目前认为AKI引起的肾纤维化可能由受损的肾小管上皮细胞与肾小管周围的成纤维细胞相互作用引起。长时间缺血或反复中毒,使肾小管上皮细胞丧失分化和再生能力,同时受损的肾小管上皮细胞分泌炎症因子和包括转化生长因子β1(transforming growth factor-β1,TGF-β1)在内的各种生长因子等,促使成纤维细胞转化为α-平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)阳性的肌成纤维细胞,合成大量细胞外基质等成分,并促进炎症细胞浸润,最终出现不可逆的肾纤维化[4]。如何有效预防和延缓AKI向CKD转化是目前肾脏病研究的重点和热点。
1 材料与方法
1.1 动物与细胞株
SPF级C57BL/6雄性小鼠,8周龄,体质量约20 g,购自上海吉辉实验动物责任有限公司,实验动物生产许可证编号为SCXK(沪)2018-0003;小鼠饲养于上海中医药大学附属岳阳中西医结合医院动物实验中心,使用许可证编号为SYXK(沪)2018-0040。正常大鼠肾小管上皮细胞株(NRK-52E)和正常大鼠肾脏成纤维细胞株(NRK-49F)购自ATCC细胞库。
1.2 主要试剂和仪器
马兜铃酸Ⅰ型(AA1,A5512)购自美国Sigma公司,淫羊藿苷(MB 2189-S)购自大连美仑生物技术有限公司,α-SMA抗体(ab5694)、Ⅰ型胶原蛋白抗体(collagen Ⅰ,cst72026)、F4/80抗体(ab16911)购自英国Abcam公司,活化的胱天蛋白酶3(cleaved caspase-3)抗体(9664)、辣根过氧化物酶(HRP)偶联的抗兔IgG(7074s),HRP偶联的抗小鼠IgG(7076s)、β-肌动蛋白(β-actin)抗体(3700T)、甘油醛-3-磷酸脱氢酶(GAPDH)抗体(5174)购自美国CST公司,聚偏二氟乙烯膜(PVDF膜,IPVH00005)购自德国Merk Millipore公司,Transwell细胞小室(3412)购自美国Corning公司,过氧化物酶体增殖物激活受体α(peroxisome proliferator activated receptor α,PPARα)抗体(MAI-822)、Lipofectamine 3000转染试剂盒(L3000150)购自美国Thermo Fisher公司,反转录试剂盒(RC112)、SYBR qPCR Master Mix(Q711)购自南京诺唯赞生物科技股份有限公司,苏木精-伊红(H-E)染液(G1005)、Masson三色染色液(G1006)、过碘酸希夫(PAS)染液(G1008)购自武汉赛维尔生物科技有限公司,BCA蛋白定量检测试剂盒(P0009)购自上海碧云天生物技术有限公司。
实时定量PCR仪(型号ABI7900)购自美国ABI公司,荧光显微镜(型号BX51)购自日本Olympus公司,台式离心机(型号5418)购自美国Eppendorf公司,蛋白条带检测仪器(型号CHEMIDOC)购自美国Bio-Rad公司,病理烘片机(型号HI1220)购自德国Leica公司,透射电子显微镜(透射电镜,型号Talos F200X S/TEM)购自美国Thermo Fisher公司,全自动生化分析仪(型号ADVIA1800)购自德国Siemens公司。
1.3 动物分组及给药
1.3.1 预防性用药
18只小鼠适应性饲养2周后,称重、排序,根据随机数字表随机分为3组,分别为对照组-1、马兜铃酸(AA1)组-1、马兜铃酸+淫羊藿苷(AA1+ICA)组-1,每组6只。AA1组-1每3 d腹腔注射1次马兜铃酸(3 mg/kg),共注射5次,约2周,之后停药2周。注射马兜铃酸2周为急性期,停药2周为重塑期。AA1+ICA组-1在造模前1周即予以250 mg/(kg·d)淫羊藿苷[溶于5%浓度的二甲基亚砜(DMSO)与生理盐水的混合液],灌胃给药5周。对照组-1与AA1组-1给予5%浓度的DMSO与生理盐水的混合液灌胃。急性期2周结束时小鼠眼眶后血管丛取血,隔日再行肾活检;重塑期2周结束时(4周)处死小鼠,眼球取血,并留取肾组织。
1.3.2 治疗用药
22只小鼠适应性饲养2周,随机分为3组(方法同“1.3.1”),分别为对照组-2(6只),马兜铃酸(AA1)组-2以及马兜铃酸+淫羊藿苷(AA1+ICA)组-2各8只。对照组-2以及AA1组-2给药方法同“1.3.1”,AA1+ICA组-2在马兜铃酸给药2周结束时给予250 mg/(kg·d)淫羊藿苷,灌胃给药2周。淫羊藿苷给药2周结束时(4周)处死小鼠,眼球取血,并留取肾组织。
