
上海交通大学学报(医学版) ›› 2024, Vol. 44 ›› Issue (11): 1439-1446.doi: 10.3969/j.issn.1674-8115.2024.11.011
刘永慧1( ), 唐莉1, 梁泰刚1, 张健2(
), 唐莉1, 梁泰刚1, 张健2( ), 冯丽2(
), 冯丽2( )
)
收稿日期:2024-06-05
接受日期:2024-06-11
出版日期:2024-11-28
发布日期:2024-11-28
通讯作者:
张 健,电子信箱:jian.zhang@sjtu.deu.cn。#为共同通信作者。作者简介:刘永慧(1999—),女,硕士生;电子信箱:QLiuYongHui@163.com。
基金资助:
LIU Yonghui1( ), TANG Li1, LIANG Taigang1, ZHANG Jian2(
), TANG Li1, LIANG Taigang1, ZHANG Jian2( ), FENG Li2(
), FENG Li2( )
)
Received:2024-06-05
Accepted:2024-06-11
Online:2024-11-28
Published:2024-11-28
Contact:
ZHANG Jian, E-mail: jian.zhang@sjtu.edu.cn.Supported by:摘要:
SIRT6是组蛋白去乙酰化酶第三家族长寿蛋白中的一员,具备依赖于烟酰胺腺嘌呤二核苷酸的脱酰基酶活性和单ADP核糖基转移酶活性。SIRT6主要位于细胞核内,可通过核心调控基因组稳定性和相关基因表达,参与控制机体能量代谢和衰老在内的关键过程。鉴于其在维持细胞稳态和机体健康中的关键作用,SIRT6作为潜在的药物靶点引起人们对其靶向调节剂开发广泛的研究兴趣。药物激动长寿蛋白的活性可能治疗与年龄相关的疾病,如衰老、代谢综合征、炎症和生殖健康等。该文综述了SIRT6的结构特征、酶活性、生物学功能,以及SIRT6激动剂的作用机制、药理活性和临床应用。
中图分类号:
刘永慧, 唐莉, 梁泰刚, 张健, 冯丽. 长寿蛋白SIRT6在衰老和代谢中作用的研究进展[J]. 上海交通大学学报(医学版), 2024, 44(11): 1439-1446.
LIU Yonghui, TANG Li, LIANG Taigang, ZHANG Jian, FENG Li. Research progress in the role of SIRT6 in aging and metabolism[J]. Journal of Shanghai Jiao Tong University (Medical Science), 2024, 44(11): 1439-1446.
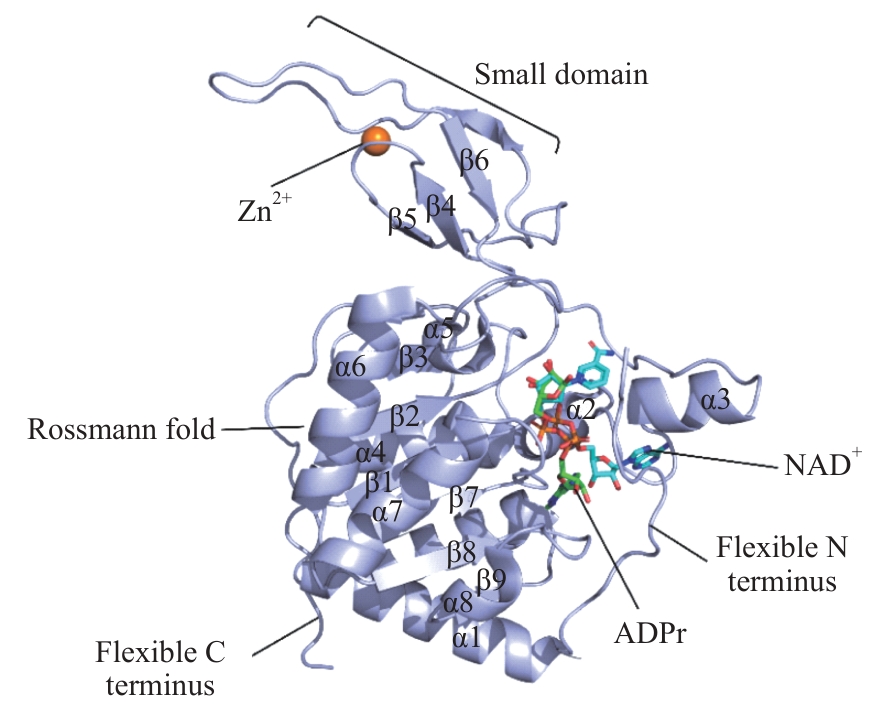
图1 人源SIRT6蛋白的二级结构Note: The structural composition of SIRT6 is basically divided into Rossmann fold structure, small structural domains (including the zinc finger module), C-terminal extension (CTE) and N-terminal extension (NTE). SIRT6 is shown as a purple cartoon structure with lake blue stick structures for NAD+ and green for ADP ribose.
