. T—tumor, N—normal. C. CYLDprotein expression in tumor and adjacent normal tissue samples from gastric cancer patients were detected by immunohistochemistry. D. Prognostic significance of CYLD in gastric cancer patients was analyzed using the Kaplan-Meier Plotter database. HR=0.68 (0.56-0.82), logrank P<0.001. E/F. CYLD expression levels in GES-1 and 7 gastric cancer cell lines were analyzed by qRT-PCR (E) and Western blotting (F). ①P=0.004, ②P<0.001, ③P=0.007, ④P=0.036, ⑤P=0.027, ⑥P=0.045, compared with GES-1.
Fig 1 Expression of CYLD in gastric cancer tissues and cell lines and its correlation with prognosis of gastric cancer patients
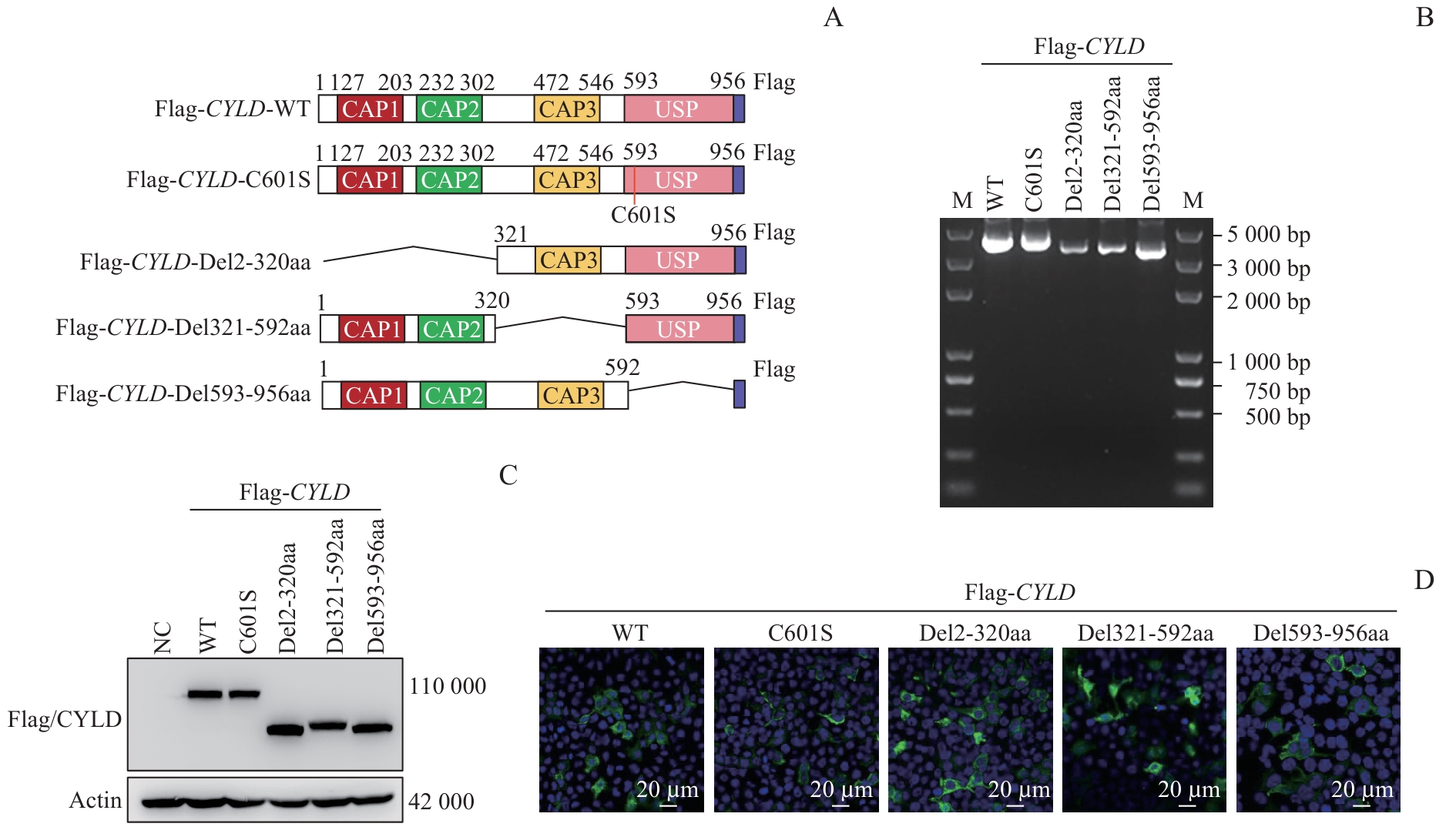
图2 去泛素化酶 CYLD 蛋白截短体的构建、表达验证及细胞定位分析Note: A. Schematic diagram showed the structure of Flag-CYLD full-length, enzymatically inactivated mutant, and truncated variants, with the three CAP domains (CAP1/CAP2/CAP3) and the USP catalytic domain marked. B. Agarose gel electrophoresis was performed to analyze the plasmids Flag-CYLD-WT, Flag-CYLD-C601S, Flag-CYLD-Del2-320aa, Flag-CYLD-Del321-592aa, and Flag-CYLD-Del593-956aa (M=DNA marker). C. Western blotting was conducted to analyze the protein expression of Flag-CYLD-WT, Flag-CYLD-C601S, Flag-CYLD-Del2-320aa, Flag-CYLD-Del321-592aa, and Flag-CYLD-Del593-956aa, with empty vector-transfected HEK-293T cells serving as controls. D. Immunofluorescence (IF) assay was carried out to detect the subcellular localization of Flag-CYLD-WT, Flag-CYLD-C601S, Flag-CYLD-Del2-320aa, Flag-CYLD-Del321-592aa, and Flag-CYLD-Del593-956aa.
