
上海交通大学学报(医学版) ›› 2025, Vol. 45 ›› Issue (8): 969-980.doi: 10.3969/j.issn.1674-8115.2025.08.004
收稿日期:2025-02-17
接受日期:2025-04-03
出版日期:2025-08-28
发布日期:2025-08-26
通讯作者:
王 颖,教授,博士;电子信箱:ywangssmu@shsmu.edu.cn。基金资助:
JIANG Qianyu, YAO Chengcheng, JI Ping, WANG Ying( )
)
Received:2025-02-17
Accepted:2025-04-03
Online:2025-08-28
Published:2025-08-26
Contact:
WANG Ying,E-mail:ywangssm@shsmu.edu.cn.Supported by:摘要:
目的·探索甲基丙烯酰化透明质酸(hyaluronic acid methacryloyl,HAMA)水凝胶在皮肤创面愈合中的作用,解析创面局部微环境特征。方法·构建小鼠全皮层切除模型,随机分为对照组(n=3)和HAMA组(n=3);HAMA组在创面覆盖100 μL HAMA水凝胶,对照组在创面覆盖100 μL苯基-2,4,6-三甲基苯甲酰基次膦酸锂(LAP),均用紫外灯照射20 s,分别在第0天、第3天、第7天、第10天和第14天对剩余创面进行测量。通过测量剩余创面面积和苏木精-伊红染色(H-E染色)分析HAMA水凝胶对创面愈合的作用;利用单细胞测序技术分析第14天创面局部皮肤的细胞特征谱,通过免疫组织荧光技术检测创面局部Ⅰ型胶原、Ⅲ型胶原、F4/80、CD206和CD86的表达水平;RT-qPCR检测与HAMA水凝胶共孵育24 h的小鼠巨噬细胞系Raw264.7中Arg1、Nos2、Itgam和Itgb2的mRNA表达水平。利用Seurat软件包对小鼠第14天创面局部皮肤的成纤维细胞和巨噬细胞进行聚类分析,并利用CellChat软件包分析成纤维细胞和巨噬细胞的相互通信状况。结果·HAMA组小鼠皮肤创面愈合的速度显著快于对照组,第14天HMAM组小鼠创面已经愈合,而对照组的创面尚未完全愈合。单细胞测序分析显示,HAMA组中Col3a1高表达的成纤维细胞亚群比例(90.2%)高于对照组(79.8%),而Col1a1高表达的成纤维细胞亚群比例(5.7%)低于对照组(15.9%)。免疫荧光分析结果证实HAMA组创面局部Ⅲ型胶原水平高于对照组(P=0.035),而Ⅰ型胶原水平则低于对照组(P=0.044)。HAMA组小鼠与对照组小鼠创面局部巨噬细胞的比例没有明显差异,但单细胞测序分析结果和HAMA水凝胶体外处理巨噬细胞Raw264.7后均显示Arg1表达水平升高(P<0.001),Nos2表达水平降低(P<0.001),同时HAMA组小鼠创面部位巨噬细胞表达较高水平的CD206(P=0.042),表达较低水平的CD86(P=0.011)。CellChat分析结果显示,相较于对照组,HAMA组小鼠创面部位巨噬细胞和特定成纤维细胞亚群间相互通信的强度增大。结论·HAMA水凝胶处理后的微环境有利于皮肤创面愈合,创面愈合组织局部汇聚更多的抑炎性巨噬细胞和分泌Ⅲ型胶原的成纤维细胞。