1.4 肾穿刺活检术
小鼠麻醉后,侧卧位,从脊柱左侧旁开一指的部位做切口进入,切取肾脏上极约0.3 cm×0.3 cm×0.3 cm大小的肾组织后,迅速予无菌棉签压迫止血10 min,确定止血后,缝合手术切口[11]。
1.5 细胞培养和处理
大鼠肾小管上皮细胞株和肾脏成纤维细胞株予以含5%胎牛血清的DMEM培养基培养。Transwell细胞小室用于共培养实验。将细胞分为3组,即DMSO组、马兜铃酸(AA1)组、马兜铃酸+淫羊藿苷(AA1+ICA)组。将肾小管上皮细胞接种在下室(第0日),次日(第1日)AA1组的上皮细胞予以40 μmol/L马兜铃酸作用24 h,AA1+ICA组在加入40 μmol/L马兜铃酸之前,提前1 h加入10-6 mol/L淫羊藿苷,DMSO组加入等体积的DMSO溶液。第1日,另将肾脏成纤维细胞接种在Transwell上室,次日(第2日)将上室转移到肾小管上皮细胞(下室)上方共培养24 h;在共培养前,用PBS冲洗肾脏上皮细胞表面3次,以避免药物影响肾脏成纤维细胞。第3日,收集各组细胞株,提取细胞总RNA及蛋白[12]。
1.6 观察指标及检测方法
1.6.1 肾功能检测
应用全自动生化分析仪检测各组小鼠血尿素氮和肌酐值。
1.6.2 肾组织病理学观察
石蜡包埋各组小鼠肾脏组织,分别行H-E染色、PAS染色、Masson染色。应用Image-pro Plus 6.0软件分析每张照片的视野,定量分析各组小鼠组织切片Masson染色的胶原纤维面积百分比。根据H-E染色的肾组织切片,对肾小管损伤进行评分,评判标准[13]为:0分,无肾小管损伤;1分,<10%肾小管受损;2分,10%~25%肾小管受损;3分,26%~50%肾小管受损;4分,51%~75%肾小管受损;5分,>75%肾小管受损。每只小鼠观察10张切片,每张组织切片观察20个视野。
1.6.3 免疫组织化学染色
制备4 μm厚度的肾组织石蜡切片,梯度二甲苯脱腊,梯度乙醇水化,予α-SMA、Ⅰ型胶原蛋白、F4/80或PPARα一抗避光4 ℃过夜,次日复温1 h后加入二抗,DAB染色。每只小鼠观察5张切片,每张组织切片观察20个视野。
1.6.4 肾组织电镜观察
将组织迅速置于2.5%戊二醛固定液中,脱水包埋,超薄切片,透射电镜观察。
1.6.5 实时荧光定量PCR法检测
采用TRIzol法,提取肾脏组织或细胞株RNA;通过反转录获得cDNA。以cDNA为模板,行实时荧光定量PCR(real-time qPCR)。反应体系(20 μL):ddH2O 7.2 μL,cDNA模板 2 μL,上、下游引物各0.4 μL,SYBR Green Ⅰ 10 μL。反应条件:95 ℃ 30 s;95 ℃ 5 s,60 ℃ 31 s,72 ℃ 30 s,共40个循环;95 ℃ 15 s,60 ℃ 15 s,95 ℃ 15 s。引物由上海华津生物科技有限公司合成,序列详见表1。小鼠肾脏以次黄嘌呤磷酸核糖转移酶(hypoxanthine phosphoribosyltransferase,Hprt)基因作为内参,大鼠细胞以β-肌动蛋白基因作为内参。采用2-ΔΔCT法计算目标基因的相对水平。
表1 实时荧光定量PCR引物序列
Tab 1
| Gene | Forward (5′→3′) | Reward (5′→3′) |
|---|---|---|
| Fn (mouse) | ATGGTACAGCTGATCCTGCC | GCCCTGGTTTGTACCTGCTA |
| Acta2 (mouse) | GGAACCCTGAGACGCTGCT | CATTCCAACCATTACTCCC |
| Col1a1 (mouse) | CCCAGCCGCAAAGAGTCTAC | AGCATACCTCGGGTTTCCAC |
| Tgfb1 (mouse) | GGGAAGCAGTGCCCGAACCC | TGGGGGTCAGCAGCCGGTTA |
| Hprt (mouse) | TATGCCGAGGATTTGGAAAA | TCCCATCTCCTTCATGACATC |
| Cpt1a (mouse) | CTTCCCATTTGACACCTTTG | ATACGTGAGGCAGAACTTGC |
| Col3a1 (mouse) | ACAGCTGGTGAACCTGG | ACCAGGAGATCCATCTCGAC |
| Vimentin (mouse) | GGATCAGCTCACCAACGACA | GGTCAAGACGTGCCAGAGAA |