Fig 2 Construction, expression verification, and subcellular localization of CYLD truncated variants
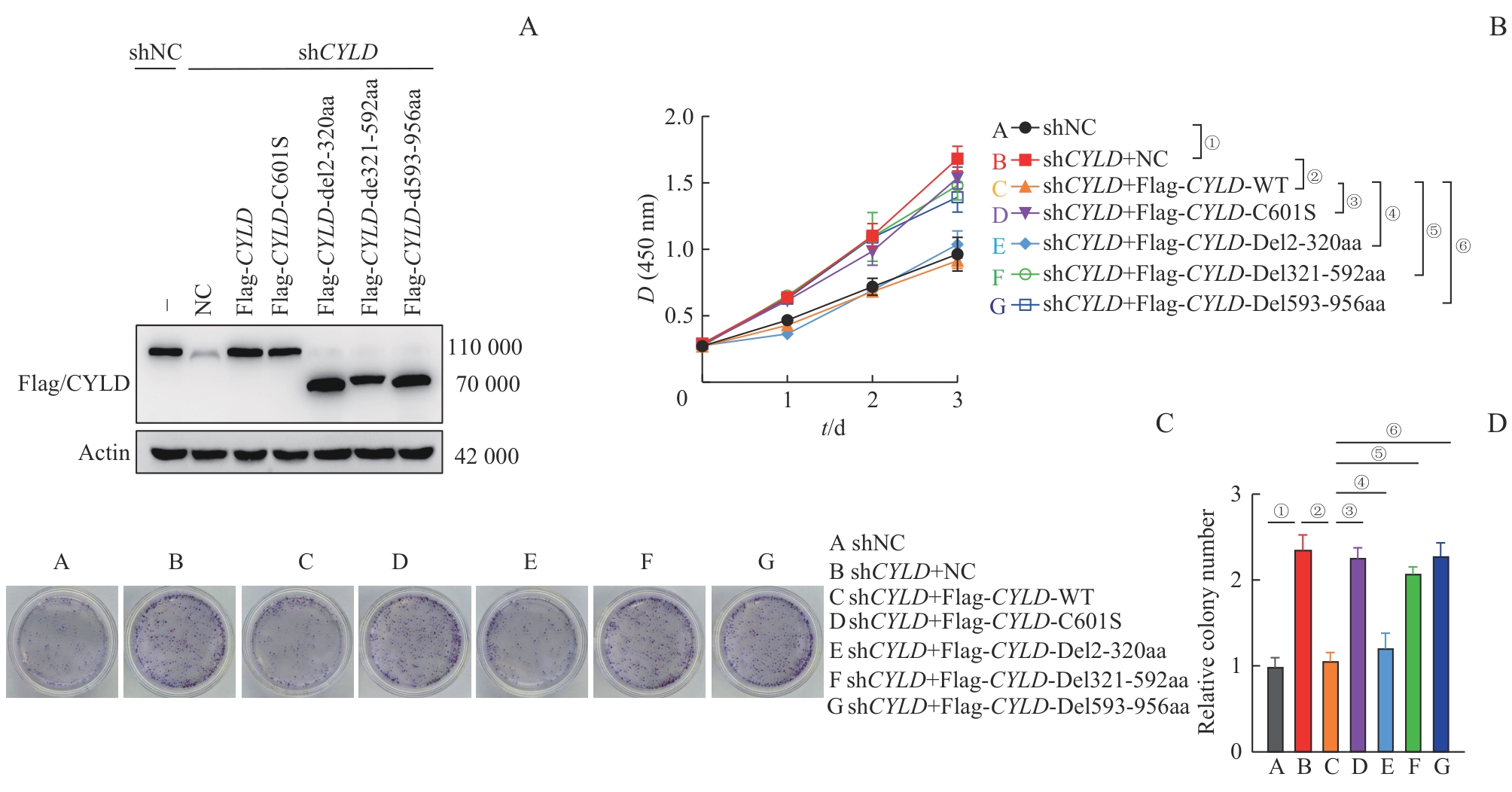
图3 CYLD 野生型、酶失活突变型及截短体对胃癌细胞系AGS增殖与克隆形成能力的影响Note: A. Western blotting was performed to analyze the basal CYLD expression in AGS cells transfected with shNC and shCYLD, as well as the protein expression after transfection of plasmids in each group. B. CCK8 assay was carried out to evaluate the cell proliferation of each group. ①P<0.001, ②P=0.007, ③P=0.004, ④P>0.05, ⑤P=0.033, ⑥P=0.041. C/D. Plate colony formation experiments were conducted to assess the colony-forming abilities of each group. ①P=0.036, ②P<0.001, ③P=0.002, ④P>0.05, ⑤P=0.002, ⑥P=0.041.
Fig 3 Effects of CYLD WT, enzyme-inactivated mutant, and truncated variants on proliferation and colony formation of AGS gastric cancer cells
| Protein ID | Candidate protein | Description |
|---|---|---|
| Q9UM82 | SPATA2 | Spermatogenesis-associated protein 2 |
| Q9UBN7 | HDAC6 | Histone deacetylase 6 |
| P04637 | P53 | Tumor protein p53 |
| Q9UQM7 | CAMK2A | Calcium/calmodulin-dependent protein kinase Ⅱα |
表4 BioGRID 数据库鉴定的与 CYLD 相互作用的候选蛋白
Tab 4 Candidate proteins predicted to interact with CYLD, as identified in the BioGRID database