中图分类号:
姜芊羽, 姚程程, 季萍, 王颖. HAMA水凝胶促进皮肤创面愈合的组织局部微环境特征[J]. 上海交通大学学报(医学版), 2025, 45(8): 969-980.
JIANG Qianyu, YAO Chengcheng, JI Ping, WANG Ying. Microenvironmental profiles of wound tissues with accelerated healing properties by HAMA hydrogel[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2025, 45(8): 969-980.
| Name | Forward (5'→3' ) | Reverse (3'→5' ) |
|---|---|---|
| Arg1 | CTCCAAGCCAAAGTCCTTAGAG | AGGAGCTGTCATTAGGGACATC |
| Nos2 | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
| Itgam | CCATGACCTTCCAAGAGAATGC | ACCGGCTTGTGCTGTAGTC |
| Itgb2 | AGGAGCATCGCTAATCCTGAG | CCTGGTCGCAAGTAAAGTGTC |
| Gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
表1 引物序列
Tab 1 Primer sequences
| Name | Forward (5'→3' ) | Reverse (3'→5' ) |
|---|---|---|
| Arg1 | CTCCAAGCCAAAGTCCTTAGAG | AGGAGCTGTCATTAGGGACATC |
| Nos2 | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
| Itgam | CCATGACCTTCCAAGAGAATGC | ACCGGCTTGTGCTGTAGTC |
| Itgb2 | AGGAGCATCGCTAATCCTGAG | CCTGGTCGCAAGTAAAGTGTC |
| Gapdh | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
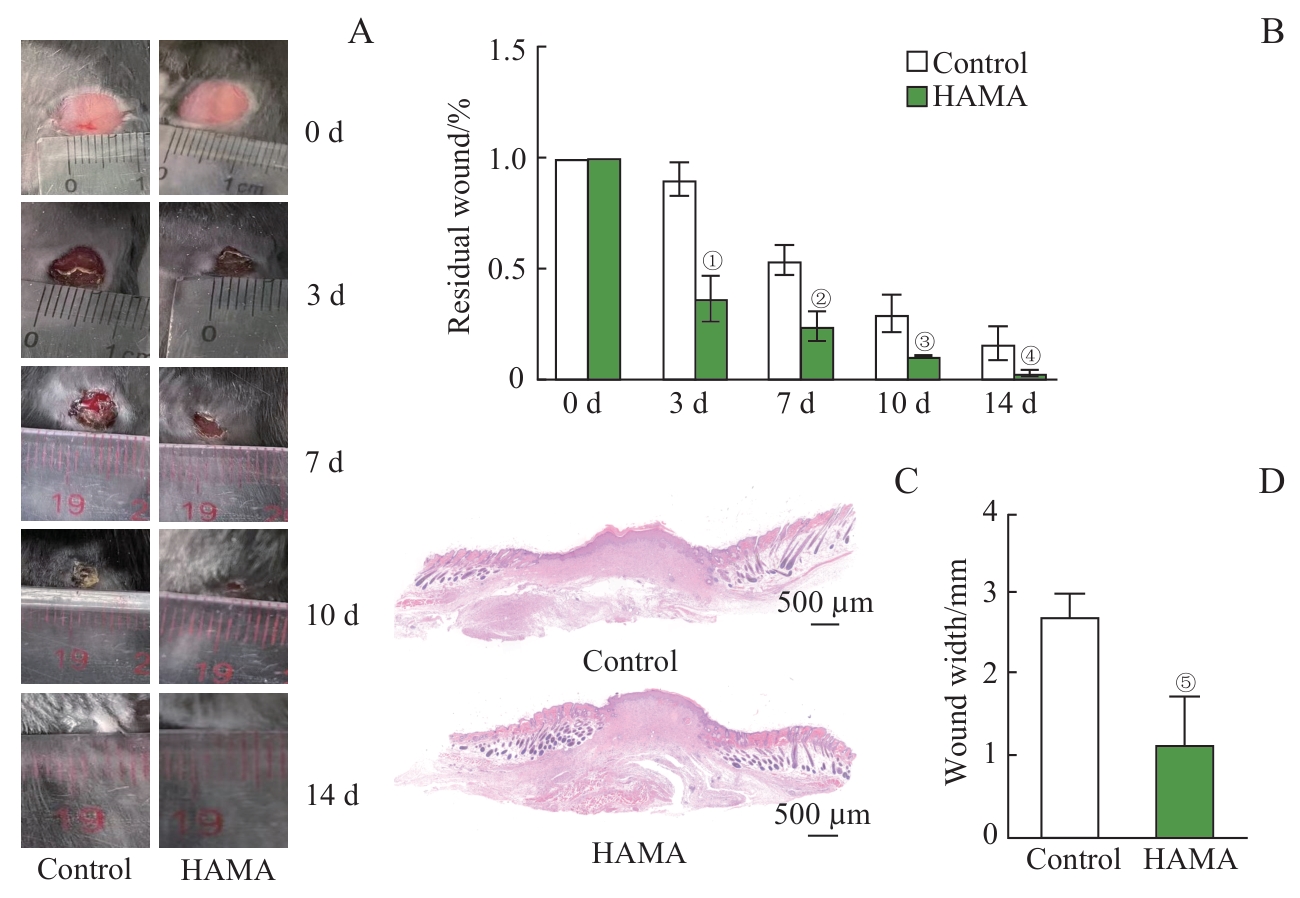