| Cpt2 (mouse) | CCCAGGCTGCCTATCCCT | TCCTTCCCAATGCCGTTC |
| Ppara (mouse) | AGAGCCCCATCTGTCCTCTC | ACTGGTAGTCTGCAAAACCAAA |
| Acox2 (mouse) | GAGGGTGAGAATACGGT | GGTCTTGGTGTGGCGAG |
| Ppara (rat) | AACATCGAGTGTCGAATATGTGG | CCGAATAGTTCGCCGAAAGAA |
| Tnfa (rat) | ATGGGCTCCCTCTCATCAGT | GCTTGGTGGTTTGCTACGAC |
| Fn (rat) | AGAGGGGAGTGGAAGTGTGA | GTTGTAGGTGAACGGGAGAA |
| Tgfb1 (rat) | CTAATGGTGGACCGCAACAAC | CACTGCTTCCCGAATGTCTGA |
| Ctgf (rat) | AACCGTGTGTCATTGTCAT | CACCTTAGTGTGCGTTCTG |
| Cpt1a (rat) | ATGGAGGTTGTCTACGA | CAGGGGTTTACTTTTTA |
| Cpt2 (rat) | CAGCTGACCAAAGAAGCAGC | GTTCAGAGTGCTGGTGGACA |
| Col1a1 (rat) | CTGACGCATGGCCAAGAAGA | TTGGGTCCCTCGACTCCTAT |
| β-actin (rat) | GTAAAGACCTCTATGCCAACA | GGACTCATCGTACTCCTGCT |
1.6.6 Western blotting
采用Western blotting检测cleaved caspase-3、α-SMA、PPARα表达情况。提取各组细胞总蛋白,采用BCA蛋白定量试剂盒检测蛋白浓度。通过SDS-PAGE、转膜、一抗二抗孵育、底物化学发光显色,采用Image J软件对条带进行定量分析。
1.7 RNA干扰序列
靶向大鼠Ppara特异性干扰小RNA(small interfering RNA,siRNA)和非特异性siRNA(NC-siRNA)均购自上海吉玛基因股份有限公司。特异性siRNA分别为siRNA-1(F:GGCGAACUAUUCGG-CUAAATT;R:UUUAGCCGAAUAGUUCGCCTT)、siRNA-2(F:GACCUGGAAAGUCCCUUAUTT;R:AUAAGGGACUUUCCAGGUCTT)、siRNA-3(F:C-CCGAGAGUUCCUAAAGAATT;R:UUCUUUAGG-AACUCUCGGGTT),选出转染效率最高的siRNA用于后续实验。
1.8 转染与细胞培养
取对数生长期的肾小管上皮细胞,用含10%胎牛血清的DMEM培养基配成单细胞悬液,计数,接种至6孔板中,于37 ℃、5% CO2细胞培养箱中过夜预培养。待融合度达60%~70%,选择合适的转染浓度,按照Lipofectamine 3000试剂盒说明书转染siRNA或NC-siRNA。转染48 h后,NC-AA1组和siRNA-AA1组加入40 μmol/L AA1处理24 h;NC-AA1+ICA组和siRNA-AA1+ICA组先提前1 h加入10-4 mol/L淫羊藿苷,然后加入40 μmol/L AA1处理细胞24 h;NC-DMSO组和siRNA-DMSO组加入等体积DMSO。
1.9 统计学分析
实验数据采用GraphPad Prism 6软件处理。分析定量资料是否符合正态性及方差齐性:若符合正态性和方差齐性,以
2 结果
2.1 淫羊藿苷预防AKI-CKD小鼠模型的肾功能损伤
图1
图1
马兜铃酸诱导的AKI-CKD小鼠模型的肾功能损伤以及淫羊藿苷的预防作用
Note: A. The grouping diagram of the AKI-CKD mice model induced by AA1 and intervened by ICA. B. Blood urea nitrogen (BUN) after 2 weeks or 4 weeks. C. Serum creatinine (Scr) after 2 weeks or 4 weeks. ip—intraperitoneal injection. ①P=0.000, ②P=0.001, ③P=0.003, ④P=0.007, ⑤P=0.006.