| Protein ID | Candidate protein | Description |
|---|---|---|
| Q9UM82 | SPATA2 | Spermatogenesis-associated protein 2 |
| Q9UBN7 | HDAC6 | Histone deacetylase 6 |
| P04637 | P53 | Tumor protein p53 |
| Q9UQM7 | CAMK2A | Calcium/calmodulin-dependent protein kinase Ⅱα |
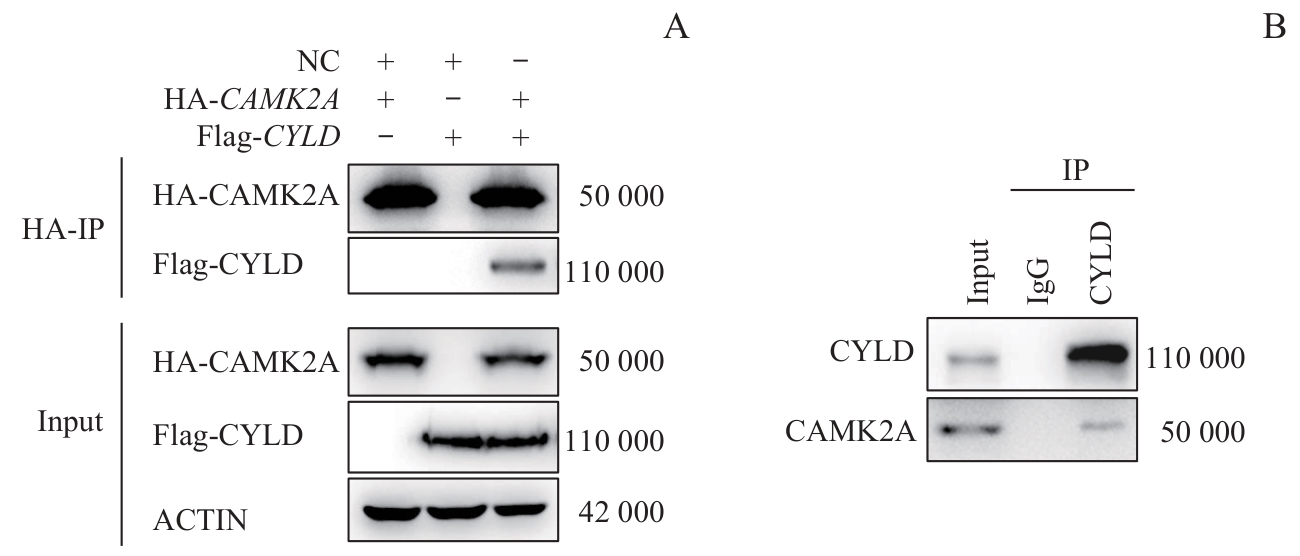
图4 去泛素化酶CYLD与CAMK2A的相互结合验证Note: A. Co-immunoprecipitation (co-IP) assays were performed in HEK-293T cells transfected with HA-CAMK2A/Flag-CYLD (or negative control plasmids) to verify the interaction between CAMK2A and CYLD. B. The interaction between endogenous CYLD and CAMK2A was confirmed by co-IP assays in AGS cells.
Fig 4 Verification of the interaction between deubiquitinase CYLD and CAMK2A
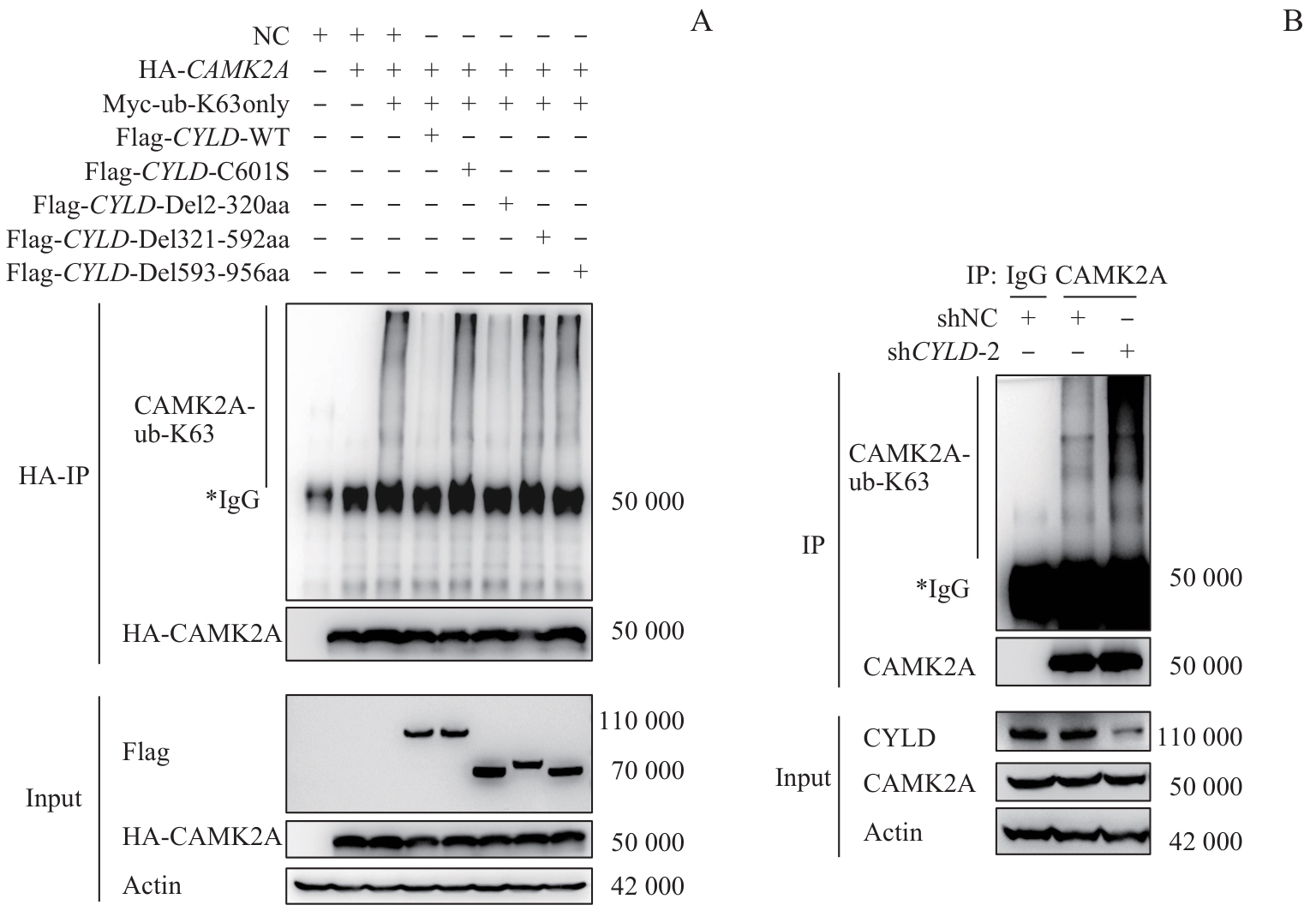
图5 CYLD全长、酶失活突变体及截短体对CAMK2A的K63位泛素化修饰水平的调控Note: A. HEK-293T cells were co-transfected with NC, HA-CAMK2A, and/or Flag-tagged CYLD (wild-type, enzyme-inactivated mutant, or truncated mutant). Co-immunoprecipitation (co-IP) assays were performed to detect changes in K63-linked ubiquitination levels of HA-CAMK2A. B. AGS cells with CYLD knockdown were used, with shNC-transfected cells as controls. Co-IP assays were conducted to analyze changes in K63-linked ubiquitination levels of endogenous CAMK2A.
Fig 5 Regulation of K63-linked ubiquitination of CAMK2A by CYLD full-length, enzyme-inactivated mutant, and truncated variants