图1 HAMA水凝胶对创面愈合的促进作用Note: A. Residual wound area in the control group and HAMA group. B. Statistical graph of residual wound area in the control group and HAMA group (n=3). C. H-E staining of the wound areas in the control group and HAMA group on day 14. D. Statistical graph of wound width (n=3). ①P=0.002, ②P=0.006,③P=0.017, ④P=0.039, ⑤P=0.013, compared with the control group.
Fig 1 Promotive effects of HAMA hydrogel on wound healing
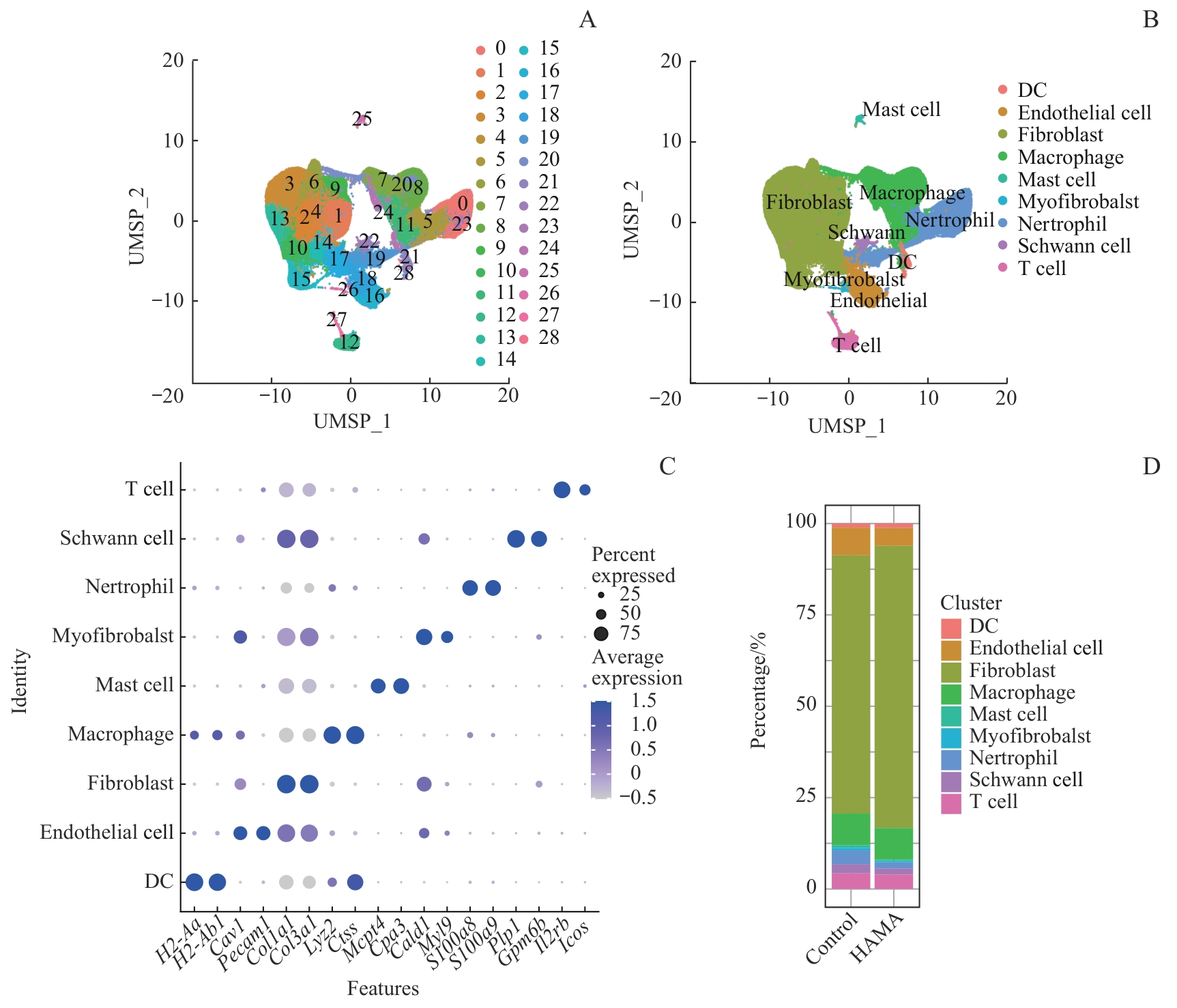
图2 单细胞测序分析小鼠皮肤创面部位细胞组成Note: A. Schematic diagram of cell clusters in the control group and HAMA group. B. Identified cell types at wound site in the control group and HAMA group. C. Marker genes for various cell types. D. Percentage of various cell types in the control group and HAMA group.
Fig 2 Single-Cell RNA sequencing analysis of cell composition at mouse skin wound sites
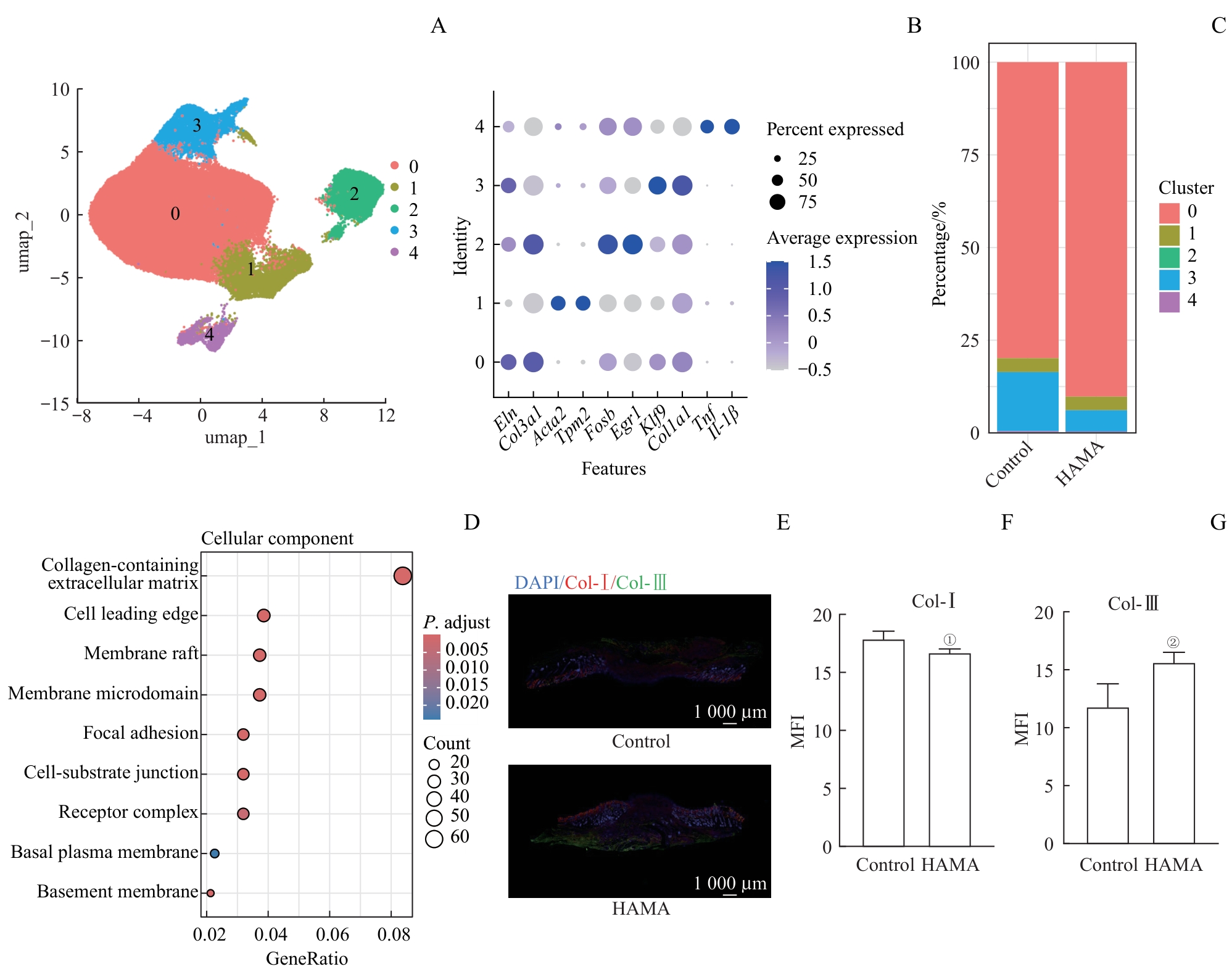
图3 创面部位成纤维细胞的组成和功能分析Note: A. Subsets of fibroblasts. B. Marker genes of fibroblast subsets. C. Percentage of fibroblast subsets in the control group and HAMA group. D. GO enrichment analysis of DEGs between fibroblasts in the control group and HAMA group. E. Immunofluorescence of collagen in the wound area of mice. F/G. Mean fluorescence intensity of Type Ⅰ collagen (F) and Type Ⅲ collagen (G) in the wound area of mice (n=3). ①P=0.044, ②P=0.035, compared with the control group. MFI—mean fluorescence intensity.