Fig 1
Renal function injury in the AA1-induced AKI-CKD murine model and preventive effect of ICA
2.2 淫羊藿苷预防AKI-CKD小鼠模型的病理损伤
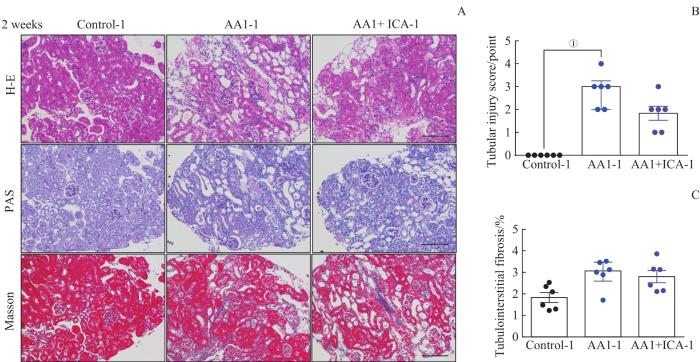
2.2.1 2周急性期结束时小鼠的肾脏病理改变
为进一步明确马兜铃酸诱导AKI-CKD模型中不同阶段肾脏病理结构的变化,以及淫羊藿苷对该模型的保护作用,我们在2周急性期结束时对各组小鼠行肾穿刺病理活检(图2)。与对照组-1比较,AA1组-1可见肾小管上皮细胞大量坏死,肾间质水肿伴大量炎症细胞浸润,肾小管损伤评分明显增高(均P<0.05)。与AA1组-1比较,AA1+ICA组-1肾小管损伤明显减轻,炎症细胞浸润减少,肾损伤评分降低,但差异无统计学意义。通过Masson染色,我们观察到各组小鼠肾组织无明显胶原沉积,证实该模型在2周时处于AKI阶段。
图2
图2
AKI-CKD小鼠模型急性期肾脏的病理损伤以及淫羊藿苷的预防效果
Note: A. Representative images of H-E, PAS and Masson staining kidney sections of AKI-CKD model after 2 weeks (×200). B. Renal tubular injury scores of the three groups after 2 weeks. C. Quantification of Masson trichrome staining after 2 weeks. Scale bar=100 μm. ①P=0.000.
Fig 2
Pathological changes of kidney in the AKI-CKD murine model at acute stage and preventive effect of ICA
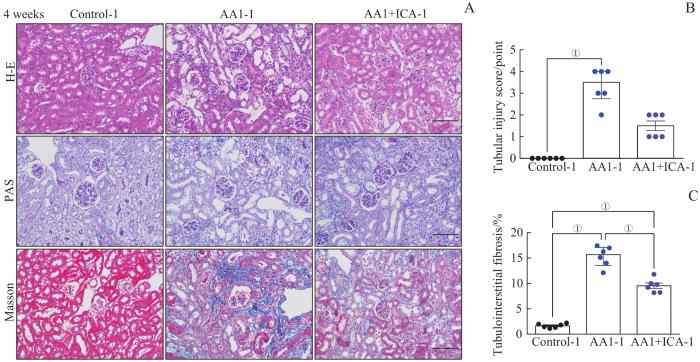
2.2.2 2周重塑期结束时小鼠的肾脏病理改变
图3
图3
AKI-CKD小鼠模型重塑期肾脏的病理损伤以及淫羊藿苷的预防效果
Note: A. Representative images of H-E, PAS and Masson staining kidney sections of AKI-CKD model after 4 weeks (×200). B. Renal tubular injury scores of the three groups after 4 weeks. C. Quantification of Masson trichrome staining after 4 weeks. Scale bar=100 μm. ①P=0.000.