Fig 1 Secondary structure of the human protein SIRT6
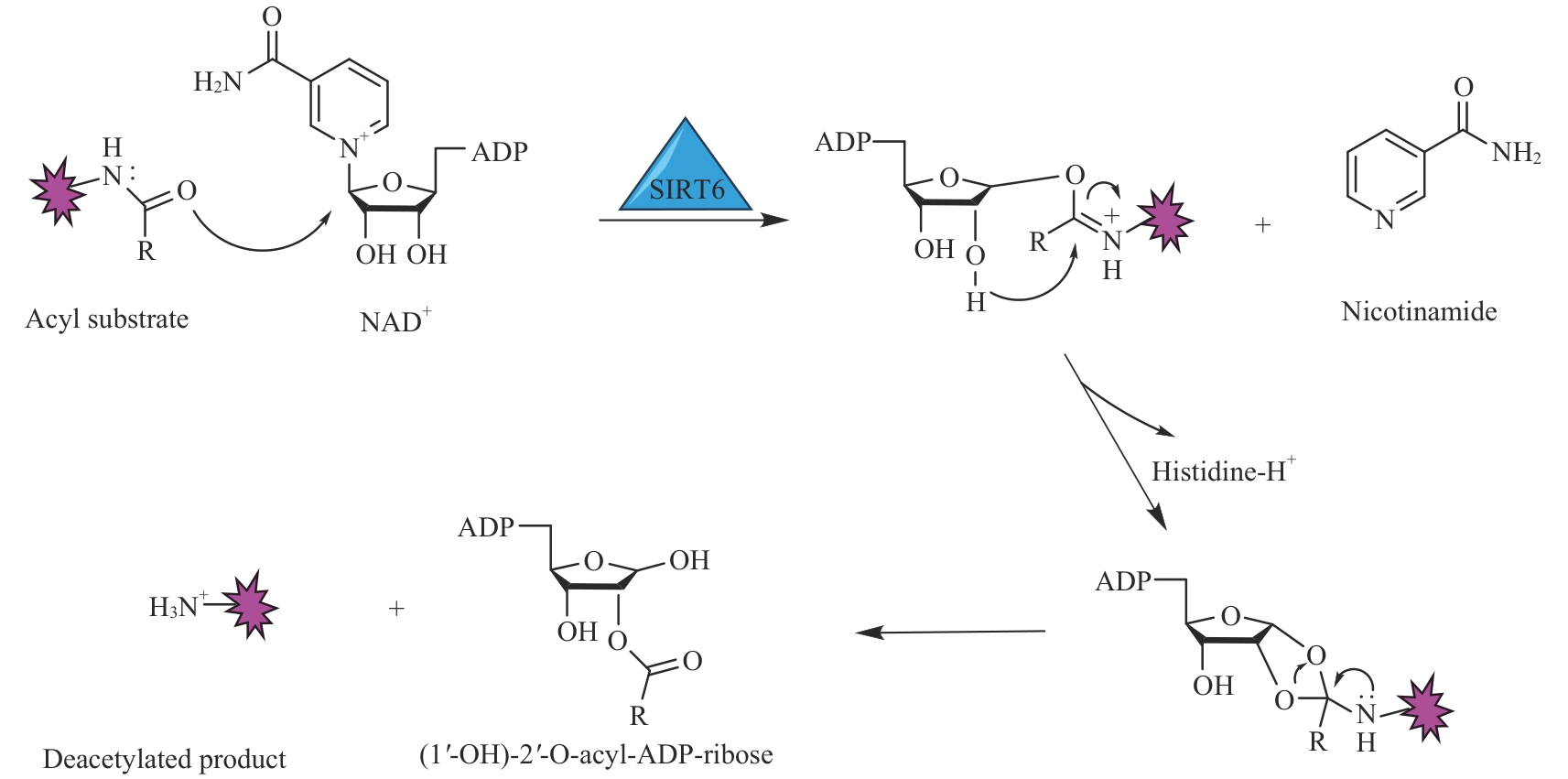
图2 SIRT6催化去酰化的分子机制Note: NAD+ acts as a cofactor to assist SIRT6 in removing acylation groups from substrate lysine residues, resulting in the generation of NAM, ADP ribose, and the deacetylated product.