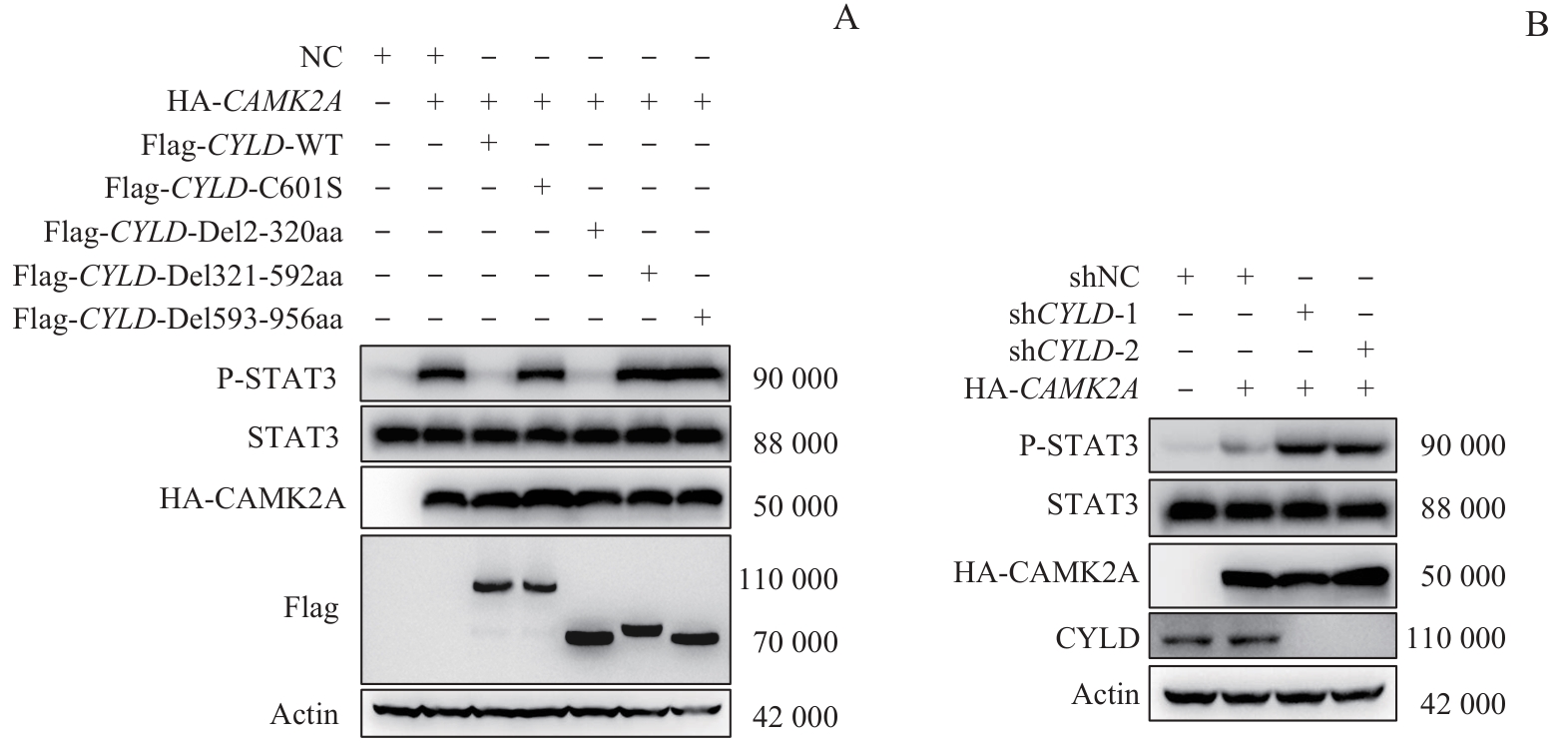
图6 CYLD全长、酶失活突变体及截短体对CAMK2A下游STAT3磷酸化修饰水平的调控Note: A. HEK-293T cells were co-transfected with NC, HA-CAMK2A, and/or Flag-tagged CYLD (wild-type, enzyme-inactivated mutant, or truncated mutant). Western blotting was performed to analyze changes in protein expression levels of p-STAT3 and STAT3. B. shNC-transfected AGS cells were used as controls. shNC, shCYLD-1, and shCYLD-2 AGS cells were transfected with HA-CAMK2A. Western blotting was conducted to detect changes in protein expression levels of p-STAT3 and STAT3.
Fig 6 Regulation of phosphorylation of STAT3 (downstream of CAMK2A) by CYLD full-length, enzyme-inactivated mutant, and truncated variants
| [1] | BRAY F, LAVERSANNE M, SUNG H, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2024, 74(3): 229-263. |
| [2] | HAQ S, SARODAYA N, KARAPURKAR J K, et al. CYLD destabilizes NoxO1 protein by promoting ubiquitination and regulates prostate cancer progression[J]. Cancer Lett, 2022, 525: 146-157. |
| [3] | HELLERBRAND C, BUMES E, BATAILLE F, et al. Reduced expression of CYLD in human colon and hepatocellular carcinomas[J]. Carcinogenesis, 2007, 28(1): 21-27. |
| [4] | YUAN Y, LIU L, WANG Y, et al. Reduced expression of CYLD promotes cell survival and inflammation in gefitinib-treated NSCLC PC-9 cells: targeting CYLD may be beneficial for acquired resistance to gefitinib therapy[J]. Cell Biol Int, 2020, 44(9): 1911-1918. |