Fig 3 Analysis on composition and function of fibroblasts in wound sites
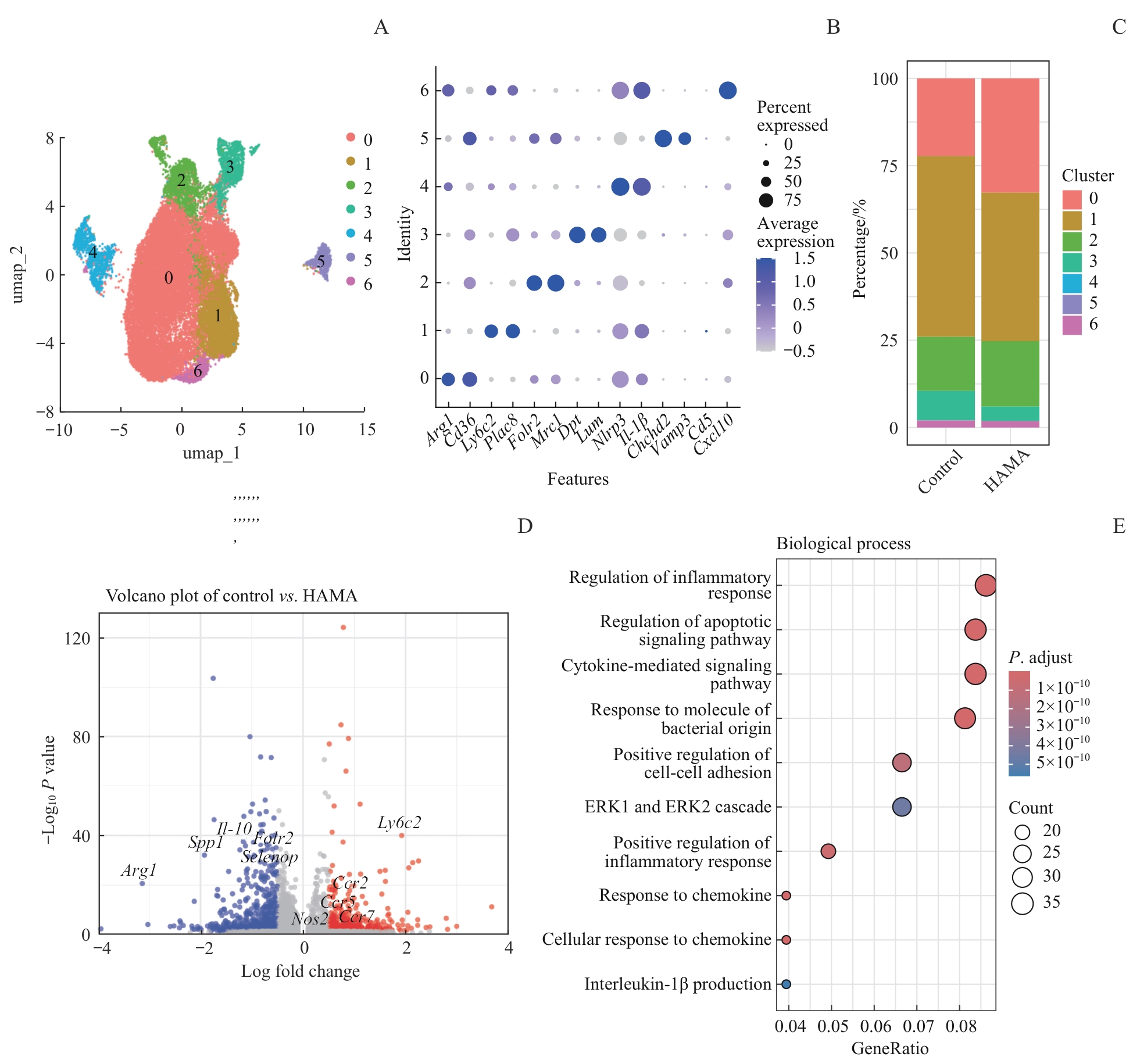
图4 创面部位巨噬细胞的组成和功能分析Note: A. Subsets of macrophages. B. Marker genes of macrophage subsets. C. Percentage of macrophage subsets in the control group and HAMA group. D. Volcano plot of DEGs in all the macrophages between the control group and HAMA group. E. GO enrichment analysis of DEGs between macrophages in the control group and HAMA group.