Fig 2 Mechanism of SIRT6 catalyzing deacylation
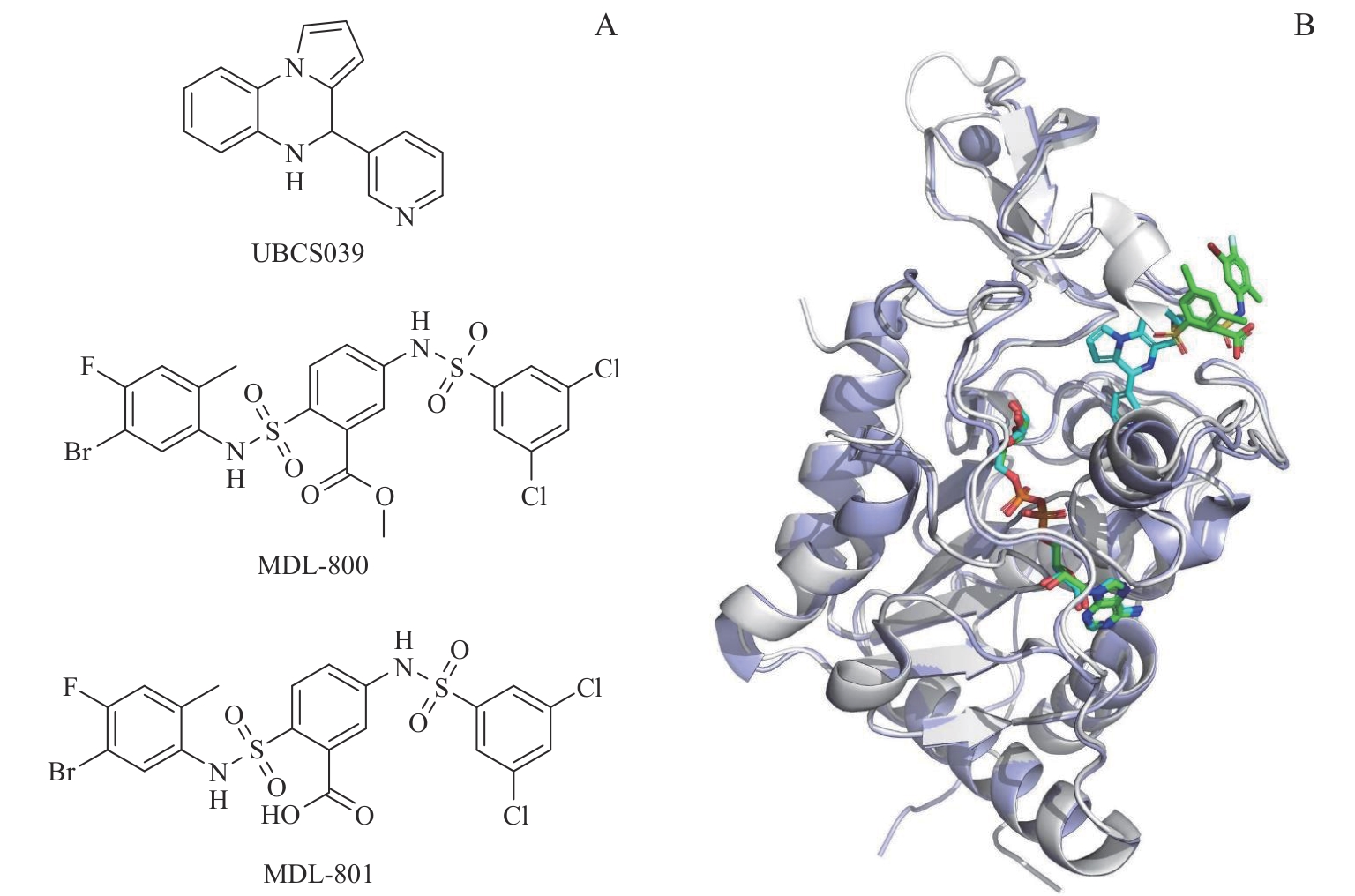
图3 SIRT6 激动剂的化学结构和结合模式Note: A. Chemical structures of SIRT6 agonist, MDL-800, MDL-801, and UBCS039. B. The binding modes of MDL-801 or UBCS039 with SIRT6. MDL-801 and UBCS039 are shown in green and blue sticks, respectively.
Fig 3 Structure and binding modes of SIRT6 activators
| 1 | WU Q J, ZHANG T N, CHEN H H, et al. The sirtuin family in health and disease[J]. Signal Transduct Target Ther, 2022, 7(1): 402. |
| 2 | SHARMA A, MAHUR P, MUTHUKUMARAN J, et al. Shedding light on structure, function and regulation of human sirtuins: a comprehensive review[J]. 3 Biotech, 2023, 13(1): 29. |
| 3 | DZIDEK A, CZERWIŃSKA-LEDWIG O, ŻYCHOWSKA M, et al. The role of increased expression of sirtuin 6 in the prevention of premature aging pathomechanisms[J]. Int J Mol Sci, 2023, 24(11): 9655. |
| 4 | KLEIN M A, DENU J M. Biological and catalytic functions of sirtuin 6 as targets for small-molecule modulators[J]. J Biol Chem, 2020, 295(32): 11021-11041. |
| 5 | HOU T Y, TIAN Y, CAO Z Y, et al. Cytoplasmic SIRT6-mediated ACSL5 deacetylation impedes nonalcoholic fatty liver disease by facilitating hepatic fatty acid oxidation[J]. Mol Cell, 2022, 82(21): 4099-4115.e9. |
| 6 | PAN P W, FELDMAN J L, DEVRIES M K, et al. Structure and biochemical functions of SIRT6[J]. J Biol Chem, 2011, 286(16): 14575-14587. |
| 7 | YOU Y Z, LIANG W. SIRT1 and SIRT6: the role in aging-related diseases[J]. Biochim Biophys Acta Mol Basis Dis, 2023, 1869(7): 166815. |
| 8 | MAHLKNECHT U, HO A D, VOELTER-MAHLKNECHT S. Chromosomal organization and fluorescence in situ hybridization of the human Sirtuin 6 gene[J]. Int J Oncol, 2006, 28(2): 447-456. |
| 9 | TENNEN R I, BERBER E, CHUA K F. Functional dissection of SIRT6: identification of domains that regulate histone deacetylase activity and chromatin localization[J]. Mech Ageing Dev, 2010, 131(3): 185-192. |
| 10 | SMIRNOVA E, BIGNON E, SCHULTZ P, et al. Binding to nucleosome poises human SIRT6 for histone H3 deacetylation[J]. Elife, 2024, 12: RP87989. |