| [5] | XIA J T, CHEN L Z, JIAN W H, et al. microRNA-362 induces cell proliferation and apoptosis resistance in gastric cancer by activation of NF-κB signaling[J]. J Transl Med, 2014, 12: 33. |
| [6] | HUANG C Q, LIU J Y, PAN X K, et al. miR-454 promotes survival and induces oxaliplatin resistance in gastric carcinoma cells by targeting CYLD[J]. Exp Ther Med, 2020, 19(6): 3604-3610. |
| [7] | WANG J G, YE J, LIU R Q, et al. TRIM47 drives gastric cancer cell proliferation and invasion by regulating CYLD protein stability[J]. Biol Direct, 2024, 19(1): 106. |
| [8] | TROMPOUKI E, HATZIVASSILIOU E, TSICHRITZIS T, et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activation by TNFR family members[J]. Nature, 2003, 424: 793-796. |
| [9] | GU Y N, WU S Q, FAN J J, et al. CYLD regulates cell ferroptosis through Hippo/YAP signaling in prostate cancer progression[J]. Cell Death Dis, 2024, 15(1): 79. |
| [10] | 杨督, 田同德, 张成辉, 等. p-STAT3和STING蛋白在胃癌组织中的表达及相关性[J]. 现代肿瘤医学, 2024, 32(1): 79-86. |
| YANG D, TIAN T D, ZHANG C H, et al. Expression and correlation of p-STAT3 and STING protein in gastric cancer tissues [J]. Journal of Modern Oncology, 2024, 32(1): 79-86. | |
| [11] | YU T J, LIU Y Y, LI X G, et al. PDSS1-mediated activation of CAMK2A-STAT3 signaling promotes metastasis in triple-negative breast cancer[J]. Cancer Res, 2021, 81(21): 5491-5505. |