Fig 4 Analysis on composition and function of macrophages in wound sites
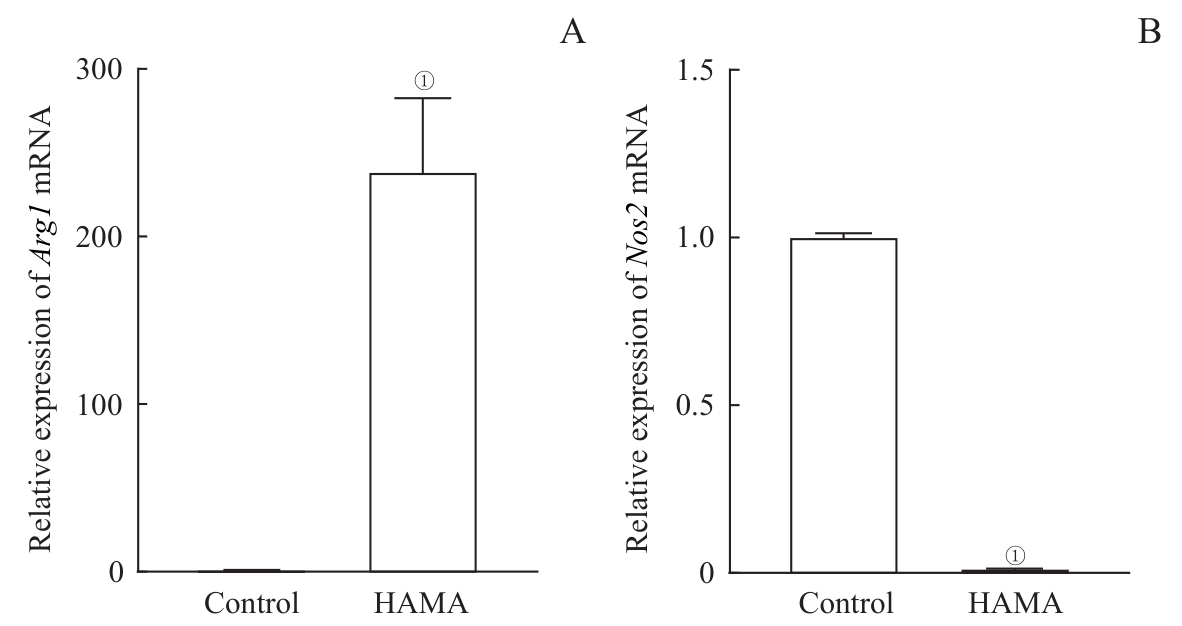
图5 HAMA水凝胶对巨噬细胞极化特征基因表达的影响Note: A. mRNA expression of Arg1. B. mRNA expression of Nos2. ①P<0.001, compared with the control group.
Fig 5 Effects of HAMA hydrogel on the expression of polarization-related genes in the macrophages
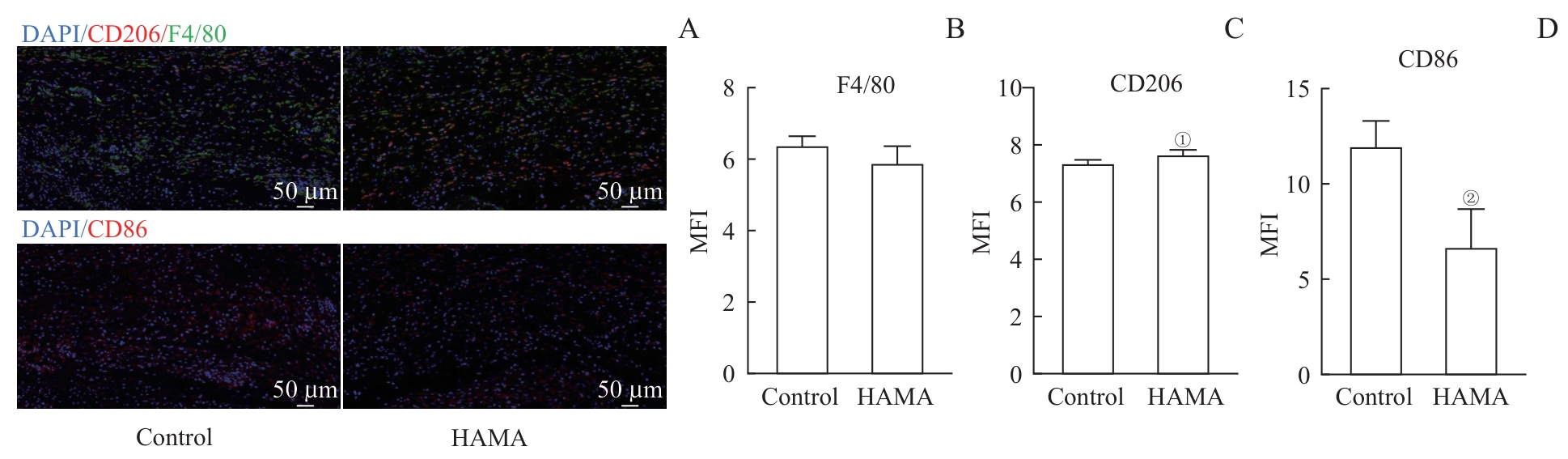
图6 HAMA水凝胶处理创面局部巨噬细胞的极化特征分析Note: A. Immunofluorescence of macrophages at the wound site of mice. B-D. Mean fluorescence intensity of macrophages F4/80 (B), CD206 (C) and CD86 (D) at the wound site. ①P=0.042, ②P=0.011, compared with the control group.