| 11 | WANG Z A, MARKERT J W, WHEDON S D, et al. Structural basis of sirtuin 6-catalyzed nucleosome deacetylation[J]. J Am Chem Soc, 2023, 145(12): 6811-6822. |
| 12 | KALOUS K S, WYNIA-SMITH S L, OLP M D, et al. Mechanism of Sirt1 NAD+-dependent protein deacetylase inhibition by cysteine S-nitrosation[J]. J Biol Chem, 2016, 291(49): 25398-25410. |
| 13 | SACCONNAY L, CARRUPT P A, NURISSO A. Human sirtuins: structures and flexibility[J]. J Struct Biol, 2016, 196(3): 534-542. |
| 14 | MIN J, LANDRY J, STERNGLANZ R, et al. Crystal structure of a SIR2 homolog-NAD complex[J]. Cell, 2001, 105(2): 269-279. |
| 15 | RONNEBAUM S M, WU Y X, MCDONOUGH H, et al. The ubiquitin ligase CHIP prevents SirT6 degradation through noncanonical ubiquitination[J]. Mol Cell Biol, 2013, 33(22): 4461-4472. |
| 16 | JIN L, WEI W T, JIANG Y B, et al. Crystal structures of human SIRT3 displaying substrate-induced conformational changes[J]. J Biol Chem, 2009, 284(36): 24394-24405. |
| 17 | BARAN M, MIZIAK P, STEPULAK A, et al. The role of sirtuin 6 in the deacetylation of histone proteins as a factor in the progression of neoplastic disease[J]. Int J Mol Sci, 2023, 25(1): 497. |
| 18 | QIU B Q, LI S, LI M T, et al. KAT8 acetylation-controlled lipolysis affects the invasive and migratory potential of colorectal cancer cells[J]. Cell Death Dis, 2023, 14(2): 164. |
| 19 | MOSTOSLAVSKY R, CHUA K F, LOMBARD D B, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6[J]. Cell, 2006, 124(2): 315-329. |
| 20 | LU Z Z, CHEN H J, WANG W Q, et al. Synthesized soliton crystals[J]. Nat Commun, 2021, 12(1): 3179. |
| 21 | TIAN X, FIRSANOV D, ZHANG Z H, et al. SIRT6 is responsible for more efficient DNA double-strand break repair in long-lived species[J]. Cell, 2019, 177(3): 622-638.e22. |
| 22 | MAO Z Y, HINE C, TIAN X, et al. SIRT6 promotes DNA repair under stress by activating PARP1[J]. Science, 2011, 332(6036): 1443-1446. |
| 23 | MICHISHITA E, MCCORD R A, BOXER L D, et al. Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human SIRT6[J]. Cell Cycle, 2009, 8(16): 2664-2666. |
| 24 | XIAO C Y, KIM H S, LAHUSEN T, et al. SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice[J]. J Biol Chem, 2010, 285(47): 36776-36784. |