| [12] | 邓成念, 周文玉, 谢斌, 等. CaMKⅡ在肿瘤中的研究进展[J]. 临床误诊误治, 2021, 34(1): 112-116. |
| DENG C N, ZHOU W Y, XIE B, et al. Research progress of CaMKⅡ in tumor [J]. Clinical Misdiagnosis & Mistherapy, 2021, 34 (1): 112-116. | |
| [13] | LIU Z L, HAN G, CAO Y, et al. Calcium/calmodulin-dependent protein kinase II enhances metastasis of human gastric cancer by upregulating nuclear factor‑κB and Akt-mediated matrix metalloproteinase-9 production[J]. Mol Med Rep, 2014, 10(5): 2459-2464. |
| [14] | CHEN W, AN P, QUAN X J, et al. Ca2+/calmodulin-dependent protein kinase II regulates colon cancer proliferation and migration via ERK1/2 and p38 pathways[J]. World J Gastroenterol, 2017, 23(33): 6111-6118. |
| [15] | ZHU G X, HERLYN M, YANG X L. TRIM15 and CYLD regulate ERK activation via lysine-63-linked polyubiquitination[J]. Nat Cell Biol, 2021, 23(9): 978-991. |
| [16] | CHENG N, TREJO J. An siRNA library screen identifies CYLD and USP34 as deubiquitinases that regulate GPCR-p38 MAPK signaling and distinct inflammatory responses[J]. J Biol Chem, 2023, 299(12): 105370. |
| [17] | RIEHEMANN K, SORG C. Sequence homologies between four cytoskeleton-associated proteins[J]. Trends Biochem Sci, 1993, 18(3): 82-83. |
| [18] | XIE S B, CHEN M, GAO S Q, et al. The B-box module of CYLD is responsible for its intermolecular interaction and cytoplasmic localization[J]. Oncotarget, 2017, 8(31): 50889-50895. |