Fig 6 Analysis of local macrophage polarization characteristics in wound sites treated with HAMA hydrogel
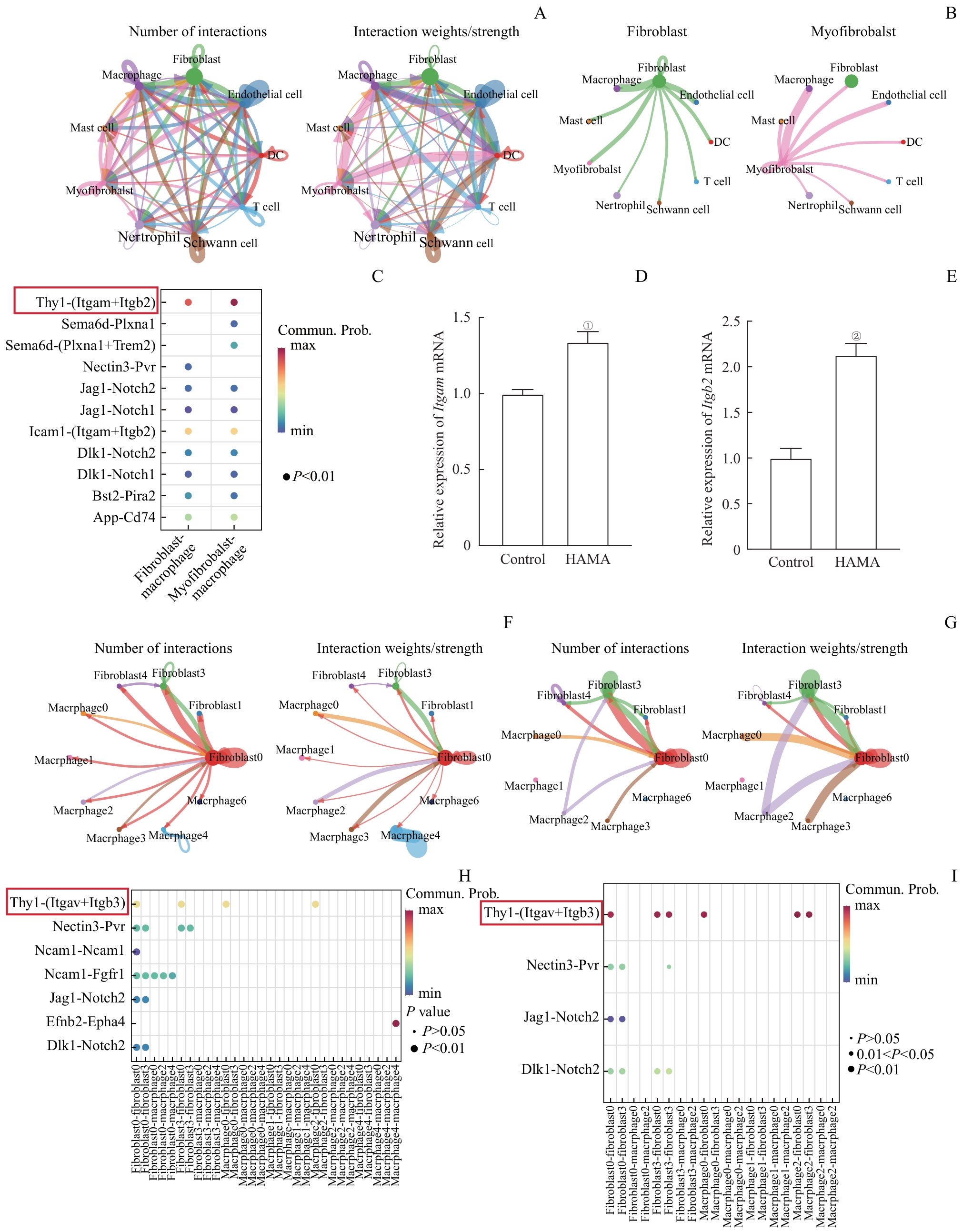
图7 创面组织成纤维细胞和巨噬细胞相互作用模式Note: A. The number and strength of interactions among different cell types in the wound tissue (Larger circles represent a greater number of cells, and thicker lines indicate stronger interaction strength). B. Interactions between fibroblasts and myofibroblasts with macrophages. C. Receptor-ligand pairs for interactions between fibroblasts and myofibroblasts with macrophages. D/E. mRNA expression levels of Itgam (D) and Itgb2 (E) in Raw264.7 cells after 24 h incubation with solidified HAMA hydrogel or control treatment. F/G. The number and strength of interactions among various subgroups in the wound sites from the control group (F) and HAMA group(G). H/I. Receptor-ligand pairs involved in interactions among various subgroups in wound sites from the control group(H) and HAMA group (I). ①P=0.001, ②P<0.001, compared with the control group.