| 25 | ROICHMAN A, ELHANATI S, AON M A, et al. Restoration of energy homeostasis by SIRT6 extends healthy lifespan[J]. Nat Commun, 2021, 12(1): 3208. |
| 26 | DONG X C. Sirtuin 6: a key regulator of hepatic lipid metabolism and liver health[J]. Cells, 2023, 12(4): 663. |
| 27 | ZHANG W Q, WAN H F, FENG G H, et al. SIRT6 deficiency results in developmental retardation in cynomolgus monkeys[J]. Nature, 2018, 560(7720): 661-665. |
| 28 | SIMON M, YANG J P, GIGAS J, et al. A rare human centenarian variant of SIRT6 enhances genome stability and interaction with Lamin A[J]. EMBO J, 2022, 41(21): e110393. |
| 29 | FERRER C M, ALDERS M, POSTMA A V, et al. An inactivating mutation in the histone deacetylase SIRT6 causes human perinatal lethality[J]. Genes Dev, 2018, 32(5/6): 373-388. |
| 30 | KIM H S, XIAO C Y, WANG R H, et al. Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis[J]. Cell Metab, 2010, 12(3): 224-236. |
| 31 | NAIMAN S, HUYNH F K, GIL R, et al. SIRT6 promotes hepatic β-oxidation via activation of PPARα[J]. Cell Rep, 2019, 29(12): 4127-4143.e8. |
| 32 | TAO R Y, XIONG X W, DEPINHO R A, et al. FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression[J]. J Biol Chem, 2013, 288(41): 29252-29259. |
| 33 | ELHANATI S, KANFI Y, VARVAK A, et al. Multiple regulatory layers of SREBP1/2 by SIRT6[J]. Cell Rep, 2013, 4(5): 905-912. |
| 34 | ZHONG L, D′URSO A, TOIBER D, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha[J]. Cell, 2010, 140(2): 280-293. |
| 35 | DOMINY J E Jr, LEE Y, JEDRYCHOWSKI M P, et al. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis[J]. Mol Cell, 2012, 48(6): 900-913. |
| 36 | BIAN C, ZHANG R J, WANG Y X, et al. Sirtuin 6 affects glucose reabsorption and gluconeogenesis in type 1 diabetes via FoxO1[J]. Mol Cell Endocrinol, 2022, 547: 111597. |
| 37 | GUO Z Y, LI P, GE J B, et al. SIRT6 in aging, metabolism, inflammation and cardiovascular diseases[J]. Aging Dis, 2022, 13(6): 1787-1822. |
| 38 | GROOTAERT M O J, BENNETT M R. Sirtuins in atherosclerosis: guardians of healthspan and therapeutic targets[J]. Nat Rev Cardiol, 2022, 19(10): 668-683. |