Fig 3
Pathological changes of kidney in the AKI-CKD murine model at remodeling stage and preventive effect of ICA
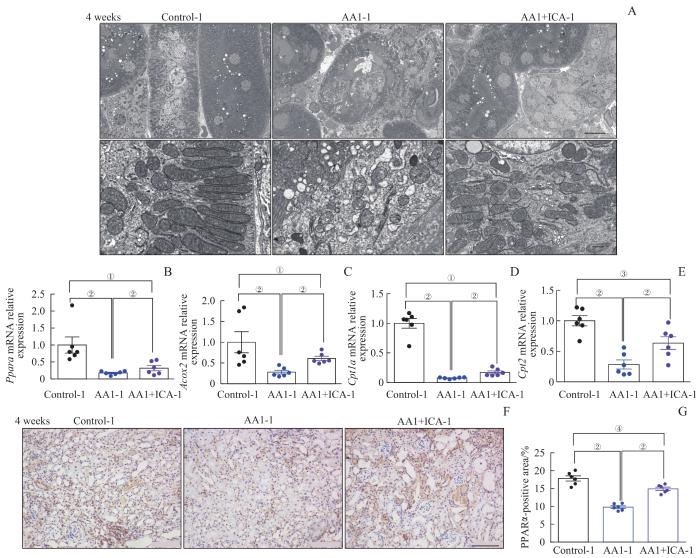
2.3 淫羊藿苷预防AKI-CKD小鼠模型肾小管上皮细胞的线粒体损伤
线粒体作为细胞能量代谢的重要场所,其功能异常与肾损伤密切相关,我们观察到重塑期结束时对照组-1的肾小管上皮细胞线粒体形态正常完整,而在AA1组-1小鼠肾脏可观察到线粒体形态不规则,内嵴稀疏断裂、排列紊乱、肿胀变形,膜融合,而AA1+ICA组-1以上病变减轻。结果提示,淫羊藿苷可以预防马兜铃酸诱导的AKI-CKD模型的线粒体结构损伤(图4A)。
图4
图4
AKI-CKD小鼠模型重塑期肾小管线粒体结构损伤与相关mRNA的表达水平以及淫羊藿苷的预防效果
Note: A. Representative electron microscopic images of mitochondria in renal tubular epithelial cells after 4 weeks (above ×2 500, scale bar=10 μm; below ×25 000, scale bar=500 nm). B‒E. Relative mRNA expression levels of Ppara (B), Acox2 (C), Cpt1a (D) and Cpt2 (E) in renal tissues after 4 weeks. F. Representative images of kidney sections with immunohistochemical staining PPARα after 4 weeks (×200). G. Quantification of PPARα-positive areas in kidney sections after 4 weeks (scale bar=100 μm). ①P=0.001, ②P=0.000, ③P=0.002, ④P=0.007.