Fig 7 Interaction patterns between fibroblasts and macrophages in wound tissues
| [1] | MASCHALIDI S, MEHROTRA P, KEÇELI B N, et al. Targeting SLC7A11 improves efferocytosis by dendritic cells and wound healing in diabetes[J]. Nature, 2022, 606(7915): 776-784. |
| [2] | LU Y Z, NAYER B, SINGH S K, et al. CGRP sensory neurons promote tissue healing via neutrophils and macrophages[J]. Nature, 2024, 628(8008): 604-611. |
| [3] | GURTNER G C, WERNER S, BARRANDON Y, et al. Wound repair and regeneration[J]. Nature, 2008, 453(7193): 314-321. |
| [4] | FISCHER A, WANNEMACHER J, CHRIST S, et al. Neutrophils direct preexisting matrix to initiate repair in damaged tissues[J]. Nat Immunol, 2022, 23(4): 518-531. |
| [5] | GALLI S J, BORREGAARD N, WYNN T A. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils[J]. Nat Immunol, 2011, 12(11): 1035-1044. |
| [6] | SINHA S, SPARKS H D, LABIT E, et al. Fibroblast inflammatory priming determines regenerative versus fibrotic skin repair in reindeer[J]. Cell, 2022, 185(25): 4717-4736.e25. |
| [7] | JIANG D S, GUO R J, MACHENS H G, et al. Diversity of fibroblasts and their roles in wound healing[J]. Cold Spring Harb Perspect Biol, 2023, 15(3): a041222. |
| [8] | ZHAO L, FENG Z P, LYU Y, et al. Electroactive injectable hydrogel based on oxidized sodium alginate and carboxymethyl chitosan for wound healing[J]. Int J Biol Macromol, 2023, 230: 123231. |
| [9] | SETHI S, THAKUR S, SHARMA D, et al. Malic acid cross-linked chitosan based hydrogel for highly effective removal of chromium (Ⅵ) ions from aqueous environment[J]. React Funct Polym, 2022, 177: 105318. |
| [10] | HELMECKE T, HAHN D, MATZKE N, et al. Inflammation-controlled anti-inflammatory hydrogels[J]. Adv Sci (Weinh), 2023, 10(7): e2206412. |
| [11] | CHENG L, CAI Z W, YE T J, et al. Injectable polypeptide-protein hydrogels for promoting infected wound healing[J]. Adv Funct Materials, 2020, 30(25): 2001196. |
| [12] | XU Z, LIU G, LIU P, et al. Hyaluronic acid-based glucose-responsive antioxidant hydrogel platform for enhanced diabetic wound repair [J]. Acta Biomater, 2022, 147: 147-157. |
| [13] | HONG S, YANG K, KANG B, et al. Hyaluronic acid catechol: a biopolymer exhibiting a pH-dependent adhesive or cohesive property for human neural stem cell engineering[J]. Adv Funct Materials, 2013, 23(14): 1774-1780. |
| [14] | SINGH A, CORVELLI M, UNTERMAN S A, et al. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid[J]. Nat Mater, 2014, 13(10): 988-995. |
| [15] | CAI Z X, ZHANG H B, WEI Y, et al. Reduction- and pH-sensitive hyaluronan nanoparticles for delivery of iridium (Ⅲ) anticancer drugs[J]. Biomacromolecules, 2017, 18(7): 2102-2117. |
| [16] | SHU X Z, LIU Y C, LUO Y, et al. Disulfide cross-linked hyaluronan hydrogels[J]. Biomacromolecules, 2002, 3(6): 1304-1311. |
| [17] | SERBAN M A, PRESTWICH G D. Synthesis of hyaluronan haloacetates and biology of novel cross-linker-free synthetic extracellular matrix hydrogels[J]. Biomacromolecules, 2007, 8(9): 2821-2828. |
| [18] | LIU B, KONG Y F, ALIMI O A, et al. Multifunctional microgel-based cream hydrogels for postoperative abdominal adhesion prevention[J]. ACS Nano, 2023, 17(4): 3847-3864. |
| [19] | ZHOU K, YANG C L, SHI K, et al. Activated macrophage membrane-coated nanoparticles relieve osteoarthritis-induced synovitis and joint damage[J]. Biomaterials, 2023, 295: 122036. |
| [20] | LEE J, KIM D, JANG C H, et al. Highly elastic 3D-printed gelatin/HA/placental-extract scaffolds for bone tissue engineering[J]. Theranostics, 2022, 12(9): 4051-4066. |
| [21] | LIU N B, ZHU S J, DENG Y Z, et al. Construction of multifunctional hydrogel with metal-polyphenol capsules for infected full-thickness skin wound healing[J]. Bioact Mater, 2022, 24: 69-80. |
| [22] | FARAHANI M, SHAFIEE A. Wound healing: from passive to smart dressings[J]. Adv Healthc Mater, 2021, 10(16): e2100477. |
| [23] | GAO S Y, CHEN T, WANG Z, et al. Immuno-activated mesenchymal stem cell living electrospun nanofibers for promoting diabetic wound repair[J]. J Nanobiotechnology, 2022, 20(1): 294. |
| [24] | WANG X, ZHAO D H, LI Y T, et al. Collagen hydrogel with multiple antimicrobial mechanisms as anti-bacterial wound dressing[J]. Int J Biol Macromol, 2023, 232: 123413. |
| [25] | PENG Z W, XUE H, LIU X, et al. Tough, adhesive biomimetic hyaluronic acid methacryloyl hydrogels for effective wound healing[J]. Front Bioeng Biotechnol, 2023, 11: 1222088. |