| [19] | MASSOUMI R, CHMIELARSKA K, HENNECKE K, et al. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling[J]. Cell, 2006, 125(4): 665-677. |
| [20] | KOMANDER D, LORD C J, SCHEEL H, et al. The structure of the CYLD USP domain explains its specificity for Lys63-linked polyubiquitin and reveals a B box module[J]. Mol Cell, 2008, 29(4): 451-464. |
| [21] | WILLIAMS E A, MONTESION M, SHARAF R, et al. CYLD-mutant Cylindroma-like basaloid carcinoma of the anus: a genetically and morphologically distinct class of HPV-related anal carcinoma[J]. Mod Pathol, 2020, 33(12): 2614-2625. |
| [22] | ALAMEDA J P, MORENO-MALDONADO R, NAVARRO M, et al. An inactivating CYLD mutation promotes skin tumor progression by conferring enhanced proliferative, survival and angiogenic properties to epidermal cancer cells[J]. Oncogene, 2010, 29(50): 6522-6532. |
| [23] | YAMANAKA S, SATO Y, OIKAWA D, et al. Subquinocin, a small molecule inhibitor of CYLD and USP-family deubiquitinating enzymes, promotes NF-κB signaling[J]. Biochem Biophys Res Commun, 2020, 524(1): 1-7. |
| [1] | 朱子俊, 钱逸斐, 李倩玉, 李松玲, 覃雯莉, 刘艳丰. 后期促进复合体亚基10调控PI3K-AKT-mTOR通路促进肝细胞癌进展的研究[J]. 上海交通大学学报(医学版), 2025, 45(9): 1171-1182. |
| [2] | 周亦凝, 叶之韵, 陈慧文, 谢欣宜, 周薇, 宋忠臣. Th17细胞特异性敲除Stat3对牙周炎小鼠焦虑抑郁样行为的影响[J]. 上海交通大学学报(医学版), 2025, 45(7): 838-845. |