Fig 4
Mitochondrial structural damage and expression levels of related mRNAs in the AKI-CKD murine model at remodeling stage and preventive effect of ICA
当肾小管上皮细胞损伤时,肾小管的主要线粒体代谢途径——脂肪酸氧化代谢通路明显被抑制。为进一步检测线粒体脂肪酸氧化代谢通路相关基因mRNA表达变化,本研究检测了4周时各组Ppara、Cpt1a、Cpt2、Acox2 mRNA的表达水平。与对照组-1比较,AA1组-1肾脏组织Ppara和脂肪酸氧化代谢酶Cpt1a、Cpt2、Acox2的表达量均显著降低(均P<0.05);与AA1组-1相比,AA1+ICA组-1以上基因表达量均显著上调(均P<0.05,图4B~E)。PPARα在肾脏脂肪酸氧化的调节中起关键作用,是直接转录调控细胞内脂质代谢的关键转录因子,能调控脂肪酸氧化代谢酶包括Cpt1a、Cpt2、Acox2等多个基因的表达。通过PPARα免疫组织化学(组化)染色,我们发现4周时AA1组-1小鼠PPARα阳性细胞面积较对照组-1显著减少,而AA1+ICA组-1较AA1组-1阳性面积显著增加(均P<0.05,图4F、G)。
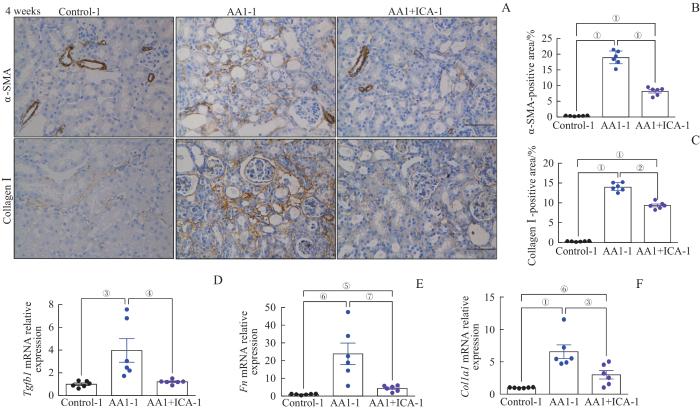
2.4 淫羊藿苷预防AKI-CKD小鼠模型的肾纤维化
图5
图5
AKI-CKD小鼠模型重塑期的肾纤维化以及淫羊藿苷的预防作用
Note: A. Representative images of kidney sections with immunohistochemical staining of α-SMA and collagen Ⅰ after 4 weeks (×200). B. Quantification of α-SMA-positive areas in kidney sections after 4 weeks. C. Quantification of collagen Ⅰ-positive areas in kidney sections after 4 weeks. D‒F. Relative mRNA expression levels of Tgfb1 (D), Fn (E) and Col1a1 (F) in renal tissues after 4 weeks. Scale bar=50 μm. ①P=0.000, ②P=0.044, ③P=0.008, ④P=0.014, ⑤P=0.007, ⑥P=0.001, ⑦P=0.003.
Fig 5
Renal fibrosis in the AKI-CKD murine model at remodeling stage and preventive effect of ICA
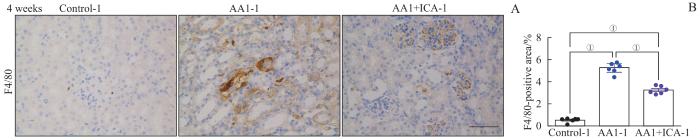
2.5 淫羊藿苷预防AKI-CKD小鼠模型肾脏的巨噬细胞浸润
目前研究表明炎症是促成AKI向CKD转化的核心因素之一,主要表现为炎症细胞的浸润和炎症介质的分泌。肾脏的炎症效应细胞主要有巨噬细胞、T淋巴细胞等。F4/80是小鼠巨噬细胞的标志物。通过F4/80免疫组化染色,可观察各组肾脏巨噬细胞的浸润情况。结果显示,重塑期结束时,AA1组-1 F4/80阳性面积较对照组-1显著增大,而AA1+ICA组-1的F4/80阳性面积较AA1组-1显著减小(均P<0.05,图6)。
图6
图6
AKI-CKD小鼠模型重塑期肾脏巨噬细胞浸润及淫羊藿苷的预防作用
Note: A. Representative images of kidney sections with immunohistochemical staining of F4/80 after 4 weeks. B. Quantification of F4/80 positive areas in kidney sections after 4 weeks. Scale bar=50 μm. ①P=0.000.