| 39 | ROE K. An inflammation classification system using cytokine parameters[J]. Scand J Immunol, 2021, 93(2): e12970. |
| 40 | SUN H L, WU Y R, FU D J, et al. SIRT6 regulates osteogenic differentiation of rat bone marrow mesenchymal stem cells partially via suppressing the nuclear factor-κB signaling pathway[J]. Stem Cells, 2014, 32(7): 1943-1955. |
| 41 | CASPER E. The crosstalk between Nrf2 and NF-κB pathways in coronary artery disease: can it be regulated by SIRT6?[J]. Life Sci, 2023, 330: 122007. |
| 42 | XU S W, YIN M M, KOROLEVA M, et al. SIRT6 protects against endothelial dysfunction and atherosclerosis in mice[J]. Aging, 2016, 8(5): 1064-1082. |
| 43 | LEE Y, KA S O, CHA H N, et al. Myeloid sirtuin 6 deficiency causes insulin resistance in high-fat diet-fed mice by eliciting macrophage polarization toward an M1 phenotype[J]. Diabetes, 2017, 66(10): 2659-2668. |
| 44 | DING Y N, WANG T T, LV S J, et al. SIRT6 is an epigenetic repressor of thoracic aortic aneurysms via inhibiting inflammation and senescence[J]. Signal Transduct Target Ther, 2023, 8(1): 255. |
| 45 | TATONE C, EMIDIO G D, BARBONETTI A, et al. Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility[J]. Hum Reprod Update, 2018, 24(3): 267-289. |
| 46 | HAN L S, GE J, ZHANG L, et al. Sirt6 depletion causes spindle defects and chromosome misalignment during meiosis of mouse oocyte[J]. Sci Rep, 2015, 5: 15366. |
| 47 | LIU W J, ZHANG X M, WANG N, et al. Calorie restriction inhibits ovarian follicle development and follicle loss through activating SIRT1 signaling in mice[J]. Eur J Med Res, 2015, 20(1): 22. |
| 48 | LI L Y, HUA R, HU K Q, et al. SIRT6 deficiency causes ovarian hypoplasia by affecting Plod1-related collagen formation[J]. Aging Cell, 2024, 23(2): e14031. |
| 49 | BARTOSCH C, MONTEIRO-REIS S, ALMEIDA-RIOS D, et al. Assessing sirtuin expression in endometrial carcinoma and non-neoplastic endometrium[J]. Oncotarget, 2016, 7(2): 1144-1154. |
| 50 | DROBINTSEVA A O, MEDVEDEV D S, MAKARENKO S V, et al. Implication of sirtuins and kisspeptin in ovarian aging[J]. Usp Gerontol, 2021, 34(1): 18-23. |
| 51 | MICHISHITA E, PARK J Y, BURNESKIS J M, et al. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins[J]. Mol Biol Cell, 2005, 16(10): 4623-4635. |