| [26] | AHMED M K, ZAYED M A, EL-DEK S I, et al. Nanofibrous ε-polycaprolactone scaffolds containing Ag-doped magnetite nanoparticles: physicochemical characterization and biological testing for wound dressing applications in vitro and in vivo[J]. Bioact Mater, 2021, 6(7): 2070-2088. |
| [27] | YAMASAKI S, ISHIKAWA E, SAKUMA M, et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells[J]. Nat Immunol, 2008, 9(10): 1179-1188. |
| [28] | CHEN J P, CHEN D F, CHEN J L, et al. An all-in-one CO gas therapy-based hydrogel dressing with sustained insulin release, anti-oxidative stress, antibacterial, and anti-inflammatory capabilities for infected diabetic wounds[J]. Acta Biomater, 2022, 146: 49-65. |
| [29] | FU Y J, SHI Y F, WANG L Y, et al. All-natural immunomodulatory bioadhesive hydrogel promotes angiogenesis and diabetic wound healing by regulating macrophage heterogeneity[J]. Adv Sci (Weinh), 2023, 10(13): e2206771. |
| [30] | PEÑA O A, MARTIN P. Cellular and molecular mechanisms of skin wound healing[J]. Nat Rev Mol Cell Biol, 2024, 25(8): 599-616. |
| [31] | HINZ B. Formation and function of the myofibroblast during tissue repair[J]. J Invest Dermatol, 2007, 127(3): 526-537. |
| [32] | WAN R, WEISSMAN J P, GRUNDMAN K, et al. Diabetic wound healing: the impact of diabetes on myofibroblast activity and its potential therapeutic treatments[J]. Wound Repair Regen, 2021, 29(4): 573-581. |
| [33] | YOUNESI F S, MILLER A E, BARKER T H, et al. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis[J]. Nat Rev Mol Cell Biol, 2024, 25(8): 617-638. |
| [34] | MOTZ K, LINA I, MURPHY M K, et al. M2 macrophages promote collagen expression and synthesis in laryngotracheal stenosis fibroblasts[J]. Laryngoscope, 2021, 131(2): E346-E353. |
| [35] | HE J H, FANG B, SHAN S Z, et al. Mechanical stretch promotes hypertrophic scar formation through mechanically activated cation channel Piezo1[J]. Cell Death Dis, 2021, 12(3): 226. |
| [36] | LI S Y, LI C, ZHANG Y T, et al. Targeting mechanics-induced fibroblast activation through CD44-RhoA-YAP pathway ameliorates crystalline silica-induced silicosis[J]. Theranostics, 2019, 9(17): 4993-5008. |
| [37] | AMUSO V M, HAAS M R, COOPER P O, et al. Fibroblast-mediated macrophage recruitment supports acute wound healing[J]. J Investig Dermatol, 2025, 145(7): 1781-1797.e8. |
| [38] | SHEN L Y, LI Y S, ZHAO H K. Fibroblast growth factor signaling in macrophage polarization: impact on health and diseases[J]. Front Immunol, 2024, 15: 1390453. |
| [39] | CHEN C, YANG J C, SHANG R Y, et al. Orchestration of macrophage polarization dynamics by fibroblast-secreted exosomes during skin wound healing[J]. J Invest Dermatol, 2025, 145(1): 171-184.e6. |
| [1] | 李景聪, 赵涵, 林巧雯, 孙宏翔, 苏冰, 伍宁波. FGF2对小鼠肠道间质细胞R-spondin 1表达的调控作用[J]. 上海交通大学学报(医学版), 2025, 45(8): 939-948. |
| [2] | 王琳, 徐萍, 张乔婷, 田军, 娄晓丽, 王静. 胱天蛋白酶募集域蛋白9在重症急性胰腺炎巨噬细胞M1极化中的作用[J]. 上海交通大学学报(医学版), 2025, 45(8): 981-989. |
| [3] | 韩龙传, 李悦, 邹智慧, 罗静, 李若伊, 张颖婷, 唐欣欣, 田丽红, 陆宇恒, 黄莺, 贺明, 付寅坤. 磷脂酰乙醇胺引起内质网应激促进巨噬细胞衰老及肝损伤[J]. 上海交通大学学报(医学版), 2025, 45(6): 693-704. |
| [4] | 黄英荷, 招冠钰, 孙阳, 侯鉴基, 左勇. 2型糖尿病创面愈合中巨噬细胞代谢调控的研究进展[J]. 上海交通大学学报(医学版), 2025, 45(6): 792-799. |
| [5] | 汤开然, 冯成领, 韩邦旻. 基于单细胞测序与转录组测序构建M2巨噬细胞基因相关的前列腺癌预后模型[J]. 上海交通大学学报(医学版), 2025, 45(5): 549-561. |
| [6] | 倪书奕, 姜钊, 汪中涛, 何树梅. 红景天苷对卡介苗感染的巨噬细胞免疫功能的影响[J]. 上海交通大学学报(医学版), 2025, 45(4): 426-433. |
| [7] | 马秀珍, 周妮, 郭思琪, 王源媛, 麦平. 大麻素受体1通过Gαi/o/RhoA信号通路促进急性肺损伤小鼠巨噬细胞M1极化[J]. 上海交通大学学报(医学版), 2025, 45(2): 161-168. |
| [8] | 梁乐斌, 陈慧芳, 赖淑静, 顾靓, 苏冰. 基于空间ATAC-seq技术的Apcmin/+小鼠结肠肿瘤表观特征分析[J]. 上海交通大学学报(医学版), 2025, 45(10): 1261-1270. |
| [9] | 张烨晟, 杨易静, 黄依雯, 施珑玙, 王曼媛, 陈思思. 肿瘤微环境免疫细胞调节肿瘤细胞耐药性的研究进展[J]. 上海交通大学学报(医学版), 2024, 44(7): 830-838. |
| [10] | 牛媛媛, 汪龙德, 胥文娟, 李正菊, 张瑞婷, 吴毓谦. 巨噬细胞M1/M2型极化在不同肝病中的作用研究进展[J]. 上海交通大学学报(医学版), 2024, 44(4): 509-517. |
| [11] | 张羽桐, 侯国俊, 沈南. 单细胞基因组学比较人诱导多能干细胞来源巨噬细胞与外周血来源巨噬细胞的异质性[J]. 上海交通大学学报(医学版), 2024, 44(12): 1477-1489. |
| [12] | 韩瑞, 吴倩, 刘丹, 程棣, 张盈, 倪嘉成, 康飘, 陈安然, 于淑洁, 方启晨, 李华婷. 超重肥胖青少年认知功能的改变及其与血清FGF21水平的关系[J]. 上海交通大学学报(医学版), 2024, 44(1): 87-97. |
| [13] | 刘思雨, 张磊. 七氟烷抑制新生小鼠前额叶皮质神经祖细胞向神经元分化发育[J]. 上海交通大学学报(医学版), 2023, 43(9): 1115-1130. |
| [14] | 宋文汀, 陶悦, 潘艺, 莫茜, 曹清. SIRT2通过组蛋白H4K8去乳酸化修饰调控巨噬细胞趋化功能[J]. 上海交通大学学报(医学版), 2023, 43(8): 1008-1016. |
| [15] | 吴淇琦, 汪豪, 林砺, 晏博, 张舒林. miR-185-5p通过抑制巨噬细胞自噬促进胞内分枝杆菌生长[J]. 上海交通大学学报(医学版), 2023, 43(6): 699-708. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||