Fig 6
Renal macrophage infiltration in the AKI-CKD murine model at remodeling stage and preventive effect of ICA
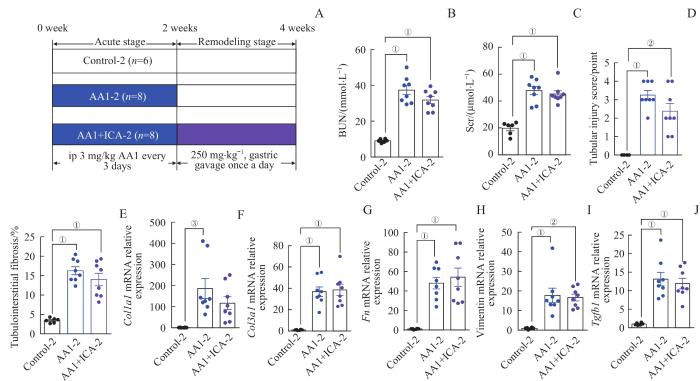
2.6 AKI-CKD小鼠模型急性期结束给予淫羊藿苷对肾脏的影响
图7
图7
AKI-CKD小鼠模型急性期结束给予淫羊藿苷对小鼠肾脏的影响
Note: A. The grouping diagram of the AKI-CKD mice model induced by AA1 and intervened by ICA. B. BUN after 4 weeks. C. Scr after 4 weeks. D. Renal tubular injury scores of the three groups after 4 weeks. E. Quantification of Masson trichrome staining after 4 weeks. F‒J. Relative mRNA expression levels of Col1a1 (F), Col3a1 (G), Fn (H), vimentin (I) and Tgfb1 (J)
Fig 7
Effect of ICA on kidney of the AKI-CKD murine model at the end of acute phase
2.7 淫羊藿苷通过保护肾小管上皮细胞抑制肾成纤维细胞活化
为进一步明确淫羊藿苷是否通过保护肾小管上皮细胞,抑制肾脏成纤维细胞合成细胞外基质,我们予AA1和淫羊藿苷共同干预肾脏上皮细胞株后,与肾脏成纤维细胞株共培养。结果发现,与DMSO组比较,AA1组肾小管上皮细胞的脂质代谢核心转录因子Ppara mRNA表达显著下降(P<0.05);与AA1组比较,AA1+ICA组Ppara mRNA表达上调(P<0.05,图8A)。AA1组肾小管上皮细胞炎症及纤维化相关的Tnfa、Ctgf、Tgfb1 mRNA表达水平亦显著高于DMSO组(均P<0.05);AA1+ICA组这3个mRNA表达水平较AA1组有下降趋势,但差异无统计学意义(图8B~D)。检测上皮细胞的凋亡相关蛋白cleaved caspase-3表达发现:与DMSO组比较,AA1组cleaved caspase-3表达显著升高(P<0.05);AA1+ICA组cleaved caspase-3表达与AA1组比较显著下降(P<0.05,图8E)。同时还检测了肾脏成纤维细胞细胞外基质相关基因表达的变化:与DMSO组比较,AA1组Fn和Col1a1 mRNA表达均显著升高(均P<0.05);AA1+ICA组Fn mRNA水平较AA1组显著降低(P<0.05),而Col1a1 mRNA表达水平下降,但差异无统计学意义(图8F、G)。Western blotting结果发现,AA1组成纤维细胞的α-SMA表达较DMSO组和AA1+ICA组均显著升高(均P<0.05,图8H)。
图8
图8
淫羊藿苷通过保护肾小管上皮细胞抑制肾成纤维细胞活化
Note: A‒D. Relative mRNA expression levels of Ppara (A), Tnfa (B), Tgfb1 (C) and Ctgf (D) in renal tubular epithelial cells. E.