| 52 | PASCOAL G F L, GERALDI M V, MARÓSTICA M R Jr, et al. Effect of paternal diet on spermatogenesis and offspring health: focus on epigenetics and interventions with food bioactive compounds[J]. Nutrients, 2022, 14(10): 2150. |
| 53 | WU Y F, YING J H, ZHU X Y, et al. Pachymic acid suppresses the inflammatory response of chondrocytes and alleviates the progression of osteoarthritis via regulating the Sirtuin 6/NF-κB signal axis[J]. Int Immunopharmacol, 2023, 124(Pt A): 110854. |
| 54 | LEE A, GU H, GWON M H, et al. Hesperetin suppresses LPS/high glucose-induced inflammatory responses via TLR/MyD88/NF-κB signaling pathways in THP-1 cells[J]. Nutr Res Pract, 2021, 15(5): 591-603. |
| 55 | PAN Z S, GUO J Y, TANG K J, et al. Ginsenoside Rc modulates SIRT6-NRF2 interaction to alleviate alcoholic liver disease[J]. J Agric Food Chem, 2022, 70(44): 14220-14234. |
| 56 | WU R Y, JIAN T, DING X Q, et al. Total sesquiterpene glycosides from loquat leaves ameliorate HFD-induced insulin resistance by modulating IRS-1/GLUT4, TRPV1, and SIRT6/Nrf2 signaling pathways[J]. Oxid Med Cell Longev, 2021, 2021: 4706410. |
| 57 | LOMBARDO G E, RUSSO C, MAUGERI A, et al. Sirtuins as players in the signal transduction of Citrus flavonoids[J]. Int J Mol Sci, 2024, 25(4): 1956. |
| 58 | IACHETTINI S, TRISCIUOGLIO D, ROTILI D, et al. Pharmacological activation of SIRT6 triggers lethal autophagy in human cancer cells[J]. Cell Death Dis, 2018, 9(10): 996. |
| 59 | JIAO F Z, ZHANG Z W, HU H T, et al. SIRT6 activator UBCS039 inhibits thioacetamide-induced hepatic injury in vitro and in vivo[J]. Front Pharmacol, 2022, 13: 837544. |
| 60 | HUANG Z M, ZHAO J X, DENG W, et al. Identification of a cellularly active SIRT6 allosteric activator[J]. Nat Chem Biol, 2018, 14(12): 1118-1126. |
| 61 | HUANG Z M, ZHAO J X, DENG W, et al. Reply to: binding site for MDL-801 on SIRT6[J]. Nat Chem Biol, 2021, 17(5): 522-523. |
| 62 | YOU W J, STEEGBORN C. Binding site for activator MDL-801 on SIRT6[J]. Nat Chem Biol, 2021, 17(5): 519-521. |