| [3] | 杨娜, 刘俊丽, 白静, 杨思怡, 韩继明, 张华华. HENMT1通过激活PI3K-AKT-mTOR信号通路促进胃癌的增殖与迁移[J]. 上海交通大学学报(医学版), 2025, 45(6): 717-726. |
| [4] | 许沐馨, 刘贤, 蒋立姗, 孙青. Nd:YAP激光生物刺激通过WNT/β-catenin信号通路促进人牙周韧带细胞增殖和成骨分化[J]. 上海交通大学学报(医学版), 2025, 45(5): 562-569. |
| [5] | 陈怡楠, 郑旸, 曾汉林, 雷鸣. Fas相关死亡结构域蛋白促进头颈部鳞状细胞癌细胞增殖能力的机制研究[J]. 上海交通大学学报(医学版), 2025, 45(4): 404-414. |
| [6] | 陈勇羽, 黄益仁, 陈哲逸, 周冰倩, 陈诗宇, 郑英霞. 丝氨酸蛋白酶抑制因子1在胃癌中的表达及其促进胃癌发展的作用机制[J]. 上海交通大学学报(医学版), 2025, 45(2): 150-160. |
| [7] | 张先洲, 杜凤麟, 吴雷, 任逸喆, 赵明娜, 娄加陶. OGT通过ERK信号通路促进非小细胞肺癌增殖的机制研究[J]. 上海交通大学学报(医学版), 2025, 45(10): 1288-1297. |
| [8] | 李想, 魏鸣, 吴文曦, 罗小琴, 姚彪, 伍思宇. 芦丁对骨肉瘤生长和转移的体内外抑制作用[J]. 上海交通大学学报(医学版), 2025, 45(1): 20-28. |
| [9] | 孙晨玮, 海汪溪, 屈骞, 席云. [18F]F-FMISO和[18F]F-FLT PET/CT双核素显像预测胰腺癌耐药性的体内研究[J]. 上海交通大学学报(医学版), 2025, 45(1): 60-68. |
| [10] | 施灵玲, 程燕咏, 张磊. 七氟烷对原代少突胶质细胞增殖和分化的影响[J]. 上海交通大学学报(医学版), 2024, 44(9): 1115-1123. |
| [11] | 钱立恒, 温凯玲, 廖颖娜, 李书鑫, 聂惠贞. 分选链接蛋白1抑制结直肠癌细胞增殖和迁移的作用和机制研究[J]. 上海交通大学学报(医学版), 2024, 44(9): 1124-1135. |
| [12] | 韩依杉, 徐梓淇, 陶梦玉, 范广建, 余波. PRMT6促进乳腺癌细胞的增殖和迁移[J]. 上海交通大学学报(医学版), 2024, 44(8): 999-1010. |
| [13] | 谭露, 沈少明, 何平. 低氧诱导的长链非编码RNA 68在肝癌中的功能及其作用机制[J]. 上海交通大学学报(医学版), 2024, 44(6): 702-712. |
| [14] | 蔡人杰, 徐明. KHSRP通过ANK3调节前列腺癌细胞对雄激素的反应性[J]. 上海交通大学学报(医学版), 2024, 44(4): 417-426. |
| [15] | 安俊伊, 陈必颖, 陈循睿, 尹姗姗, 边洲亮, 刘峰. SFXN3在头颈部鳞状细胞癌中的表达及其对细胞增殖的影响[J]. 上海交通大学学报(医学版), 2024, 44(4): 427-434. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||