Fig 8
ICA inhibiting renal fibroblast activation through protecting renal tubular epithelial cells
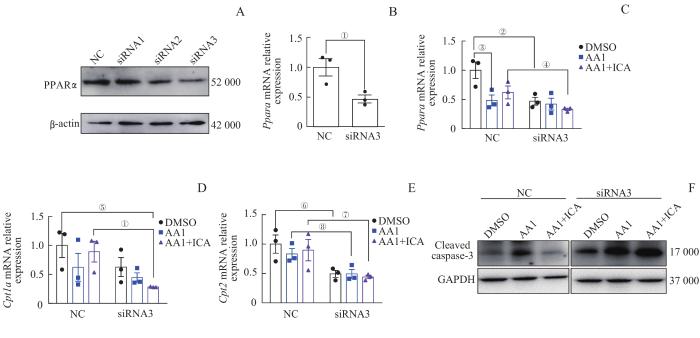
2.8 淫羊藿苷可能通过PPARα发挥对肾小管上皮细胞的保护作用
为进一步明确淫羊藿苷对肾小管上皮细胞保护作用的可能机制,采用Ppara特异性siRNA转染肾小管上皮细胞。从蛋白和mRNA水平检测发现,siRNA3可有效敲低肾小管上皮细胞中PPARα的表达(图9A、B)。与NC-AA1+ICA组比较,siRNA3-AA1+ICA组Ppara及其下游Cpt1a、Cpt2 mRNA表达水平均下降(图9C~E)。Western blotting分析发现,NC-AA1+ICA组cleaved caspase-3较NC-AA1组减少,但敲低Ppara后,siRNA3-AA1+ICA组cleaved caspase-3表达与siRNA3-AA1组无明显变化,且高于NC-AA1+ICA组(图9F)。以上结果提示,敲低肾小管上皮细胞Ppara后,淫羊藿苷对抗马兜铃酸的预防保护作用减弱。
图9
图9
敲低 Ppara 后淫羊藿苷对肾小管上皮细胞脂肪酸氧化代谢相关基因及凋亡蛋白表达水平的影响
Note: A. Western blotting analysis of PPARα protein expression in epithelial cells after siRNAs intervention. B. Relative mRNA expression level of Ppara in epithelial cells after siRNA3 intervention. C‒E. Relative mRNA expression levels of Ppara (C), Cpt1a (D)
Fig 9
Effect of ICA on expression levels of fatty acid oxidative metabolism-related mRNAs and the apoptotic protein in renal tubular epithelial cells after knocking down Ppara
3 讨论
淫羊藿苷是中药淫羊藿的主要活性成分[19]。近来多项研究表明淫羊藿苷可能通过线粒体发挥其保护作用。淫羊藿苷可缓解缺血再灌注引起的线粒体氧化应激损伤[20],并能调节线粒体跨膜电位,上调细胞质中细胞色素C含量[21]。淫羊藿苷可通过保护线粒体抑制心肌细胞及肾脏足细胞凋亡[22-23]。本次研究发现,在马兜铃酸诱导的经典AKI-CKD模型中,预防性给予淫羊藿苷对肾小管上皮细胞线粒体结构具有保护作用,并可上调脂肪酸氧化代谢通路表达,减少马兜铃酸引起的肾小管上皮细胞凋亡蛋白cleaved caspase-3的表达,从而改善急性期和重塑期的小鼠肾功能和肾脏病理损伤,减少巨噬细胞浸润及肾间质纤维化的发展。
而且此次研究发现淫羊藿苷预防性给药可以通过减轻AKI肾脏损伤,延缓或抑制AKI向CKD的转化;而在急性期结束后给药,淫羊藿苷对肾脏无明显保护作用。提示淫羊藿苷可能主要发挥预防性保护作用。
总之,此次研究初步揭示预防性给予淫羊藿苷可能通过改善线粒体脂肪酸氧化代谢通路,尤其可能通过PPARα,抑制肾小管上皮细胞凋亡,减少促炎因子和促纤维化因子的分泌,减少巨噬细胞浸润,并抑制肌成纤维细胞活化,减轻AKI肾脏损伤,抑制肾间质纤维化发生,从而发挥预防AKI向CKD转化的保护作用。
作者贡献声明
谢林、程烨、郑琦敏参与实验设计与操作,顾向晨、谢林、程烨参与论文的写作,顾向晨、谢静远、王怡、付莉莉、梅长林参与课题设计与讨论,顾向晨、谢林、程烨、张熙、陈敏参与数据分析和论文修改。所有作者均阅读并同意了最终稿件的提交。
The experiments were performed by XIE Lin, CHENG Ye and ZHENG Qimin. The manuscript was drafted by GU Xiangchen, XIE Lin and CHENG Ye. GU Xiangchen, XIE Jingyuan, WANG Yi, FU Lili, MEI Changlin participated in the project design and discussion. GU Xiangchen, XIE Lin, CHENG Ye, ZHANG Xi, CHEN Min participated in the data analysis and paper revision. All the authors have read the last version of paper and consented for submission.
利益冲突声明
所有作者声明不存在利益冲突。
All authors disclose no relevant conflict of interests.
参考文献