| 63 | WU X, LIU H, BROOKS A, et al. SIRT6 mitigates heart failure with preserved ejection fraction in diabetes[J]. Circ Res, 2022, 131(11): 926-943. |
| 64 | ZHANG J H, LI Y P, LIU Q H, et al. Sirt6 alleviated liver fibrosis by deacetylating conserved lysine 54 on Smad2 in hepatic stellate cells[J]. Hepatology, 2021, 73(3): 1140-1157. |
| 65 | CHEN Y, CHEN J Y, SUN X X, et al. The SIRT6 activator MDL-800 improves genomic stability and pluripotency of old murine-derived iPS cells[J]. Aging Cell, 2020, 19(8): e13185. |
| [1] | 朱子俊, 钱逸斐, 李倩玉, 李松玲, 覃雯莉, 刘艳丰. 后期促进复合体亚基10调控PI3K-AKT-mTOR通路促进肝细胞癌进展的研究[J]. 上海交通大学学报(医学版), 2025, 45(9): 1171-1182. |
| [2] | 王治琪, 王莹. 儿童炎症性肠病相关贫血的诊治研究进展[J]. 上海交通大学学报(医学版), 2025, 45(9): 1232-1238. |
| [3] | 杨全军, 柏丁源, 周雨萱, 白露, 郭澄. 异柠檬酸脱氢酶1突变介导D-2-羟基戊二酸代谢重编程在肿瘤免疫调控中的作用及相关药物研发进展[J]. 上海交通大学学报(医学版), 2025, 45(9): 1239-1248. |
| [4] | 黄昕, 刘家辉, 叶敬文, 钱文莉, 许万星, 王琳. 基于机器学习的小细胞肺癌代谢分子诊断模型的建立和临床应用[J]. 上海交通大学学报(医学版), 2025, 45(8): 1009-1016. |
| [5] | 赛提尔古丽·克然木, 钱蕾, 丁思怡, 哈娜提·马合力木汗, 杨雪儿, 贾浩. 精氨酸代谢调控间充质干细胞功能的研究进展[J]. 上海交通大学学报(医学版), 2025, 45(7): 910-915. |
| [6] | 宋静, 姜烁, 万方煜, 李娟, 艾迪娜·木合塔, 闵新颖, 周婧琪. 膳食模式干预对代谢相关脂肪性肝病的影响与机制研究进展[J]. 上海交通大学学报(医学版), 2025, 45(7): 926-933. |
| [7] | 赵心雨, 张文超, 陈旭卓, 宋佳琪, 黄慧, 张善勇. 亚精胺对脂多糖诱导的小鼠颅骨炎症性骨溶解的作用研究[J]. 上海交通大学学报(医学版), 2025, 45(6): 673-683. |
| [8] | 韩龙传, 李悦, 邹智慧, 罗静, 李若伊, 张颖婷, 唐欣欣, 田丽红, 陆宇恒, 黄莺, 贺明, 付寅坤. 磷脂酰乙醇胺引起内质网应激促进巨噬细胞衰老及肝损伤[J]. 上海交通大学学报(医学版), 2025, 45(6): 693-704. |
| [9] | 杨乐, 周怡, 王钶韵, 赖娅莉. 大黄素改善阿尔茨海默病认知障碍、内质网应激和神经炎症的研究[J]. 上海交通大学学报(医学版), 2025, 45(6): 727-734. |
| [10] | 张星语, 李若谷. 主动脉瘤单细胞转录组的系统性分析与探索[J]. 上海交通大学学报(医学版), 2025, 45(6): 735-744. |
| [11] | 黄英荷, 招冠钰, 孙阳, 侯鉴基, 左勇. 2型糖尿病创面愈合中巨噬细胞代谢调控的研究进展[J]. 上海交通大学学报(医学版), 2025, 45(6): 792-799. |
| [12] | 禹恺, 帅哲玮, 黄洪军, 罗艳. 小胶质细胞在中枢神经系统炎症性疾病中的作用和机制研究进展[J]. 上海交通大学学报(医学版), 2025, 45(5): 630-638. |
| [13] | 万宏劲, 胡逸斌, 王昕, 张凯, 秦安, 马培翔, 马辉, 赵杰. 甲基莲心碱通过KEAP1/NRF2/GPX4和NF-κB信号通路减轻椎间盘退行性变[J]. 上海交通大学学报(医学版), 2025, 45(3): 261-270. |
| [14] | 邹沛辰, 刘鸿宇, 阿衣娜扎尔·艾合买提, 朱亮, 唐亚斌, 雷绘敏. 索托拉西布获得性耐药肺癌细胞的代谢轮廓分析[J]. 上海交通大学学报(医学版), 2025, 45(2): 138-149. |
| [15] | PANDIT Roshan, 卢君瑶, 何立珩, 包玉洁, 季萍, 陈颖盈, 许洁, 王颖. 肿瘤坏死因子-α在新型冠状病毒感染合并肾损伤中的作用[J]. 上海交通大学学报(医学版), 2025, 45(1): 1-10. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||